Alcoholic Liver Disease and Systemic Inflammatory Response Syndrome: Mortality Prediction Using Biomarkers and Clinical Scores
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Evaluation of Infection and Septic Shock
2.3. Statistical Analysis
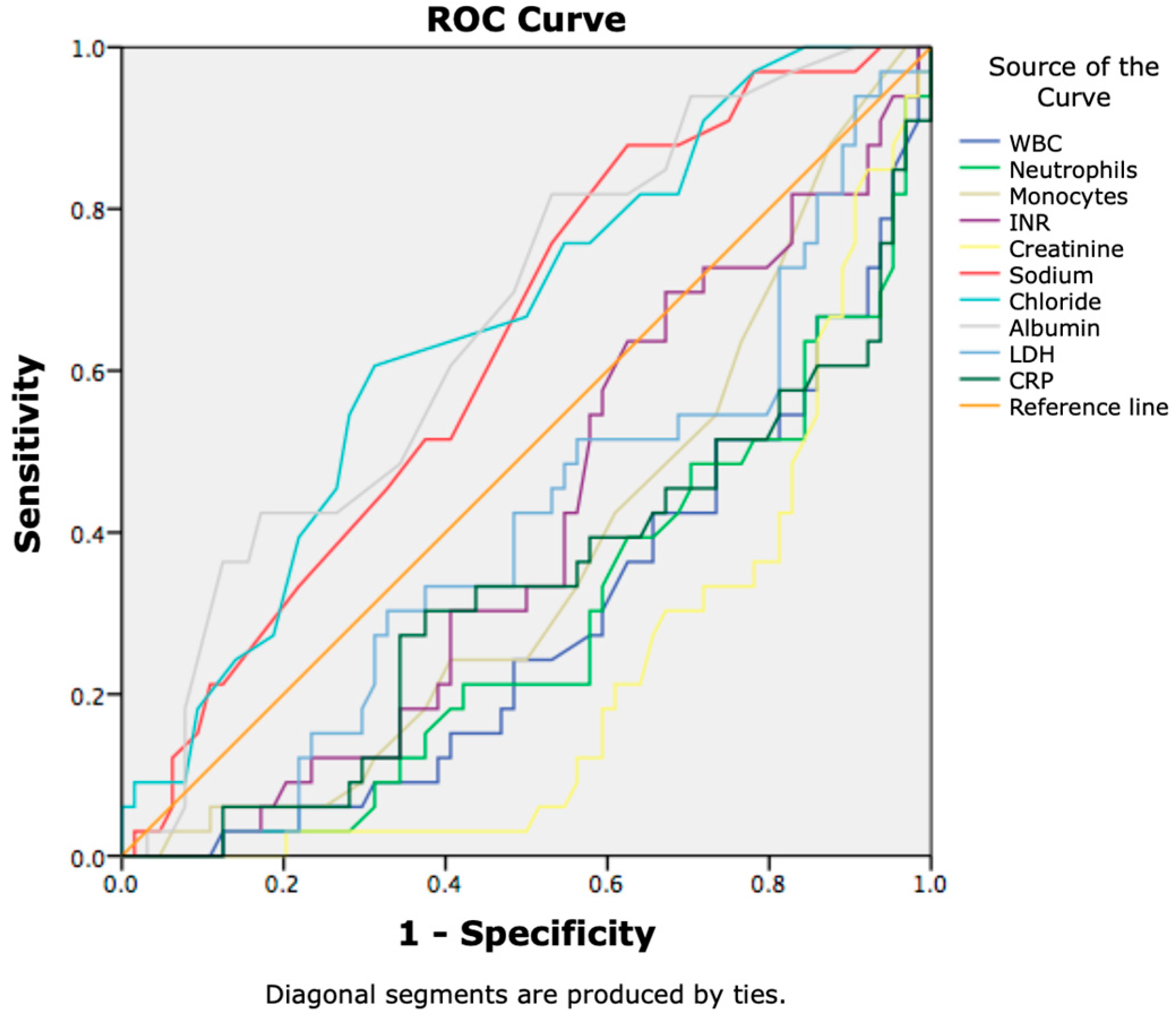
3. Results
3.1. Demographic Characteristics
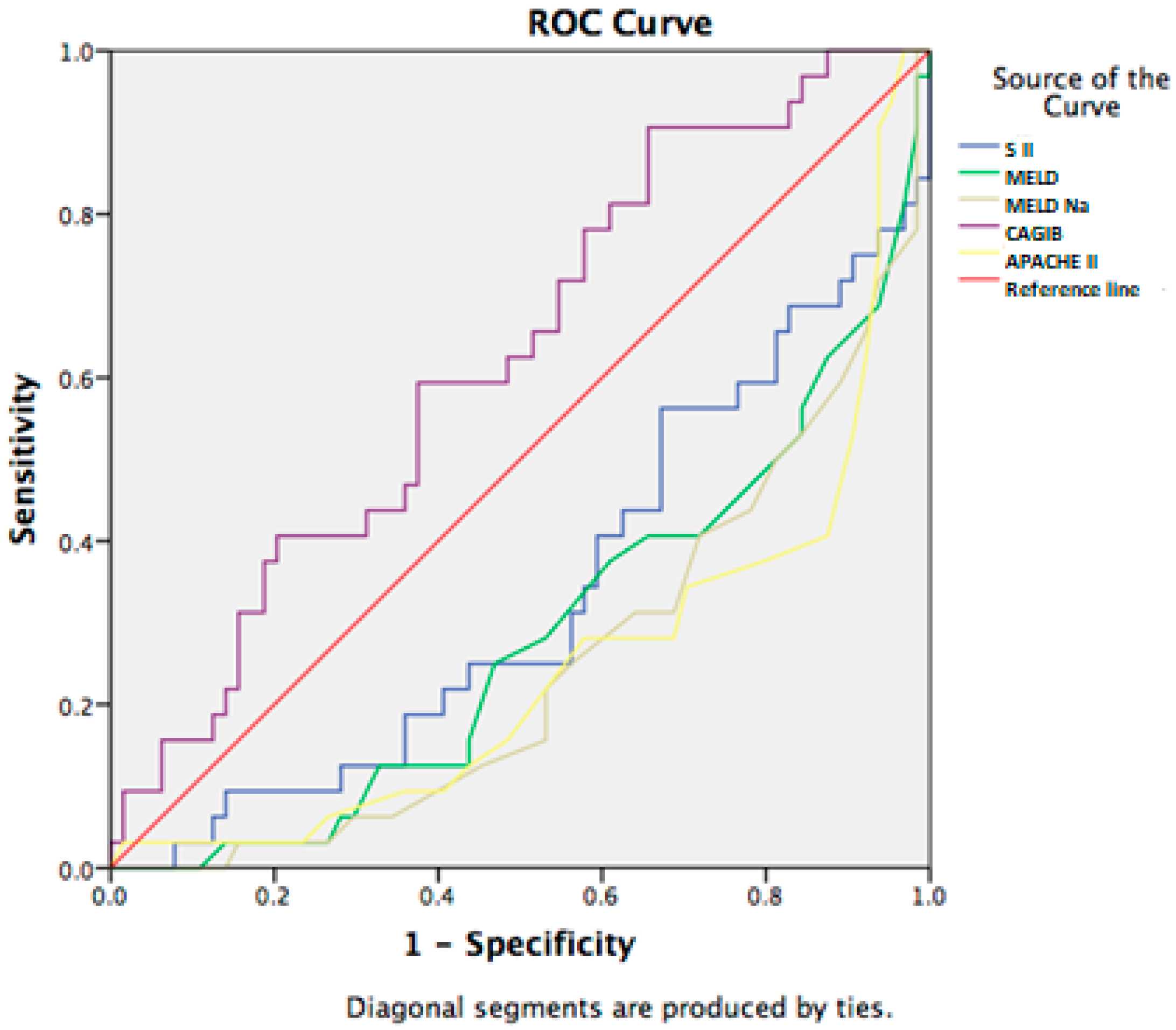
3.2. Prognostic Factors for 7-Day Lethal Outcome in Cirrhotic Patients with SIRS
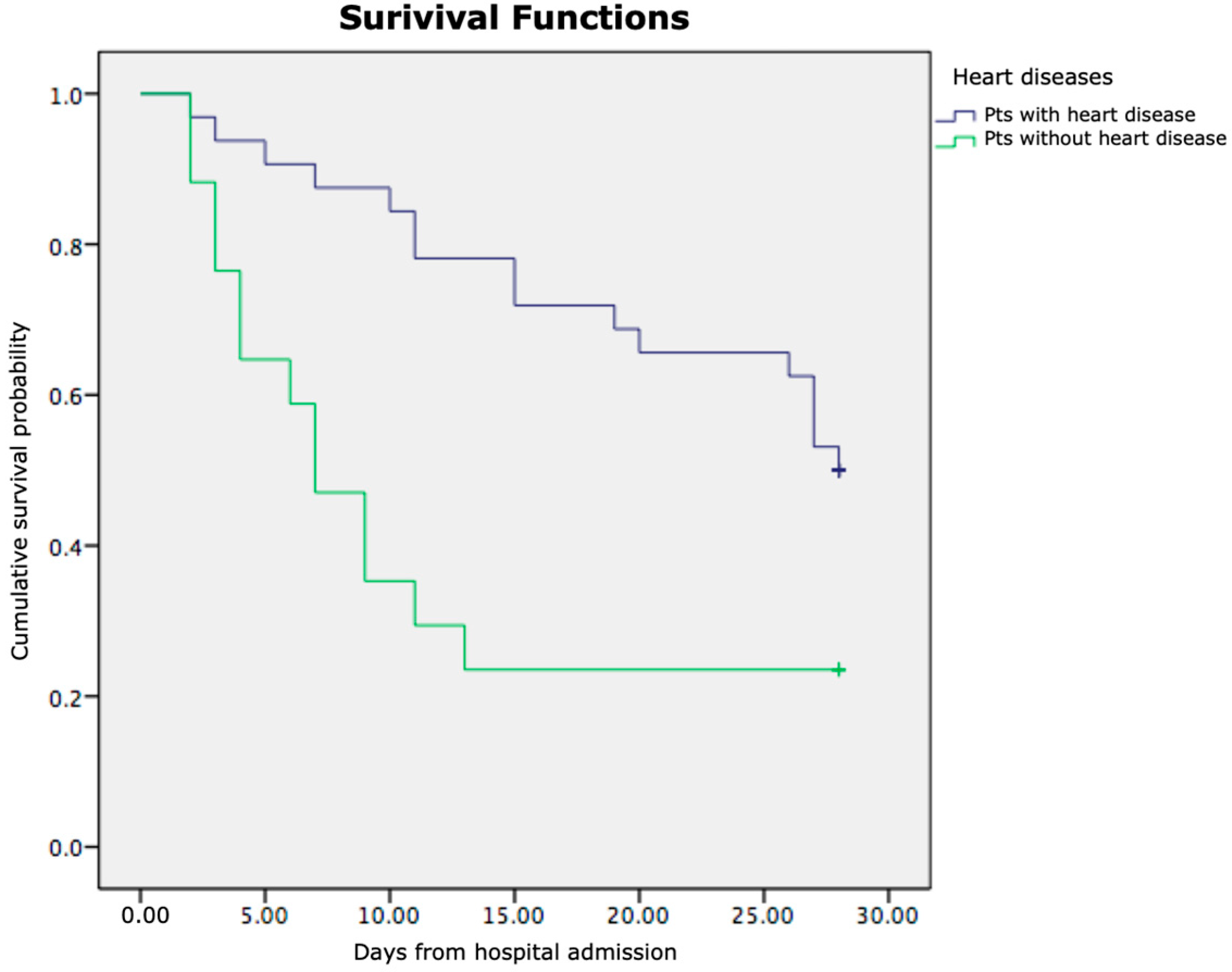
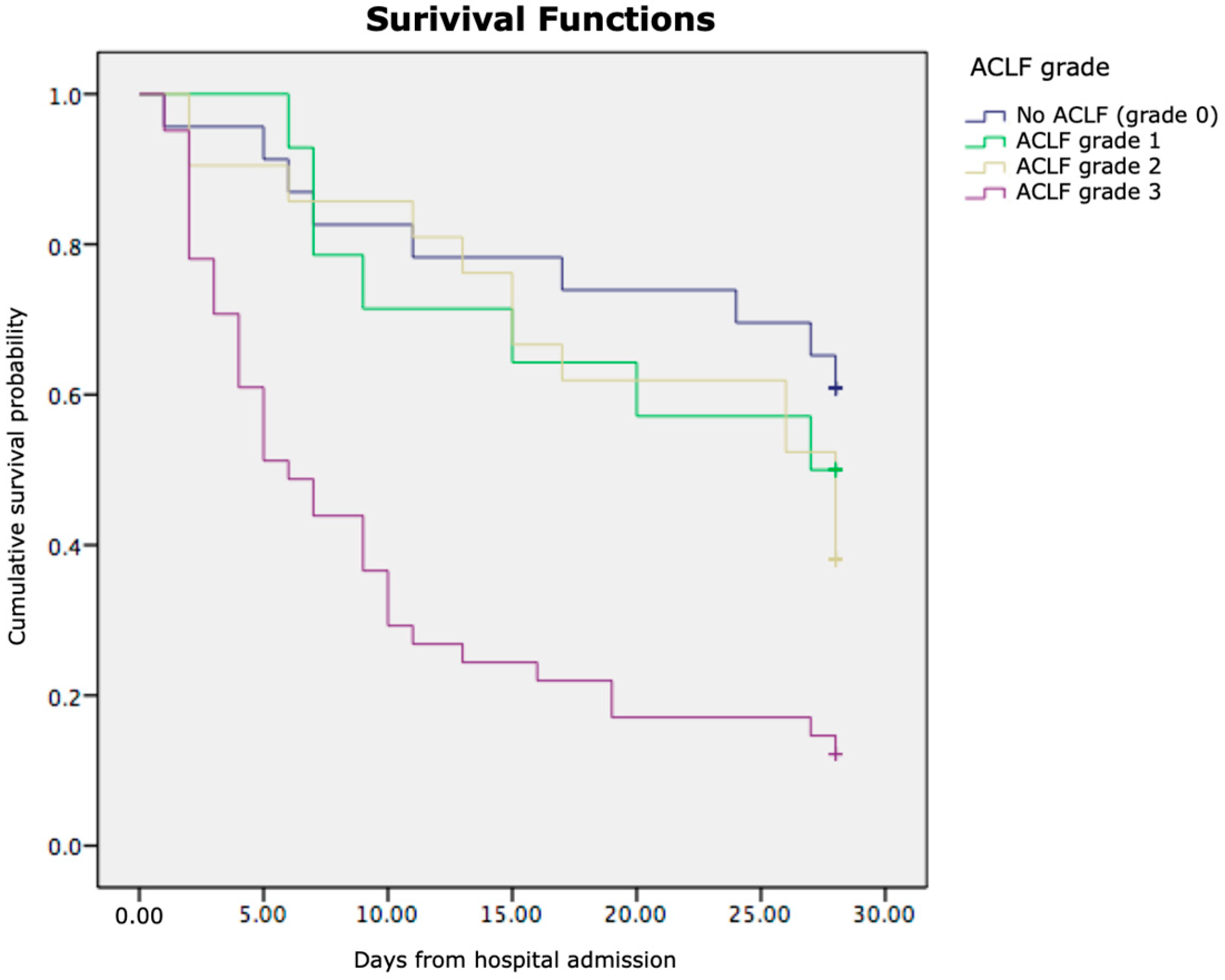
3.3. Kaplan–Meier Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Navasa, M.; Rodes, J. Bacterial infections in cirrhosis. Liver Int. 2004, 24, 277–280. [Google Scholar] [CrossRef]
- Foreman, M.G.; Mannino, D.M.; Moss, M. Cirrhosis as a risk factor for sepsis and death: Analysis of the national hospital discharge survey. Chest 2003, 124, 1016–1020. [Google Scholar] [CrossRef]
- Duo, H.; You, J.; Du, S.; Yu, M.; Wu, S.; Yue, P.; Cui, X.; Huang, Y.; Luo, J.; Pan, H.; et al. Liver cirrhosis in 2021: Global Burden of Disease study. PLoS ONE 2025, 20, e0328493. [Google Scholar] [CrossRef]
- Rehm, J.; Baliunas, D.; Borges, G.L.G.; Graham, K.; Irving, H.; Kehoe, T.; Parry, C.P.; Patra, J.; Popova, S.; Poznyak, V.; et al. The relation between different dimensions of alcohol consumption and burden of disease: An overview. Addiction 2010, 105, 817–843. [Google Scholar] [CrossRef]
- Weigand, M.A.; Hörner, C.; Bardenheuer, H.J.; Bouchon, A. The systemic inflammatory response syndrome. Best. Pract. Res. Clin. Anaesthesiol. 2004, 18, 455–475. [Google Scholar] [CrossRef]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.H.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef]
- Jalan, R.; Fernandez, J.; Wiest, R.; Schnabl, B.; Moreau, R.; Angeli, P.; Stadlbauer, V.; Gustot, T.; Bernardi, M.; Canton, R.; et al. Bacterial infections in cirrhosis: A position statement based on the EASL Special Conference 2013. J. Hepatol. 2014, 60, 1310–1324. [Google Scholar] [CrossRef] [PubMed]
- Tandon, P.; Garcia-Taso, G. Bacterial infection, sepsis, and multiorgan failure in cirrhosis. Semin. Liver Dis. 2008, 28, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Bruns, T.; Zimmermann, H.W.; Stallmach, A. Risk factors and outcome of bacterial infections in cirrhosis. World J. Gastroenterol. 2014, 20, 2542–2554. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Khalek, E.E.; El-Fakhry, A.; Helaly, M.; Hamed, M.; Elbaz, O. Systemic inflammatory response syndrome in patients with liver cirrhosis. Arab. J. Gastroenterol. 2011, 12, 173–177. [Google Scholar] [CrossRef]
