UHPLC-MS/MS for Antipsychotic Drug Monitoring: A Systematic Review of Clinical and Analytical Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources and Search Strategy
2.4. Study Selection
2.5. Data Extraction
2.6. Quality Assessment
2.7. Data Synthesis
3. Results
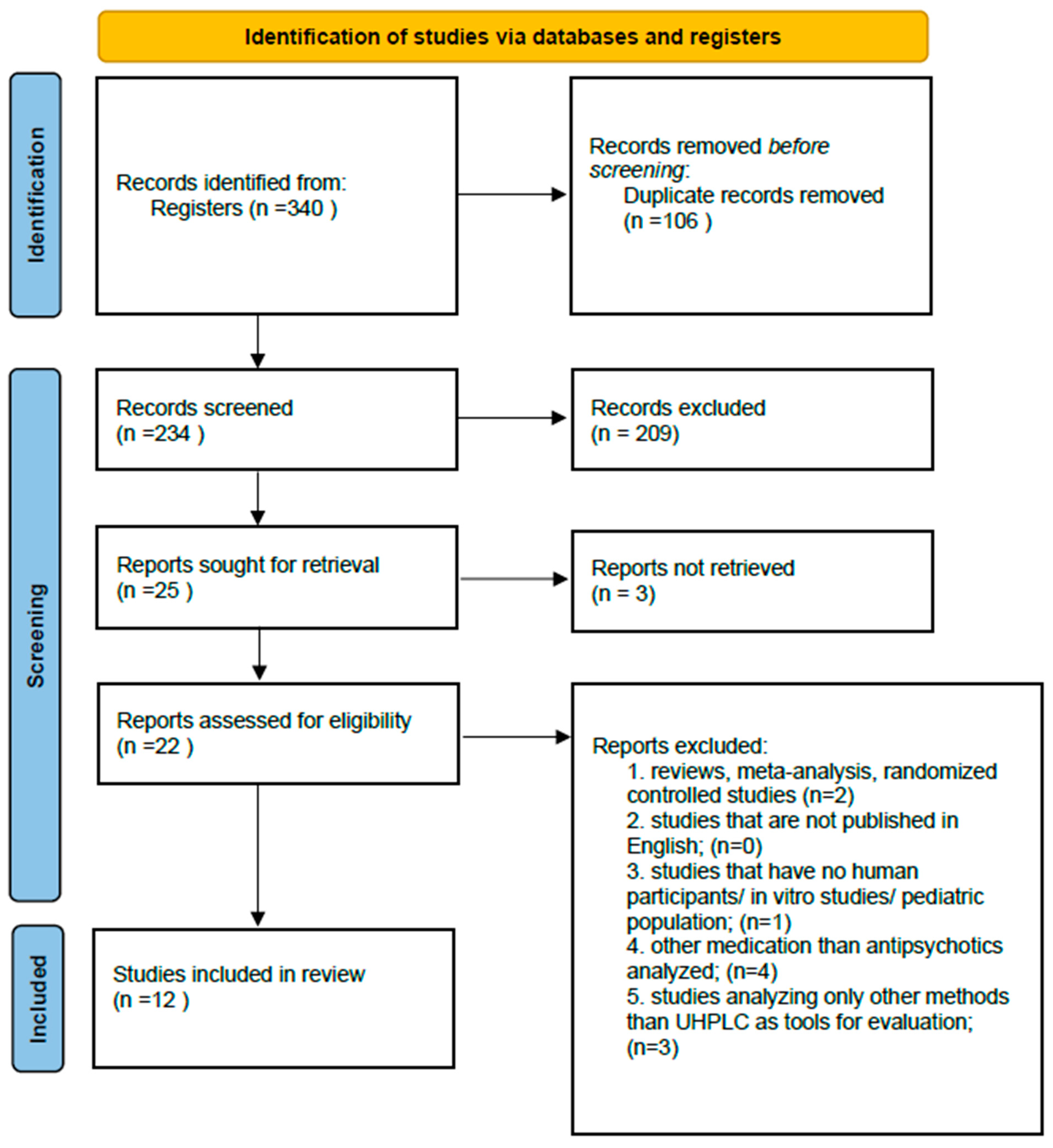
3.1. Study Selection
3.2. Characteristics of Included Studies
| Author | Matrix | Antipsychotics Investigated | Participants | Analytical Method | Main Findings | |
|---|---|---|---|---|---|---|
| 1 | Bernardo et al. (2022) [35] | DBS, Whole blood, Plasma | Aripiprazole, Clozapine, Paliperidone | 31; Schizophrenia | UHPLC–MS/MS (Acquity UPLC + Waters triple quadrupole MS; ESI+, MRM) | DBS is a reliable, minimally invasive alternative to plasma/whole blood for antipsychotic TDM; good correlation with conventional matrices |
| 2 | Cruz et al. (2020) [37] | Human plasma | Chlorpromazine, Clozapine, Olanzapine, Quetiapine | 11; Schizophrenia | UHPLC–MS/MS with RACNT microextraction; ESI+, MRM | RACNT-UHPLC–MS/MS method showed high sensitivity, precision and reusability for antipsychotic quantification in plasma; reduced matrix effects; suitable for routine TDM. |
| 3 | Millán-Santiago et al. (2023) [36] | VAMS and plasma | Cariprazine | 3; Schizophrenia | UHPLC–MS/MS (Acquity UPLC + Xevo TQ-S; ESI+, MRM; validated for VAMS and plasma) | UHPLC–MS/MS with VAMS validated for cariprazine and metabolites; VAMS showed strong agreement with plasma; supports minimally invasive TDM. |
| 4 | Patteet et al. (2014) [39] | Seum | Amisulpride, Aripiprazole, Asenapine, Bromperidol, Clozapine, Haloperidol, Iloperidone, Levosulpiride, Lurasidone, Olanzapine, Paliperidone, Pipamperone, Quetiapine, Risperidone, Sertindole, Zuclopenthixol | 12; analytical validation, not a clinical cohort | Acquity UPLC coupled to Xevo TQ-S triple quadrupole MS; ESI+; MRM; protein precipitation (high-throughput method) | High-throughput UHPLC–MS/MS validated for 16 antipsychotics + 8 metabolites in serum; accurate, precise, reproducible; suitable for routine TDM. |
| 5 | Patteet et al. (2014) [40] | Serum | Clozapine | 171; diagnosis not specified (retrospective clozapine monitoring) | UHPLC–MS/MS (Acquity UPLC + Xevo TQ-S; ESI+, MRM; serum) | Retrospective evaluation of 171 patients; UHPLC–MS/MS reliably quantified clozapine/norclozapine; confirmed interindividual variability; supports clozapine TDM in routine practice. |
| 6 | Patteet et al. (2015) [41] | DBS, Plasma | Amisulpride, Aripiprazole, Asenapine, Bromperidol, Clozapine, Haloperidol, Iloperidone, Lurasidone, (Levo)sulpiride, Olanzapine, Paliperidone, Pipamperone, Quetiapine, Risperidone, Zuclopenthixol | 171; (same clinical cohort as their serum studies) | UHPLC-MS/MS APs Metabolites Quantitative analysis TDM Bioanalysis Plasma analysis | DBS validated against plasma for 15 antipsychotics + 7 metabolites; good agreement; minimally invasive alternative for TDM. |
