Comparison of Cariogenic Organic Acid Concentrations According to Combined Use of Sucrose and Sugar Alcohols
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Saliva Sample Preparation
2.3. HPLC (IC-CD)
2.4. Method Validation
2.5. Statistical Analysis
3. Results
3.1. Analysis of the Organic Acid in Saliva Samples Using HPLC (IC-CD)
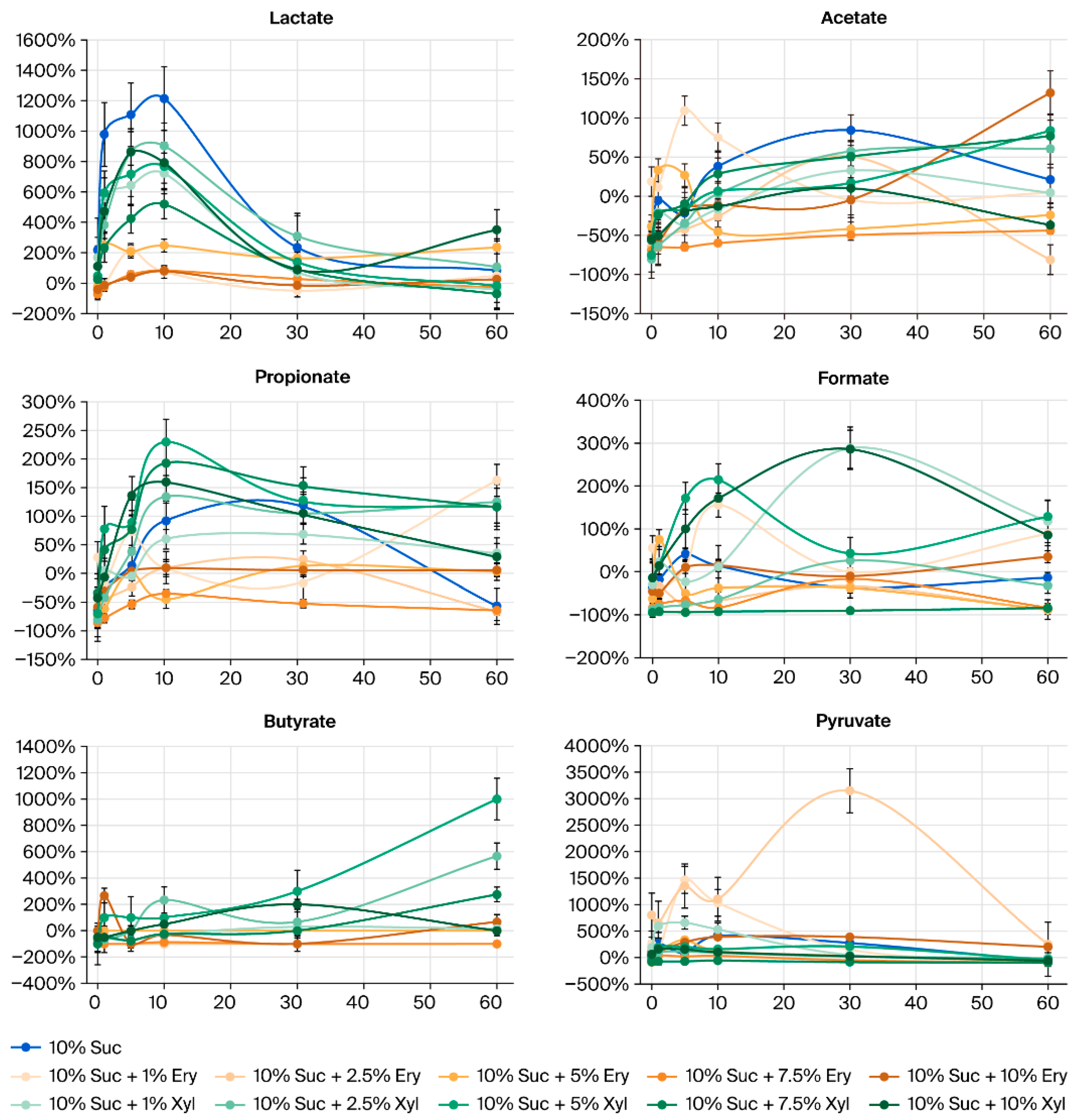
3.2. Organic Acid Levels in Saliva After Applying Sugar and Sugar Alcohols
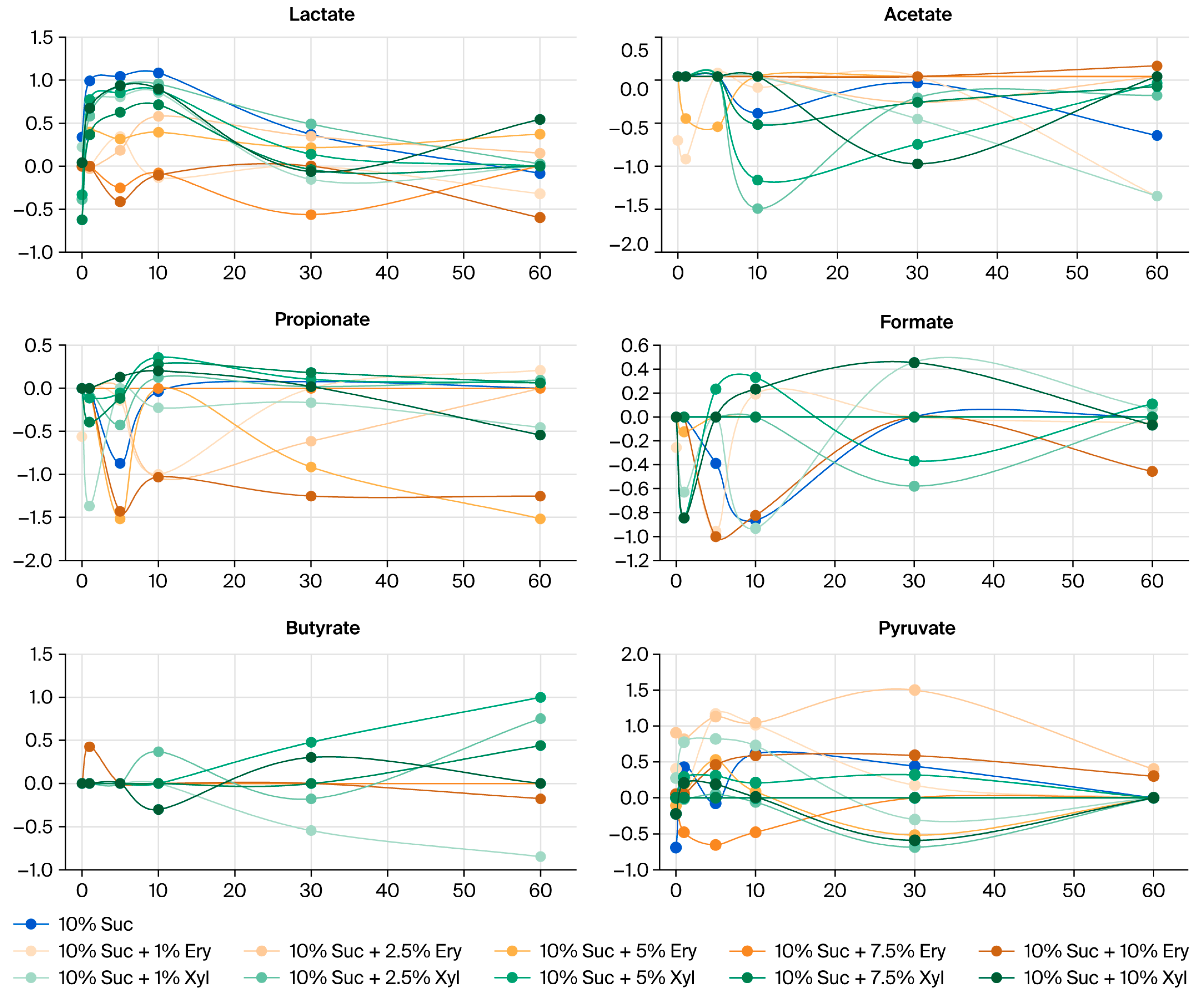
3.3. Amount of Increase/Decrease in Organic Acids in Saliva After Applying Sugar and Sugar Alcohols
3.4. Comparison of Organic Acid Levels in the Erythritol Group and Other Groups (Sucrose and Xylitol)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Variables | Min | Lactate | Acetate | Propionate | Formate | Butyrate | Pyruvate | Valerate |
|---|---|---|---|---|---|---|---|---|
| 10% Sucrose | Control | 0.68 ± 0.63 | 3.06 ± 5.38 | 0.82 ± 1.62 | 0.22 ± 0.2 | <LOQ | 0.44 ± 0.88 | <LOQ |
| 0 | 2.16 ± 1.75 | 1.47 ± 1.32 | 0.29 ± 0.28 | 0.14 ± 0.09 | <LOQ | 0.53 ± 1.08 | <LOQ | |
| 1 | 7.33 ± 4.75 | 2.89 ± 2.69 | 0.62 ± 0.48 | 0.18 ± 0.19 | <LOQ | 1.61 ± 1.61 | <LOQ | |
| 5 | 8.21 ± 7.15 | 2.4 ± 2.62 | 0.93 ± 1.45 | 0.31 ± 0.2 | <LOQ | 0.81 ± 2.44 | <LOQ | |
| 10 | 8.93 ± 3.29 | 4.23 ± 8.78 | 1.57 ± 3.38 | 0.25 ± 0.11 | <LOQ | 2.25 ± 3.39 | <LOQ | |