- Shawcross, D.L.; Davies, N.A.; Williams, R.; Jalan, R. Systemic inflammatory response exacerbates the neuropsychological effects of induced hyperammonemia in cirrhosis. J. Hepatol. 2004, 40, 247–254. [Google Scholar] [CrossRef]
- Rolando, N.; Wade, J.; Davalos, M.; Wendon, J.; Philpott-Howard, J.; Williams, R. The systemic inflammatory response syndrome in acute liver failure. Hepatology 2000, 32, 734–739. [Google Scholar] [CrossRef]
- Piano, S.; Bartoletti, M.; Tonon, M.; Baldassarre, M.; Chies, G.; Romano, A.; Viale, P.; Vettore, E.; Domenicali, M.; Stanco, M.; et al. Assessment of Sepsis-3 criteria and quick SOFA in patients with cirrhosis and bacterial infections. Gut 2018, 67, 1892–1899. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Angeli, P.; Ginès, P.; Wong, F.; Bernardi, M.; Boyer, T.D.; Gerbes, A.; Moreau, R.; Jalan, R.; Sarin, S.K.; Piano, S.; et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: Revised consensus recommendations of the International Club of Ascites. J. Hepatol. 2015, 62, 968–974. [Google Scholar] [CrossRef]
- Papp, M.; Vitalis, Z.; Altorjay, I.; Tornai, I.; Harsfalvi, J.; Vida, A.; Kappelmayer, J.; Lakatos, P.L.; Antal-Szalmas, P. Acute phase proteins in the diagnosis and prediction of cirrhosis associated bacte rial infections. Liver Int. 2012, 32, 603–611. [Google Scholar] [CrossRef] [PubMed]
- McPhail, M.J.W.; Parrott, F.; Wendon, J.A.; Harrison, D.A.; Rowan, K.A.; Bernal, W. Incidence and outcomes for patients with cirrhosis admitted to United Kingdom critical care units. Crit. Care Med. 2018, 46, 705–712. [Google Scholar] [CrossRef]
- Skurzak, S.; Carrara, G.; Rossi, C.; Nattino, G.; Crespi, D.; Giardino, M.; Bertolini, G. Cirrhotic patients admitted to the ICU for medical reasons: Analysis of 5506 patients admitted to 286 ICUs in 8 years. J. Crit. Care 2018, 45, 220–228. [Google Scholar] [CrossRef]
- Ho, Y.P.; Chen, Y.C.; Yang, C.; Lien, J.M.; Chu, Y.Y.; Fang, J.T.; Chiu, C.T.; Chen, P.C.; Tsai, M.H. Outcome prediction for critically ill cirrhotic patients: A comparison of APACHE II and Child-Pugh scoring systems. J. Intensive Care Med. 2004, 19, 105–110. [Google Scholar] [CrossRef]