| 7 | Patteet et al. (2016) [42] | Serum | Aripiprazole, Haloperidol, Risperidone, Paliperidone, Zuclopenthixol | 82 psychiatric patients (serum samples analyzed; cohort characterized for CYP2D6 genotype) | UHPLC–MS/MS (Acquity UPLC + Xevo TQ-S; ESI+, MRM; serum, CYP2D6 impact study) | CYP2D6 genotype and co-medication significantly affected serum levels of several antipsychotics; highlights importance of pharmacogenetics in TDM |
| 8 | Patteet et al. (2016) [43] | Oral fluid | Amisulpride, Aripiprazole, Clozapine, Haloperidol, Olanzapine, Quetiapine, Risperidone, Sertindole, Ziprasidone | 82 psychiatric patients (matched with serum samples from the same cohort) | UHPLC–MS/MS (Acquity UPLC + Xevo TQ-S; ESI+, MRM; validated for oral fluid) | UHPLC–MS/MS validated for 9 antipsychotics in oral fluid; variable correlation with serum; oral fluid less reliable than plasma/DBS for TDM. |
| 9 | Pouliopoulos et al. (2018) [44] | Serum, Postmortem blood | Amisulpride, Aripiprazole, Asenapine, Chlorpromazine, Clozapine, Haloperidol, Levomepromazine, Olanzapine, Paliperidone, Perphenazine, Quetiapine, Risperidone, Sulpiride, Ziprasidone, Zuclopenthixol | Method validation study; included postmortem samples and small-volume serum samples | UHPLC–MS/MS (Shimadzu Nexera X2 UHPLC system coupled to a Shimadzu 8040 triple quadrupole MS; ESI+, MRM), validated for serum and postmortem blood. | UHPLC–MS/MS validated for 15 antipsychotics in small serum/postmortem volumes; accurate and robust; applicable in clinical TDM and forensic toxicology |
| 10 | Toja-Camba et al. (2024) [2] | Plasma | Risperidone, Paliperidone | 115; 92 on risperidone, 23 on paliperidone | UHPLC–MS/MS (Acquity UPLC + Xevo TQ-S; ESI+, MRM; plasma; compared with Alinity C immunoassay) | UHPLC–MS/MS and Alinity C showed overall agreement for risperidone/paliperidone; immunoassay consistently overestimated levels; UHPLC–MS/MS remains reference method. |
| 11 | Toja-Camba et al. (2024) [11] | Plasma | Aripiprazole | 86 patients treated with aripiprazole | UHPLC–MS/MS (Acquity UPLC + Xevo TQ-S; ESI+, MRM; plasma; compared with Alinity C immunoassay) | “UHPLC–MS/MS accurately quantified aripiprazole and metabolite; immunoassay overestimated levels; supports UHPLC–MS/MS as reference method for precision psychiatry.” |
| 12 | Wang et al. (2017) [38] | Plasma | Aripiprazole, Amisulpride, Olanzapine, Paliperidone, Ziprasidone | 253 patients with schizophrenia | UHPLC–MS/MS (Ekspert ultraLC 100-XL + QTRAP 5500; ESI+, MRM; plasma) | Validated UHPLC–MS/MS for 5 antipsychotics in plasma; accurate and reproducible; suitable for routine TDM in schizophrenia. |
3.3. Quality Assessment
3.4. Quantitative Assessment
3.4.1. Biological Matrices Investigated in Included Studies
| Matrix | Study | Number of Studies |
|---|---|---|
| Serum | Patteet et al., 2014 [39], Pouliopoulos et al., 2018 [44] | 2 |
| DBS | Bernardo et al., 2022 [35], Patteet et al., 2014 [39,40] | 2 |
| Plasma | Cruz et al., 2023 [37], Wang et al., 2017 [38] | 2 |