| 30 | 2.27 ± 4.81 | 5.64 ± 1.25 | 1.8 ± 0.67 | 0.14 ± 0.24 | <LOQ | 1.65 ± 0.32 | <LOQ | |
| 60 | 1.24 ± 1.67 | 3.71 ± 4.09 | 0.35 ± 0.2 | 0.19 ± 0.12 | <LOQ | 0.14 ± 0.16 | <LOQ | |
| 10% Suc + 1% Ery | Control | 2.99 ± 3.14 | 1.39 ± 2.38 | 0.4 ± 0.59 | 0.09 ± 0.15 | <LOQ | 0.06 ± 0.08 | <LOQ |
| 0 | 1.42 ± 0.97 | 1.65 ± 0.84 | 0.51 ± 0.52 | 0.14 ± 0.17 | <LOQ | 0.21 ± 0.28 | <LOQ | |
| 1 | 2.63 ± 3.27 | 1.55 ± 1.57 | 0.33 ± 0.3 | 0.06 ± 0.07 | <LOQ | 0.13 ± 0.25 | <LOQ | |
| 5 | 9.53 ± 2.81 | 2.91 ± 1.22 | 0.69 ± 0.3 | 0.1 ± 0.1 | <LOQ | 0.94 ± 0.56 | <LOQ | |
| 10 | 5.19 ± 1.45 | 2.43 ± 1.29 | 0.44 ± 0.33 | 0.23 ± 0.09 * | <LOQ | 0.68 ± 0.25 | <LOQ | |
| 30 | 1.52 ± 3.38 | 1.32 ± 2.24 | 0.33 ± 0.63 | 0.09 ± 0.2 | <LOQ | 0.15 ± 1.05 | <LOQ | |
| 60 | 4.42 ± 2.9 | 1.45 ± 1.48 * | 1.05 ± 0.87 | 0.17 ± 0.28 | <LOQ | <LOQ | <LOQ | |
| 10% Suc + 2.5% Ery | Control | 0.77 ± 3.79 | 1.44 ± 2.06 | 0.33 ± 0.45 | 0.09 ± 0.34 | <LOQ | 0.02 ± 0.18 | <LOQ |
| 0 | 0.63 ± 1.06 | 0.51 ± 0.62 | 0.14 ± 0.21 | 0.05 ± 0.2 | 0.02 ± 0.07 | 0.18 ± 0.26 | <LOQ | |
| 1 | 1.49 ± 1.94 | 0.65 ± 0.54 | 0.18 ± 0.36 | 0.06 ± 0.1 | 0.01 ± 0.21 | 0.15 ± 0.23 | <LOQ | |
| 5 | 1.95 ± 2.75 | 0.83 ± 1.24 | 0.25 ± 0.28 | 0.02 ± 0.37 | 0 ± 0.03 | 0.29 ± 0.4 | <LOQ | |
| 10 | 3.7 ± 1.43 | 1.07 ± 1.19 | 0.36 ± 0.32 | 0.03 ± 0.22 * | <LOQ | 0.24 ± 0.63 | <LOQ | |
| 30 | 2.48 ± 3.54 | 2.17 ± 1.45 | 0.41 ± 0.58 | 0.06 ± 0.3 | <LOQ | 0.65 ± 0.62 | <LOQ | |