- Churpek, M.M.; Zadravecz, F.J.; Winslow, C.; Howell, M.D.; Edelson, D.P. Incidence and prognostic value of the systemic inflammatory response syndrome and organ dysfunctions in ward patients. Am. J. Respir. Crit. Care Med. 2015, 192, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Pugh, R.N.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef]
- Malinchoc, M.; Kamath, P.S.; Fredric, G.D.; Peine, P.J.; Jeffrey, R.; Borg, T.; Pieter, C.J. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000, 31, 864–871. [Google Scholar] [CrossRef]
- Jalan, R.; Gines, P.; Olson, J.C.; Mookerjee, R.P.; Moreau, R.; Garcia-Tsao, G.; Arroyo, V.; Kamath, P.S. Acute-on chronic liver failure. J. Hepatol. 2012, 57, 1336–1348. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure: On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Bai, Z.; Li, B.; Lin, S.; Liu, B.; Li, Y.; Zhu, Q.; Wu, Y.; Yang, Y.; Tang, S.; Meng, F.; et al. Development and Validation of CAGIB Score for Evaluating the Prognosis of Cirrhosis with Acute Gastrointestinal Bleeding: A Retrospective Multicenter Study. Adv. Ther. 2019, 36, 3211–3220. [Google Scholar] [CrossRef]
- Dong, T.S.; Kalani, A.; Aby, E.S.; Le, L.; Luu, K.; Hauer, M.; Kamath, R.; Lindor, K.D.; Tabibian, J.H. Machine learning-based development and validation of a scoring system for screening high-risk esophageal varices. Clin. Gastroenterol. Hepatol. 2019, 17, 1894–1901. [Google Scholar] [CrossRef] [PubMed]
- Moreau, R.; Jalan, R.; Gines, P.; Pavesi, M.; Angeli, P.; Cordoba, J.; Durand, F.; Gustot, T.; Saliba, F.; Domenicali, M.; et al. Acute-on chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013, 144, 1426–1437. [Google Scholar] [CrossRef]
- Vilstrup, H.; Amodio, P.; Bajaj, J.; Cordoba, J.; Ferenci, P.; Mullen, K.D.; Weissenborn, K.; Wong, P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014, 60, 715–735. [Google Scholar] [CrossRef]
- Kim, W.R.; Biggins, S.W.; Kremers, W.K.; Wiesner, R.H.; Kamath, P.S.; Benson, J.T.; Edwards, E.; Therneau, T.M. Hyponatremia and Mortality among Patients on the Liver-Transplant Waiting List. N. Engl. J. Med. 2008, 359, 1018–1026. [Google Scholar] [CrossRef]
- Zahorec, R. Ratio of neutrophil to lymphocyte counts—Rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl. Lek. Listy 2001, 102, 5–14. [Google Scholar]