| WB | Bernardo et al., 2022 [35], Millán-Santiago et al., 2023 [36] | 2 |
| SPE | Pouliopoulos et al., 2018 [44] | 1 |
| OF | Patteet et al., 2016 [42,43] | 1 |
| VAMS | Millán-Santiago et al., 2023 [36] | 1 |
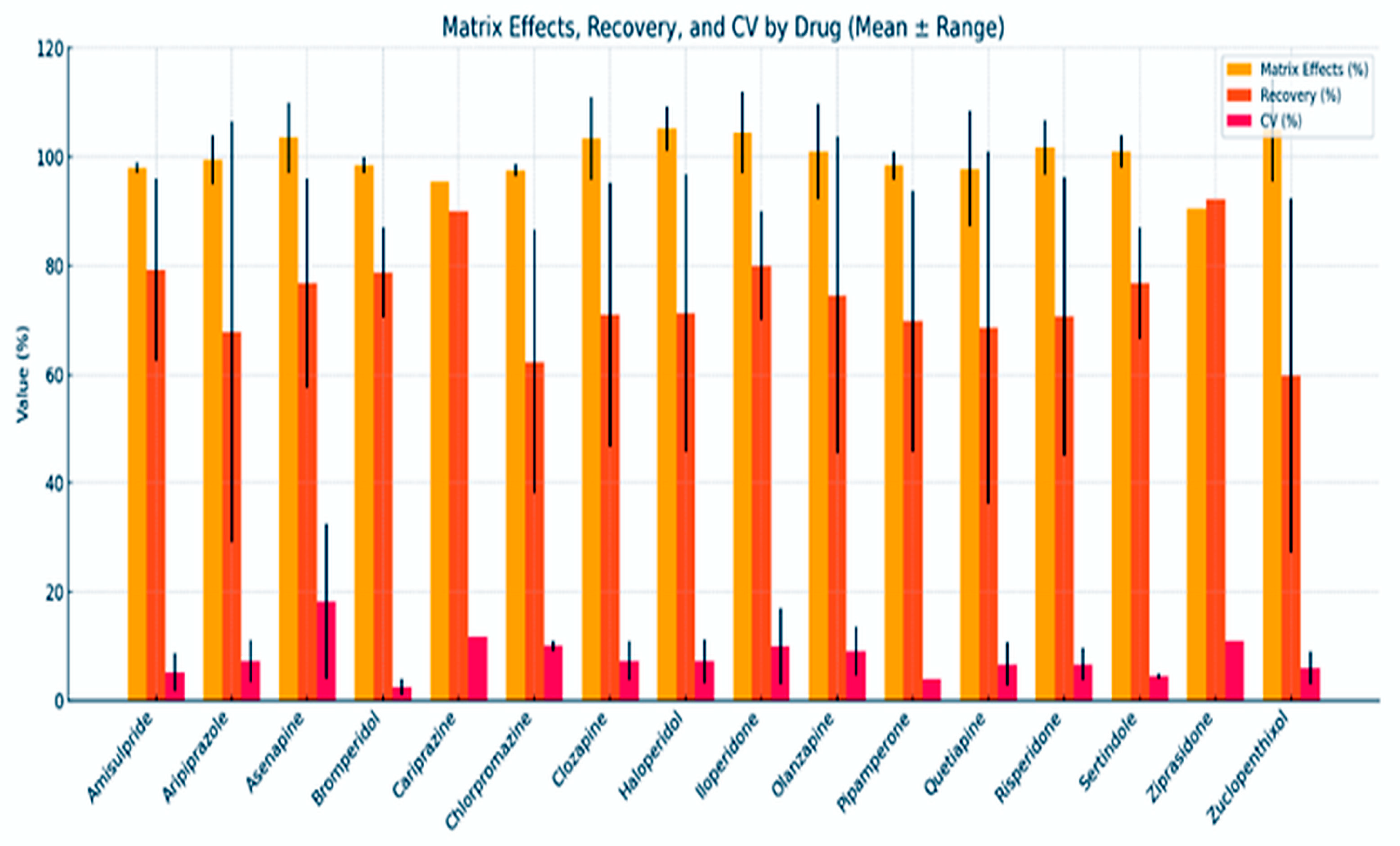
3.4.2. Analytical Method Performance Across Antipsychotics
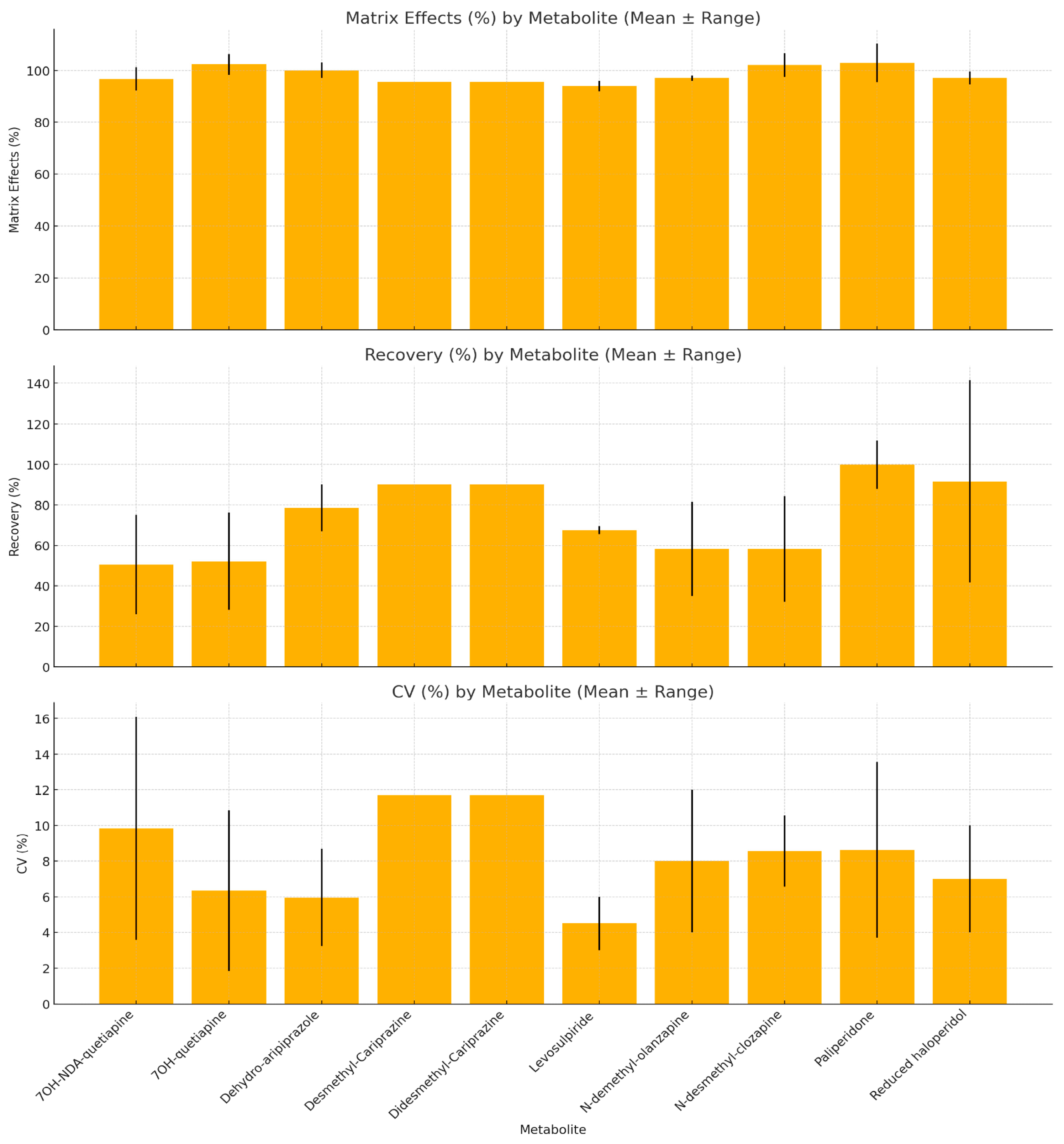
3.4.3. Analytical Method Performance Across Antipsychotic Metabolites
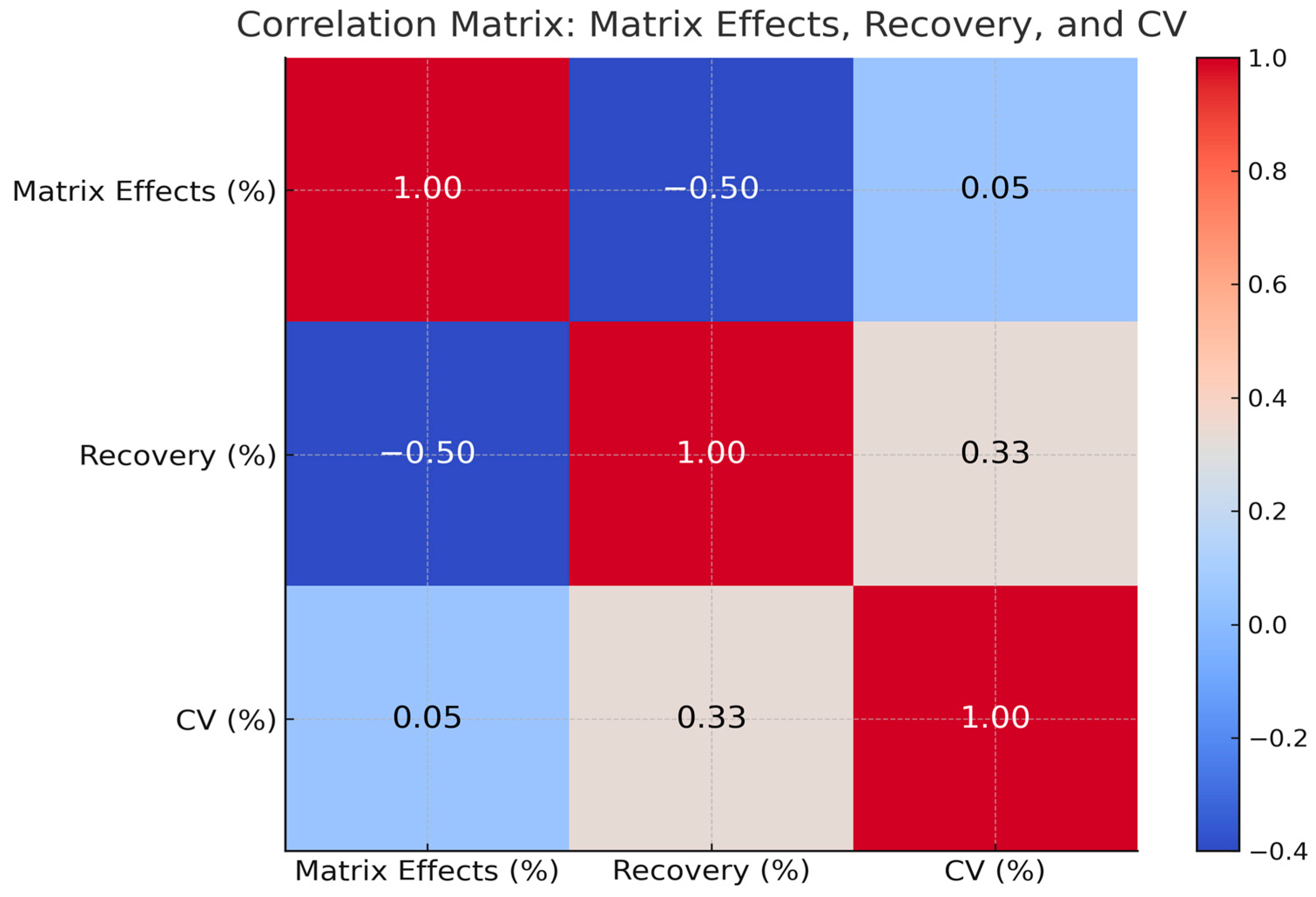
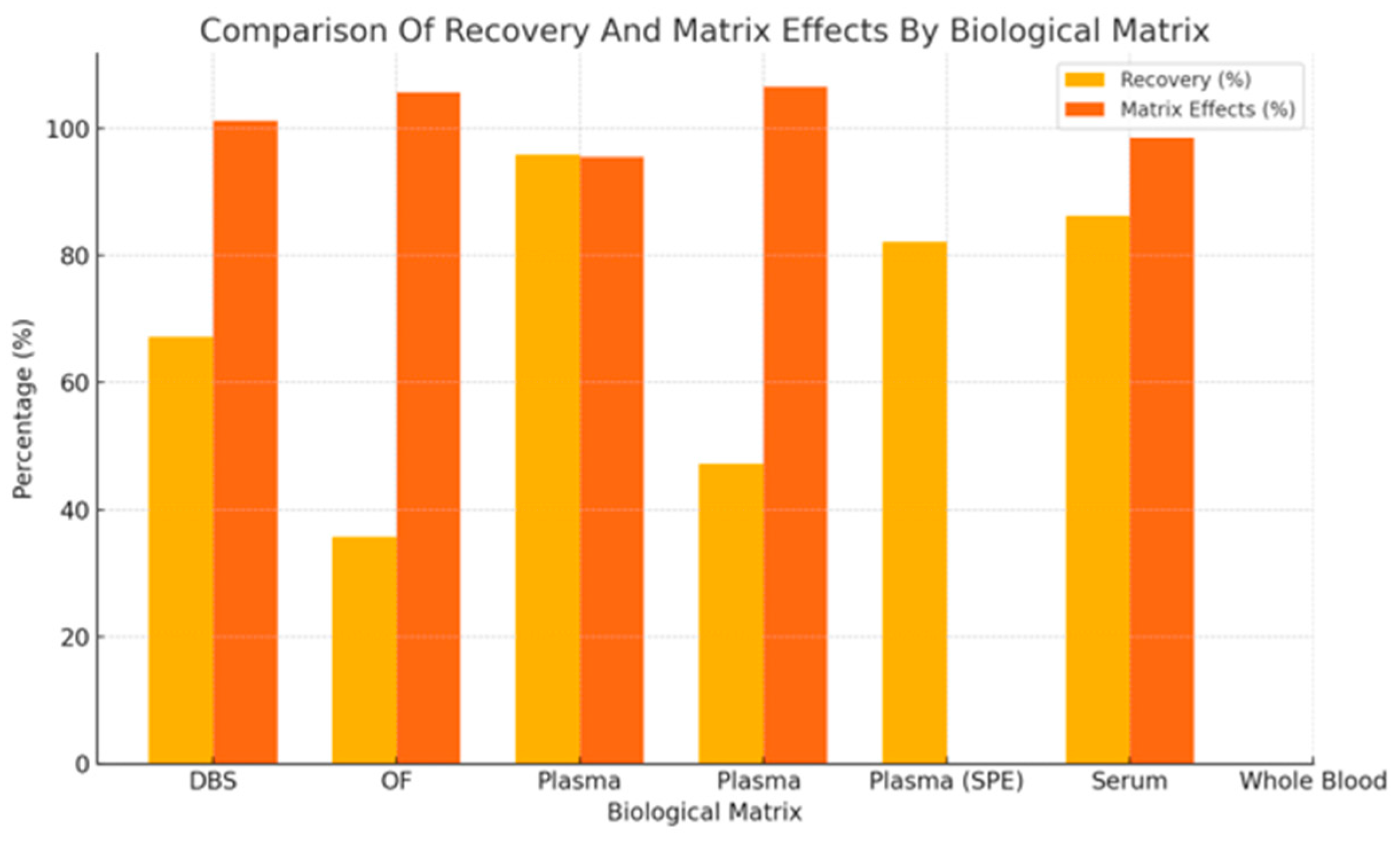
3.4.4. Analytical Performance Relationships Across Matrices
3.4.5. Recovery in Relation to Matrix Effects for Antipsychotics
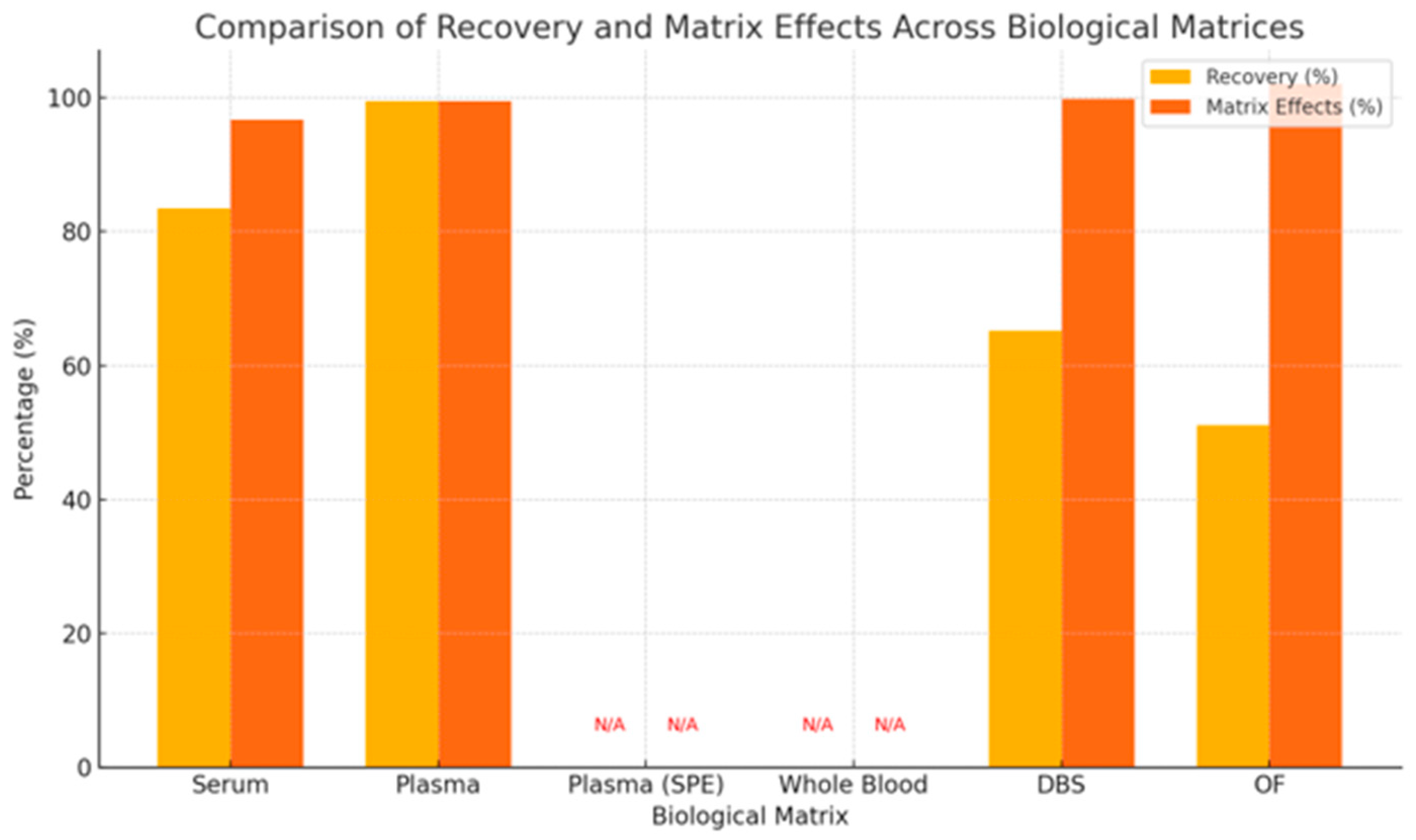
3.4.6. Recovery in Relation to Matrix Effects for Metabolites of Antipsychotics
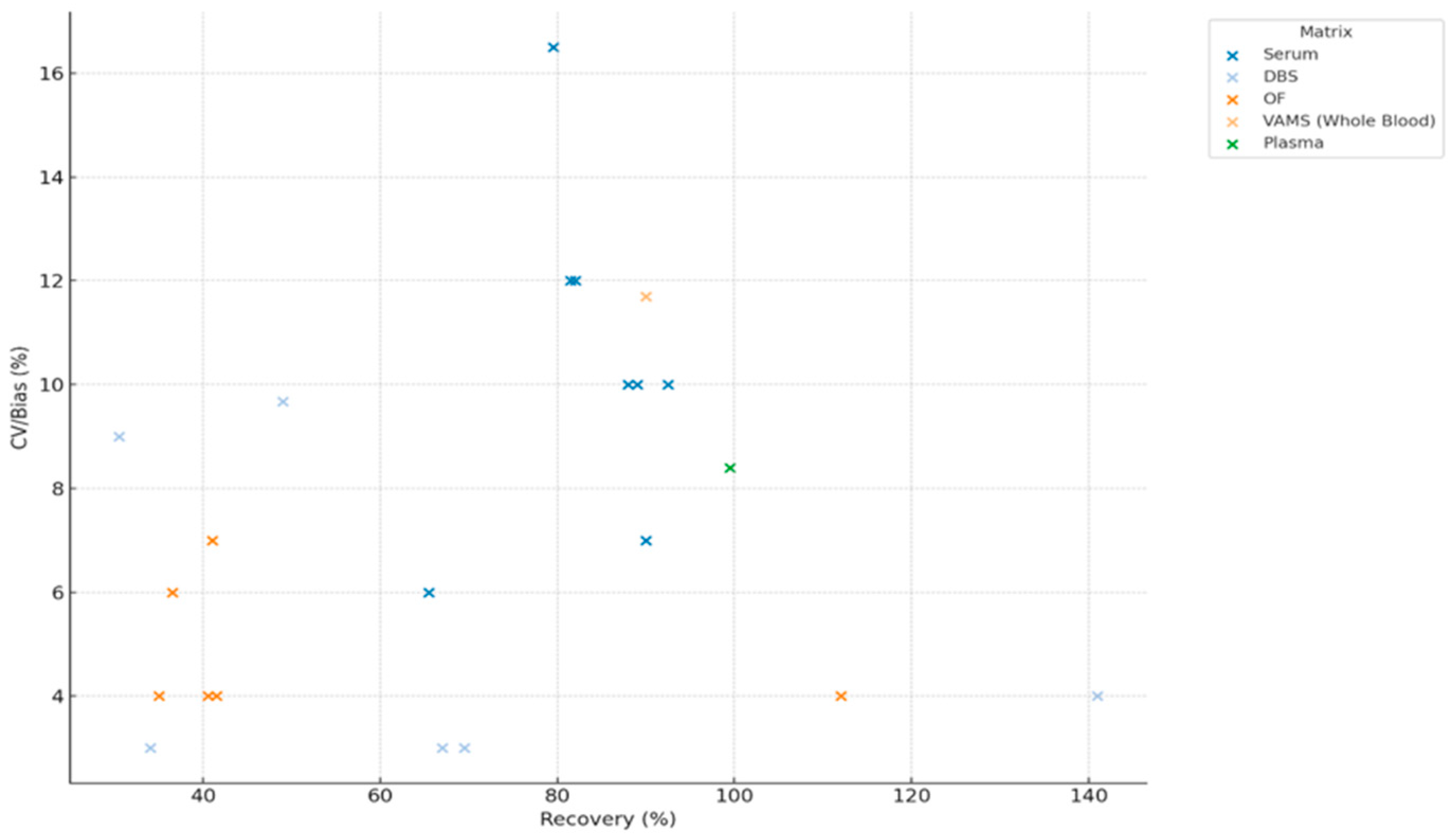
3.4.7. Impact of Recovery on Method Precision (CV/Bias) on Parent Drugs and Metabolites
3.5. Qualitative Assessment
3.5.1. Study Populations and Antipsychotic Medications
3.5.2. Comparative Assessment of UHPLC-MS/MS and Alinity C Methods for Antipsychotic Quantification
3.5.3. UHPLC-MS/MS in Personalized Therapeutic Drug Monitoring
3.5.4. Assessment of UHPLC Sample Preparation Techniques for Routine Clinical Implementation and Storage Conditions
4. Discussion
4.1. Biological Matrices and Analytical Variability: A Multidimensional Challenge
4.2. Analytical Performance Across Drugs and Metabolites: A Case for UHPLC-MS/MS
4.3. Recovery, Matrix Effects, and Precision: Navigating Analytical Trade-Offs
4.4. Toward Clinical Integration: The Role of UHPLC in Personalized Medicine
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| 1. Study Design and Reporting | |
| Clear description of study objectives and validation purpose | Adequate/Inadequate/Not reported |
| Detailed description of sample type and matrix (clinical relevance) | |
| Description of sample preparation procedures | |
| 2. Reference Standards and Controls | |
| Use of certified reference materials or appropriate standards | |
| Inclusion of quality controls and blank samples | |
| 3. Instrumentation and Method Parameters | |
| Detailed reporting of UHPLC instrumentation, column type, mobile phase, flow rate, detection method, etc. | |
| 4. Analytical Performance | |
| Calibration curve described with appropriate concentration range | |
| Linearity assessed and reported (e.g., correlation coefficient R2) | |
| Sensitivity parameters reported (Limit of Detection, Limit of Quantification) | |
| Accuracy evaluated (e.g., recovery studies, comparison with reference methods) | |
| Precision assessed (intra- and inter-day variability, CV or SD) | |
| Specificity/selectivity evaluated (ability to separate analyte from interfering substances) | |
| Robustness or ruggedness testing reported (e.g., slight method variations) | |
| 5. Sample Analysis and Validation | |
| Validation performed on real clinical samples, not only spiked or artificial samples | |
| Stability studies reported (short- and long-term, freeze-thaw, autosampler) | |