| 60 | 1.86 ± 2.75 | 0.27 ± 2.5 * | 0.11 ± 0.3 | 0.01 ± 0.23 | 0.02 ± 0.07 | 0.07 ± 0.31 | <LOQ | |
| 10% Suc + 5% Ery | Control | 1.39 ± 0.55 | 2.2 ± 2.38 | 0.66 ± 0.56 | 0.08 ± 0.15 | 0.12 ± 0.00 | 0.23 ± 0.04 | <LOQ |
| 0 | 1.09 ± 0.37 | 1.35 ± 0.29 | 0.18 ± 0.07 | 0.03 ± 0.03 | 0.01 ± 0.04 | 0.41 ± 0.19 | <LOQ | |
| 1 | 4.87 ± 1.53 | 2.93 ± 0.54 | 0.25 ± 0.17 | 0.14 ± 0.06 | 0 ± 0.01 | 0.5 ± 0.12 | <LOQ | |
| 5 | 4.26 ± 2.99 | 2.79 ± 1.35 | 0.68 ± 0.37 | 0.04 ± 0.04 | 0 ± 0.01 | 1.00 ± 0.3 | <LOQ | |
| 10 | 4.83 ± 1.49 | 1.2 ± 2.32 | 0.36 ± 0.36 | 0.05 ± 0.08 | <LOQ | 0.51 ± 0.92 | <LOQ | |
| 30 | 3.67 ± 1.54 | 1.28 ± 0.79 | 0.74 ± 0.25 | 0.05 ± 0.01 | 0 ± 0.01 | 0.3 ± 0.4 | <LOQ | |
| 60 | 4.66 ± 1.87 | 1.67 ± 0.26 * | 0.68 ± 0.06 | 0.01 ± 0.01 | 0 ± 0.04 | 0.06 ± 0.11 | <LOQ | |
| 10% Suc + 7.5% Ery | Control | 1.79 ± 1.82 | 2.37 ± 2.33 | 0.65 ± 0.88 | 0.12 ± 0.1 | 0.08 ± 0.28 | 0.18 ± 0.51 | <LOQ |
| 0 | 0.47 ± 0.7 | 0.73 ± 1.33 | 0.09 ± 0.12 | 0.02 ± 0.05 | 0 ± 0.02 | 0.02 ± 0.35 | <LOQ | |
| 1 | 1.32 ± 4.84 | 0.82 ± 2.77 | 0.14 ± 0.26 | 0.03 ± 0.21 | <LOQ | 0.24 ± 0.6 | <LOQ | |
| 5 | 2.79 ± 5.19 | 0.82 ± 1.46 | 0.3 ± 0.49 | 0.04 ± 0.09 | <LOQ | 0.22 ± 0.72 | <LOQ | |
| 10 | 3.28 ± 3.88 | 0.95 ± 1.23 | 0.42 ± 0.94 | 0.02 ± 0.1 * | 0.01 ± 0.00 | 0.24 ± 0.49 | <LOQ | |