- Proctor, M.J.; McMillan, D.C.; Morrison, D.S.; Fletcher, C.D.; Horgan, P.G.; Clarke, S.J. A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br. J. Cancer 2012, 107, 695–699. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, J.; Wang, J.; Xie, T.; Zhang, Q.; Feng, S.; Deng, H.; Zhong, B. Platelet-to-lymphocyte ratio (PLR) and neutrophil to-lymphocyte ratio (NLR) are associated with chronic hepatitis B virus (HBV) infection. Int. Immunopharmacol. 2017, 51, 1–8. [Google Scholar] [CrossRef]
- Hu, B.; Yang, X.R.; Xu, Y.; Sun, Y.F.; Sun, C.; Guo, W.; Zhang, X.; Wang, W.M.; Qiu, S.J.; Zhou, J.; et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 2014, 20, 6212–6222. [Google Scholar] [CrossRef] [PubMed]
- Jalan, R.; Saliba, F.; Pavesi, M.; Amoros, A.; Moreau, R.; Ginès, P.; Levesque, E.; Durand, F.; Angeli, P.; Caraceni, P.; et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J. Hepatol. 2014, 61, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Michelena, J.; Altamirano, J.; Abraldes, J.G.; Affò, S.; Morales-Ibanez, O.; Sancho-Bru, P.; Dominguez, M.; García-Pagán, J.C.; Fernández, J.; Arroyo, V.; et al. Systemic inflammatory response and serum lipopolysaccharide levels predict multiple organ failure and death in alcoholic hepatitis. Hepatology 2015, 62, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; O’Leary, J.G.; Reddy, K.R.; Wong, F.; Biggins, S.W.; Patton, H.; Fallon, M.B.; Garcia-Tsao, G.; Maliakkal, B.; Malik, R.; et al. Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology 2014, 60, 250–256. [Google Scholar] [CrossRef]
- Chebl, R.B.; Assaf, M.; Kattouf, N.; Haidar, S.; Khamis, M.; Abdeldaem, K.; Makki, M.; Tamim, H.; Abou Dagher, G. The association between the neutrophil to lymphocyte ratio and in-hospital mortality among sepsis patients: A prospective study. Medicine 2022, 101, e29343. [Google Scholar] [CrossRef]
- Conway Morris, A.; Datta, D.; Shankar-Hari, M.; Stephen, J.; Weir, C.J.; Rennie, J.; Antonelli, J.; Bateman, A.; Warner, N.; Judge, K.; et al. Cell-surface signa tures of immune dysfunction risk-stratify critically ill patients: INFECT study. Intensive Care Med. 2018, 44, 627–635. [Google Scholar] [CrossRef]
- Chung, H.; Lee, J.H.; Jo, Y.H.; Hwang, J.E.; Kim, J. Circulating monocyte counts and its impact on outcomes in patients with severe sepsis including septic shock. Shock 2019, 51, 423–429. [Google Scholar] [CrossRef]
- Soni, J.R.; Marrapu, S.; Kumar, R. Hypochloremia is an underutilised prognostic marker in patients with advanced liver cirrhosis and liver failure. World J. Hepatol. 2025, 17, 103807. [Google Scholar] [CrossRef]