| Number of replicates adequate to support conclusions | |
| 6. Data Reporting and Interpretation | |
| Transparent reporting of all validation results | |
| Discussion of limitations and potential sources of bias | |
Appendix B
| Parameter | Risperidone and Paliperidone Toja-Camba et al. (2024) [2] | Aripiprazole and Dehydroaripiprazole Toja-Camba et al. (2024) [11] |
|---|---|---|
| UHPLC-MS/MS [2] | ||
| Instrument | Xevo TQDR triple quadrupole MS | Xevo TQDR triple quadrupole MS |
| Internal standard | Risperidone-d4 | Aripiprazole-d8 |
| Detection transitions (m/z) | 441.2→191.1 (RIS), 427.24→207.05 (PAL), 415.2→195.1 (IS) | 448.2→285.2 (ARI), 446.04→285.02 (DHA), 6.3→293.07 (IS) |
| Calibration range | 1–200 ng/mL (R2 = 0.99) | 25–1000 ng/mL (R2 = 0.998) |
| LOD/LOQ | 0.5/1 ng/mL | 10/25 ng/mL |
| Column/temp | BEH Shield RP18, 40 °C | BEH C18, 40 °C |
| Flow rate/inj. Volume | 0.6 mL/min, 3 μL | 0.6 mL/min, 5 μL |
| Alinity C [11] | ||
| Detection principle | Competitive immunoassay with photometric detection | Competitive immunoassay with photometric detection |
| Wavelength | 604 nm | 604 nm |
| Kit used | MyCare Psychiatry Kit for risperidone | MyCare Psychiatry Kit for aripiprazole |
| LOQ range | 16–120 ng/mL | 45–1000 ng/mL |
| Cross-reactivity tested | >150 drugs, RF, lipids, hemolysis | >150 drugs, RF, lipids, hemolysis |
| Study characteristics | ||
| No. of samples (total) | 115 plasma samples | 86 plasma samples |
| Samples analyzed by both methods | Yes | Yes (60 samples) |
| Therapeutic range stratification | <20 ng/mL, 20–60 ng/mL, >60 ng/mL | <150 ng/mL, 150–500 ng/mL, >500 ng/mL |
| Ratio analysis | PAL/RIS ratio impact on method agreement | ARI/DHA ratio impact on method agreement |
| Additional analysis | - | Re-analysis after 6 months to assess reproducibility |
References
- Galson, S.K. Mental health matters. Public Health Rep. 2009, 124, 189. [Google Scholar] [CrossRef] [PubMed]
- Toja-Camba, F.J.; Hermelo-Vidal, G.; Feitosa-Medeiros, C.; Vidal-Millares, M.; Durán-Maseda, M.J.; Fernández-Ferreiro, A.; Mondelo-García, C. Head-to-Head Comparison of UHPLC-MS/MS and Alinity C for Plasma Analysis of Risperidone and Paliperidone. Pharmaceuticals 2024, 17, 1446. [Google Scholar] [CrossRef]
- Ansermot, N.; Brawand-Amey, M.; Kottelat, A.; Eap, C.B. Fast Quantification of Ten Psychotropic Drugs and Metabolites in Human Plasma by Ultra-High Performance Liquid Chromatography Tandem Mass Spectrometry for Therapeutic Drug Monitoring. J. Chromatogr. A 2013, 1292, 160–172. [Google Scholar] [CrossRef]
- Vintilă, B.I.; Anghel, C.E.; Sava, M.; Bereanu, A.S.; Codru, I.R.; Stoica, R.; Vulcu Mihai, A.M.; Grama, A.M.; Cătană, A.C.; Boicean, A.G.; et al. Evaluating Anesthesia Practices, Patient Characteristics, and Outcomes in Electroconvulsive Therapy: A Two-Year Retrospective Study. J. Clin. Med. 2024, 13, 6253. [Google Scholar] [CrossRef]