- Shrimanker, I.; Bhattarai, S. Electrolytes. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Lindvig, K.P.; Teisner, A.S.; Kjeldsen, J.; Strøm, T.; Toft, P.; Furhmann, V.; Krag, A. Allocation of patients with liver cirrhosis and organ failure to intensive care: Systematic review and a proposal for clinical practice. World J. Gastroenterol. 2015, 21, 8964–8973. [Google Scholar] [CrossRef]
- D’Amico, G.; Garcia-Tsao, G.; Pagliaro, L. Natural history and prognostic indicators of survival in cirrhosis: A systematic review of 118 studies. J. Hepatol. 2006, 44, 217–231. [Google Scholar] [CrossRef]
- Ahmed, N.A.; Gouda, T.E.; Hasan, A.S.; Elsaeed, A.; Atalla, H. The performance of different prognostic scores in cirrhotic patients admitted to intensive care unit. Egypt. Liver J. 2024, 14, 91. [Google Scholar] [CrossRef]
- Yazdanyar, A.; Maqsood, M.H.; Pelayo, J.; Sanon, J.; Quintero, E.; Lo, K.B.; Mathew, R.O.; Rangaswami, J. Clinical outcomes in patients with heart failure with and without cirrhosis: An analysis from the national inpatient sample. Rev. Cardiovasc. Med. 2021, 22, 925–929. [Google Scholar] [CrossRef]
- Bishay, K.; Tandon, P.; Fisher, S.; Yelle, D.; Carrigan, I.; Wooller, K.; Kelly, E. Clinical Factors Associated with Mortality in Cirrhotic Patients Presenting with Upper Gastrointestinal Bleeding. J. Can. Assoc. Gastroenterol. 2020, 3, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Rondon-Berrios, H.; Velez, J.C.Q. Hyponatremia in Cirrhosis. Clin. Liver Dis. 2022, 26, 149–164. [Google Scholar] [CrossRef]
- Ruf, A.E.; Kremers, W.K.; Chavez, L.L.; Descalzi, V.I.; Podesta, L.G.; Villamil, F.G. Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone. Liver Transplant. 2005, 11, 336–343. [Google Scholar] [CrossRef]
- Semmler, G.; Scheiner, B.; Balcar, L.; Paternostro, R.; Simbrunner, B.; Pinter, M.; Trauner, M.; Bofill Roig, M.; Meyer, E.L.; Hofer, B.S.; et al. Disturbances in sodium and chloride homeostasis predict outcome in stable and critically ill patients with cirrhosis. Aliment. Pharmacol. Ther. 2023, 58, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Li, L. Lower serum chloride concentrations are associated with increased risk of mortality in critically ill cirrhotic patients: An analysis of the MIMIC-III database. BMC Gastroenterol. 2021, 21, 200. [Google Scholar] [CrossRef] [PubMed]
- Gomez, E.V.; Rodriguez, Y.S.; Bertot, L.C.; Gonzalez, A.T.; Perez, Y.M.; Soler, E.A.; Garcia, A.Y.; Blanco, L.P. The natural history of compensated HCV-related cirrhosis: A prospective long-term study. J. Hepatol. 2013, 58, 434–444. [Google Scholar] [CrossRef]