- Biso, L.; Aringhieri, S.; Carli, M.; Scarselli, M.; Longoni, B. Therapeutic Drug Monitoring in Psychiatry: Enhancing Treatment Precision and Patient Outcomes. Pharmaceuticals 2024, 17, 642. [Google Scholar] [CrossRef]
- Moretti, M.; Freni, F.; Valentini, B.; Vignali, C.; Groppi, A.; Visonà, S.D.; Osculati, A.M.M.; Morini, L. Determination of Antidepressants and Antipsychotics in Dried Blood Spots (DBSs) Collected from Post-Mortem Samples and Evaluation of the Stability over a Three-Month Period. Molecules 2019, 24, 3636. [Google Scholar] [CrossRef] [PubMed]
- Tron, C.; Kloosterboer, S.M.; Van Der Nagel, B.C.H.; Wijma, R.A.; Dierckx, B.; Dieleman, G.C.; Van Gelder, T.; Koch, B.C.P. Dried Blood Spots Combined with Ultra-High-Performance Liquid Chromatography-Mass Spectrometry for the Quantification of the Antipsychotics Risperidone, Aripiprazole, Pipamperone, and Their Major Metabolites. Ther. Drug Monit. 2017, 39, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Bǎcilǎ, C.-I.; Marcu, G.M.; Vintilă, B.I.; Anghel, C.E.; Lomnasan, A.; Cornea, M.; Grama, A.M. Applications of Functional Near-Infrared Spectroscopy (FNIRS) in Monitoring Treatment Response in Psychiatry: A Scoping Review. J. Clin. Med. 2025, 14, 5197. [Google Scholar] [CrossRef]
- Chesnut, S.M.; Salisbury, J.J. The Role of UHPLC in Pharmaceutical Development. J. Sep. Sci. 2007, 30, 1183–1190. [Google Scholar] [CrossRef]
- Grand-Guillaume Perrenoud, A.; Veuthey, J.L.; Guillarme, D. Comparison of Ultra-High Performance Supercritical Fluid Chromatography and Ultra-High Performance Liquid Chromatography for the Analysis of Pharmaceutical Compounds. J. Chromatogr. A 2012, 1266, 158–167. [Google Scholar] [CrossRef]
- Toja-Camba, F.J.; Bandín-Vilar, E.; Hermelo-Vidal, G.; Feitosa-Medeiros, C.; Cañizo-Outeiriño, A.; Castro-Balado, A.; Varela-Rey, I.; Zarra-Ferro, I.; Fernández-Ferreiro, A.; Mondelo-García, C. Towards Precision Medicine in Clinical Practice: Alinity C vs. UHPLC-MS/MS in Plasma Aripiprazole Determination. Pharmaceutics 2024, 16, 104. [Google Scholar] [CrossRef]
- Geers, L.M.; Cohen, D.; Wehkamp, L.M.; Van Hateren, K.; Koster, R.A.; Fedorenko, O.Y.; Semke, A.V.; Bokhan, N.; Ivanova, S.A.; Kosterink, J.G.W.; et al. Dried Blood Spot Analysis for Therapeutic Drug Monitoring of Clozapine. J. Clin. Psychiatry 2017, 78, e1211–e1218. [Google Scholar] [CrossRef] [PubMed]
- Rathod, R.H.; Chaudhari, S.R.; Patil, A.S.; Shirkhedkar, A.A. Ultra-High Performance Liquid Chromatography-MS/MS (UHPLC-MS/MS) in Practice: Analysis of Drugs and Pharmaceutical Formulations. Future J. Pharm. Sci. 2019, 5, 6. [Google Scholar] [CrossRef]
- Čabovska, B.; Cox, S.L.; Vinks, A.A. Determination of Risperidone and Enantiomers of 9-Hydroxyrisperidone in Plasma by LC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 852, 497. [Google Scholar] [CrossRef]
- Qi, Y.; Liu, G. Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry for Simultaneous Determination of Antipsychotic Drugs in Human Plasma and Its Application in Therapeutic Drug Monitoring. Drug Des. Dev. Ther. 2021, 15, 463. [Google Scholar] [CrossRef]
- Dziurkowska, E.; Wesolowski, M. Simultaneous Quantification of Antipsychotic and Antiepileptic Drugs and Their Metabolites in Human Saliva Using UHPLC-DAD. Molecules 2019, 24, 2953. [Google Scholar] [CrossRef]
- The Difference Between Narrative Review and Systematic Review—DistillerSR. Available online: https://www.distillersr.com/resources/systematic-literature-reviews/the-difference-between-narrative-review-and-systematic-review?utm_source=chatgpt.com (accessed on 7 July 2025).
- Velligan, D.I.; Sajatovic, M.; Hatch, A.; Kramata, P.; Docherty, J.P. Why Do Psychiatric Patients Stop Antipsychotic Medication? A Systematic Review of Reasons for Nonadherence to Medication in Patients with Serious Mental Illness. Patient Prefer. Adherence 2017, 11, 449–468. [Google Scholar] [CrossRef]
- What Are the Different Types of Literature Review? Available online: https://www.litmaps.com/learn/what-are-the-different-types-of-literature-review?utm_source=chatgpt.com (accessed on 7 July 2025).
- Gritti, F.; Meyyappan, S.; Leveille, W.; Hill, J. Improved Performance of UHPLC–MS Hyphenated Systems. LC-GC N. Am. 2022, 40, 296–303. [Google Scholar] [CrossRef]
- Huang, H.; Liu, M.; Chen, P. Recent advances in ultra-high performance liquid chromatography for the analysis of traditional chinese medicine. Anal. Lett. 2014, 47, 1835. [Google Scholar] [CrossRef]
- Shah, J. Liquid Chromatography in Clinical Diagnostics: Current Trends and Innovations. J. Chromatogr. Sep. Tech. 2024, 15, 1–2. [Google Scholar] [CrossRef]
- Patteet, L.; Cappelle, D.; Maudens, K.E.; Crunelle, C.L.; Sabbe, B.; Neels, H. Advances in Detection of Antipsychotics in Biological Matrices. Clin. Chim. Acta 2015, 441, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Lomnasan, A.; Vintilă, B.I.; Bucuța, M.; Ștef, L.; Anghel, C.E.; Grama, A.M.; Cornea, M.; Boicean, A.; Ichim, C.; Paziuc, L.C.; et al. The Use of Phototherapy for the Treatment of Non-Seasonal Depression: A Systematic Review of Efficacy and Safety. J. Clin. Med. 2025, 14, 1756. [Google Scholar] [CrossRef]
- Pergantis, P.; Bamicha, V.; Doulou, A.; Christou, A.I.; Bardis, N.; Skianis, C.; Drigas, A. Assistive and Emerging Technologies to Detect and Reduce Neurophysiological Stress and Anxiety in Children and Adolescents with Autism and Sensory Processing Disorders: A Systematic Review. Technologies 2025, 13, 144. [Google Scholar] [CrossRef]
- Comparing HPLC vs. UHPLC—Creative Proteomics. Available online: https://www.creative-proteomics.com/resource/comparing-hplc-vs-uhplc.htm?utm_source=chatgpt.com (accessed on 7 July 2025).
- Charlier, B.; Coglianese, A.; Operto, F.F.; Coppola, G.; de Grazia, U.; Menna, P.; Filippelli, A.; Dal Piaz, F.; Izzo, V. Development and Validation of a UHPLC–MS/MS-Based Method to Quantify Cenobamate in Human Plasma Samples. Molecules 2022, 27, 7325. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- PROSPERO. Available online: https://www.crd.york.ac.uk/prospero/ (accessed on 11 July 2025).