- Li, H.J.; Guo, Z.M.; Yang, X.Y.; Sun, D.X. Mean arterial pressure drop is an independent risk factor of death in patients with HBV-related cirrhosis ascites. Turk. J. Gastroenterol. 2017, 28, 26–30. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, L.; Wang, Z.; Han, D.; Li, C.; Zheng, S.; Yin, H.; Lyu, J. A New Scoring System for Predicting In-hospital Death in Patients Having Liver Cirrhosis with Esophageal Varices. Front. Med. 2021, 8, 678646. [Google Scholar] [CrossRef]
- Moller, S.; Bernardi, M. Interactions of the heart and the liver. Eur. Heart J. 2013, 34, 2804. [Google Scholar] [CrossRef]
- Samsky, M.D.; Patel, C.B.; DeWald, T.A.; Smith, A.D.; Felker, G.M.; Rogers, J.G.; Hernandez, A.F. Cardiohepatic interactions in heart failure: An overview and clinical implications. JACC 2013, 61, 2397. [Google Scholar] [CrossRef] [PubMed]
- Xanthopoulos, A.R.C.; Kitai, T.; Triposkiadis, F. Heart Failure and Liver Disease: Cardiohepatic Interactions. JACC Heart Fail. 2019, 7, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Naser, J.; Lin, G.; Lee, S.S. Cardiomyopathy in cirrhosis: From pathophysiology to clinical care. JHEP Rep. 2023, 6, 100911. [Google Scholar] [CrossRef] [PubMed]

| Calculation Formulas of Non-Invasive Scores and Ratios |
|---|
| MELDscore = 9.57 × ln (Cr) + 3.78 × ln (TBIL) + 11.2 × ln (INR) + 6.43 |
| MELD-Na = MELD + 1.32 × (137-Na) − [0.033 × MELD × (137-Na)] |
| NLR = Neu/Ly |
| dNLR = Neu/(WBC − Neu) |
| PLR = Plt/Ly |
| S II = (Neu × Plt)/Ly |
| CAGIB = (Diabetes (yes = 1, no = 0) × 1.040) + (HCC (yes = 1, no = 0) × 0.974) + (TBIL × 0.005) − (ALB × 0.091) + (ALT × 0.001) + (Cr × 0.012) − 3.964. |
| CLIF-C ACLF = 10 × [0.33 × CLIF-C OF + 0.04 × age + 0.63 × ln (WBC) − 2] |
| Variables | Total Patients (n = 102) |
|---|---|
| Sex (m/f) | 72/30 |
| Age (years) | 56.6 ± 13.86 |
| SIRS | |
| SIRS 2 criteria | 63 (61.76%) |
| SIRS 3 criteria | 36 (35.29%) |
| SIRS 3 criteria | 3 (2.94%) |
| Comorbidities | |
| Heart disease (yes/no) | 17/85 |
| HBP (yes/no) | 27/75 |
| Diabetes mellitus (yes/no) | 13/89 |
| Previous hospitalisation (yes/no) | 41/61 |
| Ascites (yes/no) | 83/19 |
| Variceal bleeding (yes/no) | 19/79 |
| Hepatic encephalopathy (yes/no) | 72/30 |
| Septic shock (yes/no) | 13/89 |
| Laboratory test | |
| Hgb (g/L) | 94.33 ± 27.78 |
| WBC (109/L) | 13.62 ± 7.47 |