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Food and Drug Administration; Center for Drug Evaluation and Research. Bioanalytical Method Validation Guidance for Industry Biopharmaceutics Bioanalytical Method Validation Guidance for Industry Biopharmaceutics Contains Nonbinding Recommendations; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2018. [Google Scholar]
- EMA European Medicines Agency. 2** Committee for Medicinal Products for Human Use (CHMP) Guideline on Bioanalytical Method Validation; EMA European Medicines Agency: Amsterdam, The Netherlands, 1922. [Google Scholar]
- Ich Harmonised Tripartite Guideline Validation of Analytical Procedures: Text and Methodology Q2(R1). Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-q2r1-validation-analytical-procedures-text-methodology-step-5-first-version_en.pdf (accessed on 11 July 2025).
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.; Mezquida, G.; Ferré, P.; Cabrera, B.; Torra, M.; Lizana, A.M.; Brunet, M. Dried Blood Spot (DBS) as a Useful Tool to Improve Clozapine, Aripiprazole and Paliperidone Treatment: From Adherence to Efficiency. Rev. Psiquiatr. Salud Ment. 2022, 15, 230–237. [Google Scholar] [CrossRef]
- Millán-Santiago, J.; Vitagliano, R.; Mondella, F.; Mandrioli, R.; Sardella, R.; Vovk, T.; Lucena, R.; Cárdenas, S.; Boaron, F.; Serretti, A.; et al. Volumetric Absorptive Microsampling for the Therapeutic Drug Monitoring of Psychiatric Patients Treated with Cariprazine. J. Pharm. Biomed. Anal. 2023, 236, 115740. [Google Scholar] [CrossRef]
- Cruz, J.C.; de Faria, H.D.; Figueiredo, E.C.; Queiroz, M.E.C. Restricted Access Carbon Nanotube for Microextraction by Packed Sorbent to Determine Antipsychotics in Plasma Samples by High-Performance Liquid Chromatography-Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2020, 412, 2465–2475. [Google Scholar] [CrossRef]
- Wang, S.T.; Li, Y. Development of a UPLC-MS/MS Method for Routine Therapeutic Drug Monitoring of Aripiprazole, Amisulpride, Olanzapine, Paliperidone and Ziprasidone with a Discussion of Their Therapeutic Reference Ranges for Chinese Patients. Biomed. Chromatogr. 2017, 31, e3928. [Google Scholar] [CrossRef]
- Patteet, L.; Maudens, K.E.; Sabbe, B.; Morrens, M.; De Doncker, M.; Neels, H. High Throughput Identification and Quantification of 16 Antipsychotics and 8 Major Metabolites in Serum Using Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry. Clin. Chim. Acta 2014, 429, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Patteet, L.; Maudens, K.E.; Vermeulen, Z.; Dockx, G.; De Doncker, M.; Morrens, M.; Sabbe, B.; Neels, H. Retrospective Evaluation of Therapeutic Drug Monitoring of Clozapine and Norclozapine in Belgium Using a Multidrug UHPLC-MS/MS Method. Clin. Biochem. 2014, 47, 336–339. [Google Scholar] [CrossRef]
- Patteet, L.; Maudens, K.E.; Stove, C.P.; Lambert, W.E.; Morrens, M.; Sabbe, B.; Neels, H. The Use of Dried Blood Spots for Quantification of 15 Antipsychotics and 7 Metabolites with Ultra-High Performance Liquid Chromatography—Tandem Mass Spectrometry. Drug Test. Anal. 2015, 7, 502–511. [Google Scholar] [CrossRef]
- Patteet, L.; Haufroid, V.; Maudens, K.; Sabbe, B.; Manuel, M.; Neels, H. Genotype and Co-Medication Dependent CYP2D6 Metabolic Activity: Effects on Serum Concentrations of Aripiprazole, Haloperidol, Risperidone, Paliperidone and Zuclopenthixol. Eur. J. Clin. Pharmacol. 2016, 72, 175–184. [Google Scholar] [CrossRef]
- Patteet, L.; Maudens, K.E.; Morrens, M.; Sabbe, B.; Dom, G.; Neels, H. Determination of Common Antipsychotics in Quantisal-Collected Oral Fluid by UHPLC-MS/MS: Method Validation and Applicability for Therapeutic Drug Monitoring. Ther. Drug Monit. 2016, 38, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Pouliopoulos, A.; Tsakelidou, E.; Krokos, A.; Gika, H.G.; Theodoridis, G.; Raikos, N. Quantification of 15 Psychotropic Drugs in Serum and Postmortem Blood Samples after a Modified Mini-QuEChERS by UHPLC–MS-MS. J. Anal. Toxicol. 2018, 42, 337–345. [Google Scholar] [CrossRef]
- Toja-Camba, F.J.; Gesto-Antelo, N.; Maroñas, O.; Echarri Arrieta, E.; Zarra-Ferro, I.; González-Barcia, M.; Bandín-Vilar, E.; Mangas Sanjuan, V.; Facal, F.; Arrojo Romero, M.; et al. Review of Pharmacokinetics and Pharmacogenetics in Atypical Long-Acting Injectable Antipsychotics. Pharmaceutics 2021, 13, 935. [Google Scholar] [CrossRef]
- Lyu, H.; Chen, B.; Xu, X.; Zhu, C.; Ma, C.; Du, Y.; Liu, F.; Wu, C. Rapid Simultaneous Determination of 14 Antidepressants and 13 Antipsychotics in Human Plasma by Using High-Performance Liquid Chromatography-Tandem Mass Spectrometry with Dynamic Multiple Reaction Monitoring and Its Application to Therapeutic Drug Monitoring. Ther. Drug Monit. 2021, 43, 577–588. [Google Scholar] [CrossRef]
- Cherie, N.; Berta, D.M.; Tamir, M.; Yiheyis, Z.; Angelo, A.A.; Tarekegn, A.M.; Chane, E.; Nigus, M.; Teketelew, B.B. Improving Laboratory Turnaround Times in Clinical Settings: A Systematic Review of the Impact of Lean Methodology Application. PLoS ONE 2024, 19, e0312033. [Google Scholar] [CrossRef]
- Zailani, N.N.B.; Ho, P.C.L. Dried Blood Spots—A Platform for Therapeutic Drug Monitoring (TDM) and Drug/Disease Response Monitoring (DRM). Eur. J. Drug Metab. Pharmacokinet. 2023, 48, 467. [Google Scholar] [CrossRef]
- Cornea, M.; Vintilă, B.I.; Bucuța, M.; Ștef, L.; Anghel, C.E.; Grama, A.M.; Lomnasan, A.; Stetiu, A.A.; Boicean, A.; Sava, M.; et al. Efficacy of Transcranial Direct Current Stimulation and Photobiomodulation in Improving Cognitive Abilities for Alzheimer’s Disease: A Systematic Review. J. Clin. Med. 2025, 14, 1766. [Google Scholar] [CrossRef]
- Kumari, M.; Andrayas, A.; Al Baghal, T.; Burton, J.; Crossley, T.F.; Jones, K.S.; Parkington, D.A.; Koulman, A.; Benzeval, M. A Randomised Study of Nurse Collected Venous Blood and Self-Collected Dried Blood Spots for the Assessment of Cardiovascular Risk Factors in the Understanding Society Innovation Panel. Sci. Rep. 2023, 13, 13008. [Google Scholar] [CrossRef] [PubMed]
- Francke, M.I.; Peeters, L.E.J.; Hesselink, D.A.; Kloosterboer, S.M.; Koch, B.C.P.; Veenhof, H.; De Winter, B.C.M. Best Practices to Implement Dried Blood Spot Sampling for Therapeutic Drug Monitoring in Clinical Practice. Ther. Drug Monit. 2022, 44, 696. [Google Scholar] [CrossRef]
- Ververi, C.; Gentile, C.; Massano, M.; Salomone, A.; Vincenti, M. Quantitative Determination by UHPLC-MS/MS of 18 Common Drugs of Abuse and Metabolites, Including THC and OH-THC, in Volumetric Dried Blood Spots: A Sustainable Method with Minimally Invasive Sampling. J. Chromatogr. B 2024, 1247, 124337. [Google Scholar] [CrossRef]