| Neutrophils | 11.91 ± 10.49 |
| Lymphocytes | 1.15 ± 0.83 |
| Monocytes | 1.03 ± 0.81 |
| Eosinophils | 0.92 ± 2.67 |
| Platelets (109/L) | 120.68 ± 92.75 |
| TBIL (mmol/L) | 132.44 ± 155.61 |
| Alb (g/L) | 29.19 ± 6.47 |
| AST (U/L) | 261.85 ± 874.91 |
| ALT (U/L) | 112.27 ± 478.76 |
| ALP (U/L) | 110.26 ± 49.90 |
| GGT (U/L) | 234.67 ± 359 |
| sCr (µmol/L) | 169.69 ± 135.16 |
| BUN (mmol/L) | 16.56 ± 30.74 |
| LDH(U/L) | 674.38 ± 580.28 |
| CRP (mg/L) | 61.06 ± 63.13 |
| Pct (ng/L) | 5.91 ± 15.59 |
| Na (mmol/L) | 134.91 ± 6.20 |
| K (mmol/L) | 4.23 ± 1.03 |
| Cl (mmol/L) | 99.1 ± 7.81 |
| INR | 2.14 ± 0.91 |
| MELD score | 24.68 ± 8.43 |
| MELD-Na score | 26.23 ± 7.96 |
| ACLF (yes/no) | 77/25 |
| ACLF gr.1 | 14 (13.73%) |
| ACLF gr.2 | 21 (20.59%) |
| ACLF gr.3 | (41.18%) |
| CLIF-C-OF score | 10.99 ± 3.1 |
| APACHE II score | 21.23 ± 7.66 |
| EVendo score | 8.71 ± 4.96 |
| S II | 1731.03 ± 2710.45 |
| CAGIB score | −6.93 ± 2.93 |
| 7-day survived/non-survived | 69/33 |
| 28-day survived/non-survived | 37/65 |
| Variables | Survived Pts | Non-Survived Pts | p-Value |
|---|---|---|---|
| WBC | 11.89 ± 6.84 | 17.1 ± 7.56 | 0.001 |
| PT | 22.94 ± 8.04 | 30.14 ± 13.59 | 0.007 |
| INR | 1.96 ± 0.73 | 2.51 ± 1.11 | 0.012 |
| sCr | 129.95 ± 87.99 | 249.14 ± 174.45 | 0.001 |
| Cl | 100.58 ± 6.97 | 95.91 ± 8.63 | 0.010 |
| LDH | 512.85 ± 192.83 | 1012.59 ± 900.47 | 0.004 |
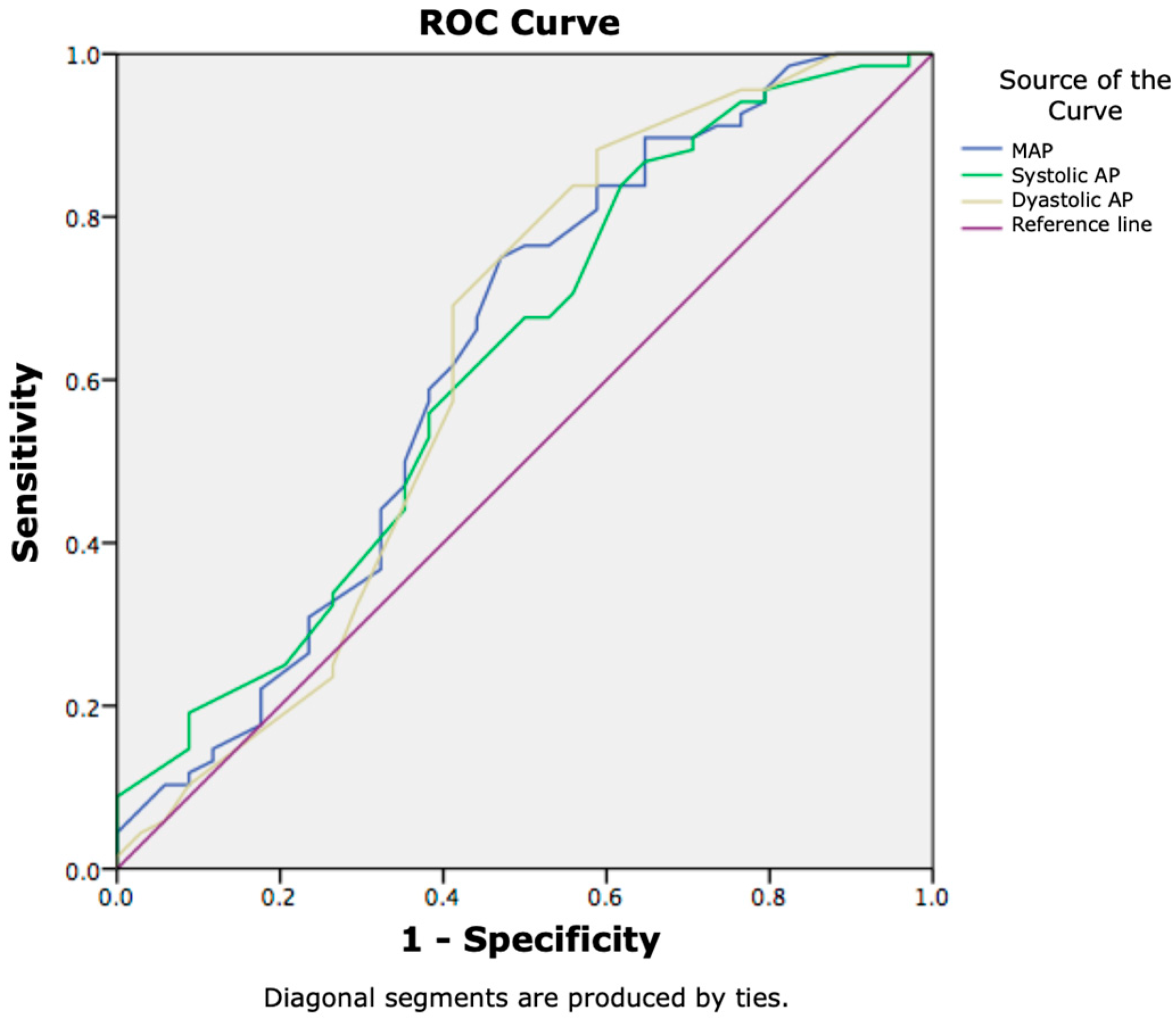
| MAP | 84.28 ± 16.19 | 74.44 ± 20.98 | 0.020 |
| sAP | 114.89 ± 22.87 | 102.26 ± 26.01 | 0.019 |
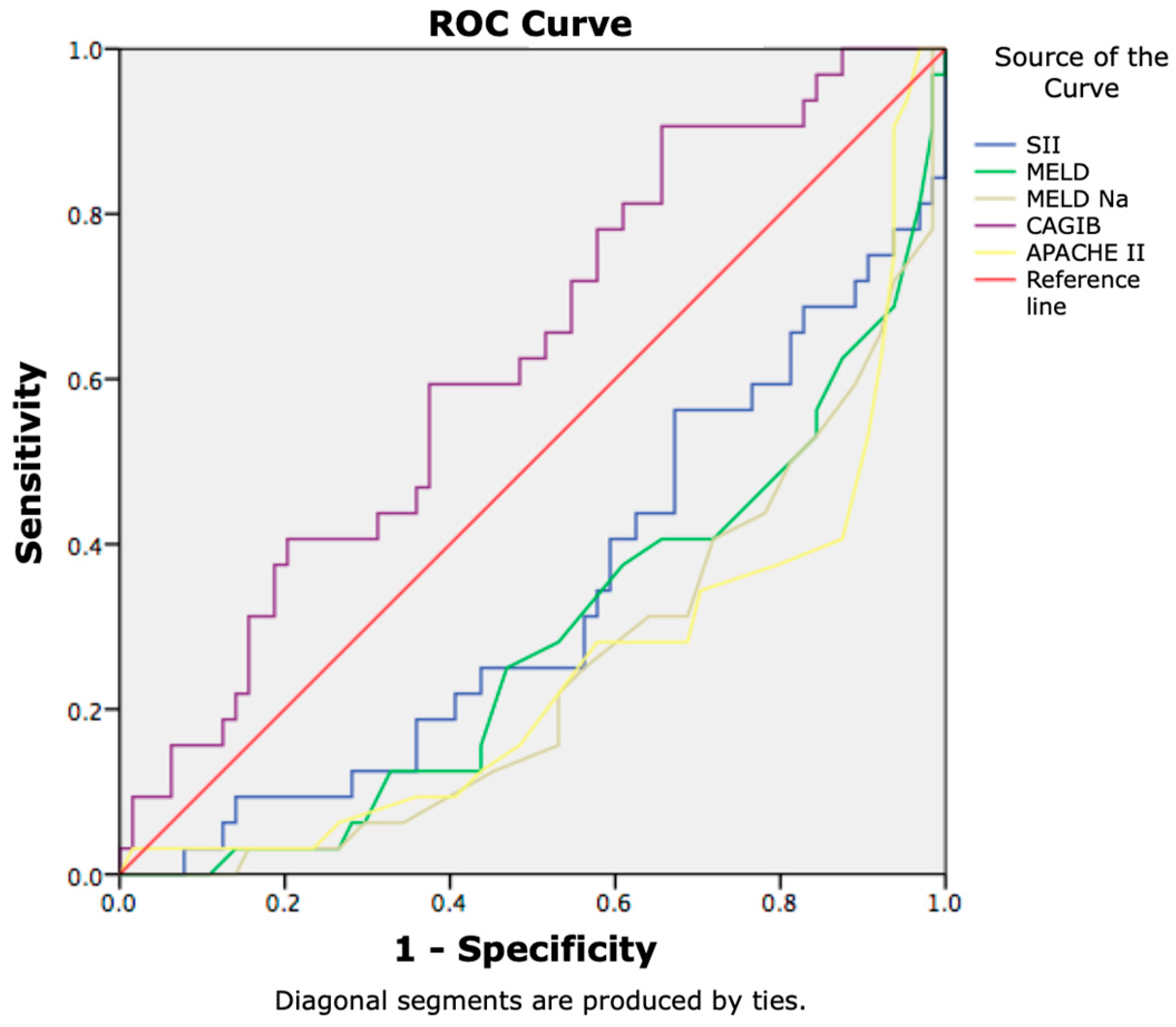
| APACHE II score | 18.92 ± 6.73 | 25.91 ± 7.37 | 0.000 |
| MELD score | 22.45 ± 7.29 | 29.15 ± 8.86 | 0.000 |
| MELD-Na score | 24.17 ± 7.14 | 30.45 ± 7.98 | 0.000 |
| CAGIB score | −6.42 ± 2.76 | −7.94 ± 3.03 | 0.018 |
| Variables | Survived Pts | Non-Survived Pts | p-Value |
|---|---|---|---|
| WBC | 9.93 ± 6.07 | 15.57 ± 7.49 | 0.000 |
| Neutrophils | 7.68 ± 5.37 | 14.11 ± 11.84 | 0.000 |
| Monocytes | 0.76 ± 0.54 | 1.15 ± 0.88 | 0.009 |
| INR | 1.88 ± 0.54 | 2.29 ± 1.02 | 0.009 |
| sCr | 94.48 ± 51.65 | 210.84 ± 148.71 | 0.000 |
| Albumin | 31.65 ± 5.80 | 27.84 ± 6.49 | 0.004 |
| Na | 137.05 ± 5.25 | 133.56 ± 6.23 | 0.004 |
| Cl | 102.45 ± 6.76 | 97.09 ± 7.70 | 0.001 |
| LDH | 509.52 ± 187.27 | 765.12 ± 694.07 | 0.007 |
| CRP | 39.29 ± 40.28 | 73.01 ± 70.23 | 0.003 |
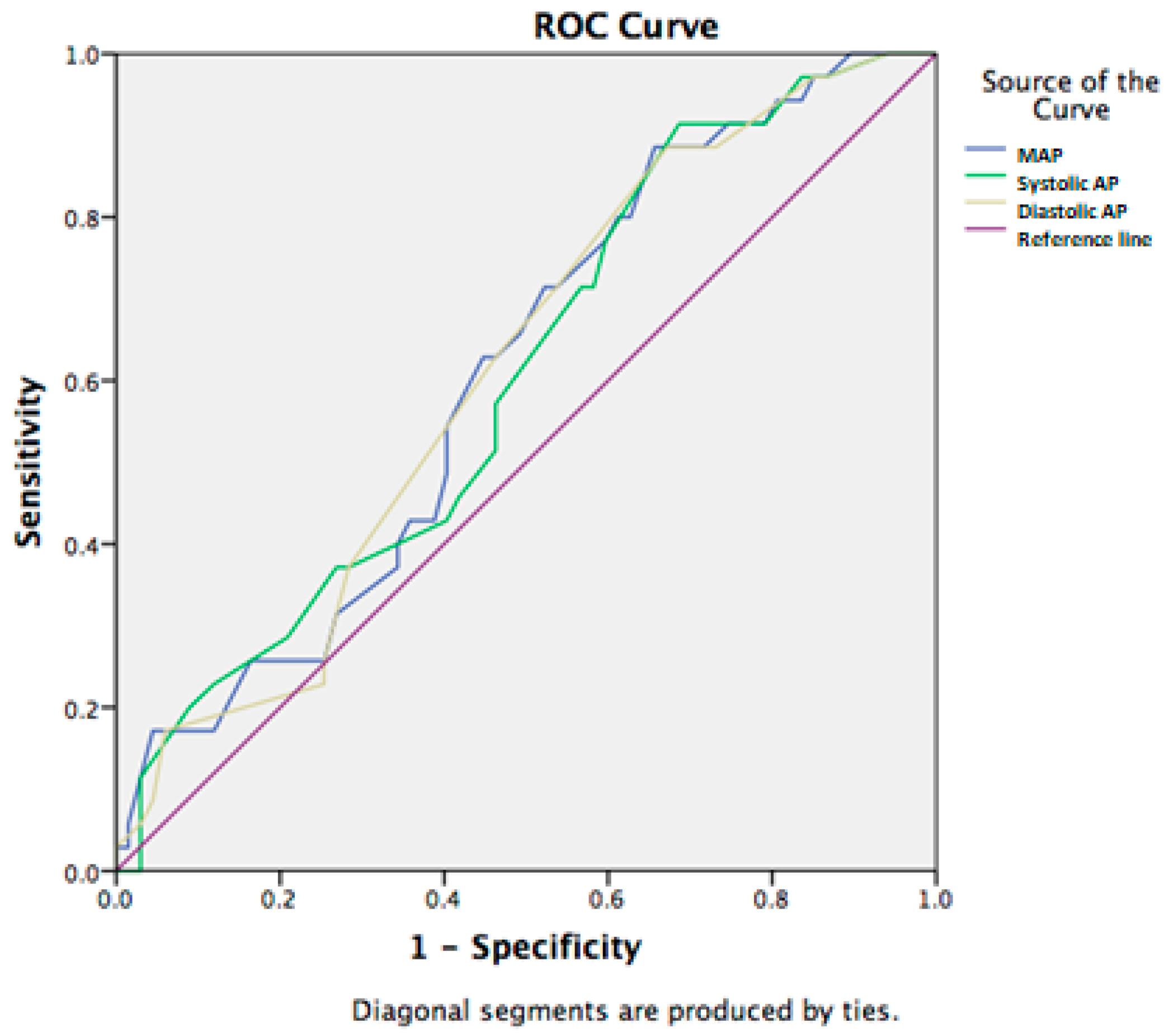
| MAP | 86.49 ± 16.41 | 77.82 ± 18.86 | 0.019 |
| sAP | 117.37 ± 20.93 | 106.77 ± 25.70 | 0.028 |
| dAP | 71.06 ± 14.83 | 63.34 ± 16.79 | 0.020 |
| APACHE II score | 17.14 ± 6.55 | 23.36 ± 7.43 | 0.000 |
| MELD score | 20.37 ± 6.78 | 27.24 ± 8.05 | 0.000 |
| MELD-Na score | 21.80 ± 6.37 | 28.91 ± 7.31 | 0.000 |
| CAGIB score | −6.03 ± 2.58 | −7.40 ± 3.03 | 0.021 |
| Blood oxygen saturation | 95.81 ± 3.33 | 90.50 ± 13.17 | 0.000 |
| Variable | B | S.E. | Wald | Df | Sig. | Exp (B) | 95% C.I. for EXP (B) | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Heart disease | 2.064 | 0.722 | 8.161 | 1 | 0.004 | 7.875 | 1.911 | 32.444 |
| Constant | −0.118 | 0.486 | 0.59 | 1 | 0.808 | 0.889 | ||
| Variable | B | S.E. | Wald | Df | Sig. | Exp (B) | 95% C.I. for EXP (B) | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| GI bleeding | 0.934 | 0.480 | 3.796 | 1 | 0.051 | 2.545 | 0.994 | 6.516 |
| Constant | 0.000 | 0.408 | 0.000 | 1 | 1.000 | 1.000 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glisic, T.; Korica, B.; Stojkovic Lalosevic, M.; Baljosevic, N.; El Mezeni, J.; Kartal, M.; Popovic, D.D.; Martinov Nestorov, J.; Lukic, S.; Mijac, D. Alcoholic Liver Disease and Systemic Inflammatory Response Syndrome: Mortality Prediction Using Biomarkers and Clinical Scores. J. Clin. Med. 2025, 14, 7580. https://doi.org/10.3390/jcm14217580
Glisic T, Korica B, Stojkovic Lalosevic M, Baljosevic N, El Mezeni J, Kartal M, Popovic DD, Martinov Nestorov J, Lukic S, Mijac D. Alcoholic Liver Disease and Systemic Inflammatory Response Syndrome: Mortality Prediction Using Biomarkers and Clinical Scores. Journal of Clinical Medicine. 2025; 14(21):7580. https://doi.org/10.3390/jcm14217580
Chicago/Turabian StyleGlisic, Tijana, Bojan Korica, Milica Stojkovic Lalosevic, Nevena Baljosevic, Jasna El Mezeni, Marko Kartal, Dusan Dj Popovic, Jelena Martinov Nestorov, Snezana Lukic, and Dragana Mijac. 2025. "Alcoholic Liver Disease and Systemic Inflammatory Response Syndrome: Mortality Prediction Using Biomarkers and Clinical Scores" Journal of Clinical Medicine 14, no. 21: 7580. https://doi.org/10.3390/jcm14217580
APA StyleGlisic, T., Korica, B., Stojkovic Lalosevic, M., Baljosevic, N., El Mezeni, J., Kartal, M., Popovic, D. D., Martinov Nestorov, J., Lukic, S., & Mijac, D. (2025). Alcoholic Liver Disease and Systemic Inflammatory Response Syndrome: Mortality Prediction Using Biomarkers and Clinical Scores. Journal of Clinical Medicine, 14(21), 7580. https://doi.org/10.3390/jcm14217580