- Hiemke, C.; Bergemann, N.; Clement, H.W.; Conca, A.; Deckert, J.; Domschke, K.; Eckermann, G.; Egberts, K.; Gerlach, M.; Greiner, C.; et al. Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology: Update 2017. Pharmacopsychiatry 2018, 51, 9–62. [Google Scholar] [CrossRef]
- Wang, B.T.; Colby, J.M.; Wu, A.H.B.; Lynch, K.L. Cross-Reactivity of Acetylfentanyl and Risperidone with a Fentanyl Immunoassay. J. Anal. Toxicol. 2014, 38, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Lee, M.H. Overview of Therapeutic Drug Monitoring. Korean J. Intern. Med. 2009, 24, 1–10. [Google Scholar] [CrossRef]
- Tuzimski, T.; Petruczynik, A. Review of Chromatographic Methods Coupled with Modern Detection Techniques Applied in the Therapeutic Drugs Monitoring (TDM). Molecules 2020, 25, 4026. [Google Scholar] [CrossRef]
- Yaegashi, H.; Kirino, S.; Remington, G.; Misawa, F.; Takeuchi, H. Adherence to Oral Antipsychotics Measured by Electronic Adherence Monitoring in Schizophrenia: A Systematic Review and Meta-Analysis. CNS Drugs 2020, 34, 579–598. [Google Scholar] [CrossRef]
- Mei, H.; Hsieh, Y.; Nardo, C.; Xu, X.; Wang, S.; Ng, K.; Korfmacher, W.A. Investigation of Matrix Effects in Bioanalytical High-Performance Liquid Chromatography/Tandem Mass Spectrometric Assays: Application to Drug Discovery. Rapid Commun. Mass Spectrom. 2003, 17, 97–103. [Google Scholar] [CrossRef]
- Langel, K.; Gjerde, H.; Favretto, D.; Lillsunde, P.; Øiestad, E.L.; Ferrara, S.D.; Verstraete, A.G. Comparison of Drug Concentrations between Whole Blood and Oral Fluid. Drug Test. Anal. 2014, 6, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Desrosiers, N.A.; Huestis, M.A. Oral Fluid Drug Testing: Analytical Approaches, Issues and Interpretation of Results. J. Anal. Toxicol. 2019, 43, 415–443. [Google Scholar] [CrossRef] [PubMed]
- de Sá e Silva, D.M.; Thaitumu, M.; Theodoridis, G.; Witting, M.; Gika, H. Volumetric Absorptive Microsampling in the Analysis of Endogenous Metabolites. Metabolites 2023, 13, 1038. [Google Scholar] [CrossRef] [PubMed]
- Binnicker, M.J. Multiplex Molecular Panels for Diagnosis of Gastrointestinal Infection: Performance, Result Interpretation, and Cost-Effectiveness. J. Clin. Microbiol. 2015, 53, 3723–3728. [Google Scholar] [CrossRef]
- Clarke, W.; Rhea, J.M.; Molinaro, R. Challenges in Implementing Clinical Liquid Chromatography-Tandem Mass Spectrometry Methods—Seeing the Light at the End of the Tunnel. J. Mass Spectrom. 2013, 48, 755–767. [Google Scholar] [CrossRef]
- Amponsah, S.K.; Pathak, Y.V. Therapeutic Drug Monitoring and Clinical Toxicology: Challenges and Future Directions. In Recent Advances in Therapeutic Drug Monitoring and Clinical Toxicology; Springer: Berlin/Heidelberg, Germany, 2023; pp. 369–377. [Google Scholar] [CrossRef]
- Petrick, L.M.; Niedzwiecki, M.M.; Dolios, G.; Guan, H.; Tu, P.; Wright, R.O.; Wright, R.J. Effects of Storage Temperature and Time on Metabolite Profiles Measured in Dried Blood Spots, Dried Blood Microsamplers, and Plasma. Sci. Total Environ. 2024, 912, 169383. [Google Scholar] [CrossRef]
- Shop UHPLC Systems For Sale, New and Used Prices|LabX.Com. Available online: https://www.labx.com/categories/uhplc-systems?utm_source=chatgpt.com (accessed on 9 October 2025).
- Rates|Harvard Center for Mass Spectrometry. Available online: https://massspec.fas.harvard.edu/pages/rates?utm_source=chatgpt.com (accessed on 9 October 2025).


| Nr. | Study | Sample Type | Sample Preparation | Clinical Context | Suitability for Standard Clinical Practice |
|---|---|---|---|---|---|
| 1 | Patteet et al. [40] | Serum | LLE with MTBE (pH 9.5), evaporation, reconstitution in ACN | Invasive blood samples | Yes |
| 2 | Patteet et al. [42] | Serum | Similar robust LLE method, low matrix effect | Invasive blood samples | Yes |
| 3 | Patteet et al. [41] | DBS | DBS dried 3 h, extracted with MeOH + MTBE | Non-invasive blood samples | Partial |
| 4 | Patteet et al. [43] | Oral fluid | OF buffer + LLE, evaporation, reconstitution | Non-invasive oral fluid samples | Partial |
| 5 | Patteet et al. [39] | Serum | LLE with MTBE, IS mix covering multiple antipsychotics | Invasive blood samples | Yes |
| 6 | Millan-Santiago et al. [36] | Whole blood (VAMS) | VAMS microsampling, UAE in MeOH, direct UHPLC-MS | Minimally invasive blood samples | Partial |
| 7 | Bernardo et al. [35] | DBS and WB | DBS dried overnight, WB with standard LLE | Non-invasive and Minimally Invasive blood samples | Partial |
| 8 | Toja-Camba et al. [11] | Plasma | IS spiked, ACN precipitation, quick prep | Invasive blood samples | Yes |
| 9 | Toja-Camba et al. [2] | Plasma | Same protein precipitation workflow for risperidone monitoring | Invasive blood samples | Yes |
| 10 | Cruz et al. [37] | Plasma | MEPS with RACNT, novel mini SPE, minimal prep | Invasive blood samples | Partial |
| 11 | Wang et al. [38] | Plasma | Small plasma volume, ACN or MeOH precipitation, direct analysis | Invasive blood samples | Yes |
| 12 | Pouliopoulos et al. [44] | Serum and postmortem blood | ACN + salts for protein precipitation, forensic + standard use | Clinical and forensic toxicology labs | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Băcilă, C.-I.; Macavei, B.-M.; Cornea, M.; Vintilă, B.I.; Lomnășan, A.; Anghel, C.E.; Grama, A.M.; Dobre, C.E.; Ichim, C.M.; Cioca, G. UHPLC-MS/MS for Antipsychotic Drug Monitoring: A Systematic Review of Clinical and Analytical Performance. J. Clin. Med. 2025, 14, 7544. https://doi.org/10.3390/jcm14217544
Băcilă C-I, Macavei B-M, Cornea M, Vintilă BI, Lomnășan A, Anghel CE, Grama AM, Dobre CE, Ichim CM, Cioca G. UHPLC-MS/MS for Antipsychotic Drug Monitoring: A Systematic Review of Clinical and Analytical Performance. Journal of Clinical Medicine. 2025; 14(21):7544. https://doi.org/10.3390/jcm14217544
Chicago/Turabian StyleBăcilă, Ciprian-Ionuț, Bianca-Maria Macavei, Monica Cornea, Bogdan Ioan Vintilă, Andrei Lomnășan, Claudia Elena Anghel, Andreea Maria Grama, Cristina Elena Dobre, Claudia Marina Ichim, and Gabriela Cioca. 2025. "UHPLC-MS/MS for Antipsychotic Drug Monitoring: A Systematic Review of Clinical and Analytical Performance" Journal of Clinical Medicine 14, no. 21: 7544. https://doi.org/10.3390/jcm14217544
APA StyleBăcilă, C.-I., Macavei, B.-M., Cornea, M., Vintilă, B. I., Lomnășan, A., Anghel, C. E., Grama, A. M., Dobre, C. E., Ichim, C. M., & Cioca, G. (2025). UHPLC-MS/MS for Antipsychotic Drug Monitoring: A Systematic Review of Clinical and Analytical Performance. Journal of Clinical Medicine, 14(21), 7544. https://doi.org/10.3390/jcm14217544