| 30 | 2.28 ± 6.1 | 1.19 ± 4.01 | 0.31 ± 1.1 | 0.1 ± 0.09 | <LOQ | 0.09 ± 1.73 | <LOQ | |
| 60 | 1.26 ± 4.57 | 1.33 ± 2.48 * | 0.23 ± 1.24 | 0.02 ± 0.02 | <LOQ | <LOQ | <LOQ | |
| 10% Suc + 10% Ery | Control | 2.1 ± 1.44 | 1.9 ± 1.68 | 0.54 ± 0.44 | 0.2 ± 0.07 | 0.03 ± 0.11 | 0.08 ± 0.16 | <LOQ |
| 0 | 1.2 ± 0.52 | 0.91 ± 0.6 | 0.22 ± 0.08 | 0.11 ± 0.02 | 0.03 ± 0.00 | 0.17 ± 0.05 | <LOQ | |
| 1 | 1.81 ± 0.91 | 0.79 ± 0.83 | 0.37 ± 0.04 | 0.1 ± 0.02 | 0.11 ± 0.00 | 0.17 ± 0.22 | <LOQ | |
| 5 | 2.91 ± 1.9 | 1.6 ± 0.53 | 0.56 ± 0.26 | 0.22 ± 0.03 | <LOQ | 0.31 ± 0.26 | <LOQ | |
| 10 | 3.75 ± 1.96 | 1.68 ± 0.98 | 0.59 ± 0.2 | 0.23 ± 0.07 * | 0.02 ± 0.00 | 0.39 ± 0.12 | <LOQ | |
| 30 | 1.78 ± 1.75 | 1.81 ± 0.61 | 0.57 ± 0.2 | 0.18 ± 0.03 | <LOQ | 0.39 ± 0.22 | <LOQ | |
| 60 | 2.63 ± 1.01 | 4.41 ± 1.96 * | 0.57 ± 0.28 | 0.27 ± 0.04 | 0.05 ± 0.00 | 0.24 ± 0.00 | <LOQ | |
| 10% Suc + 1% Xyl | Control | 0.98 ± 0.81 | 3.68 ± 2.47 | 0.94 ± 0.43 | 0.17 ± 0.1 | 0.07 ± 0.07 | 0.26 ± 0.23 | <LOQ |
| 0 | 2.62 ± 2.08 | 1.6 ± 1.34 | 0.55 ± 0.64 | 0.12 ± 0.11 | 0.03 ± 0.06 | 0.75 ± 0.67 | <LOQ | |
| 1 | 6.46 ± 3.83 | 2.92 ± 1.73 | 0.98 ± 0.53 | 0.21 ± 0.16 | 0.03 ± 0.04 | 1.8 ± 2.37 | <LOQ | |
| 5 | 7.27 ± 6.29 | 2.31 ± 1.99 | 0.89 ± 0.7 | 0.13 ± 0.1 | 0.02 ± 0.06 | 1.97 ± 2.84 | <LOQ | |
| 10 | 8.05 ± 1.38 | 3.07 ± 5.16 | 1.5 ± 1.64 | 0.19 ± 1.02 | 0.05 ± 0.14 | 1.64 ± 0.31 | <LOQ | |
| 30 | 1.67 ± 6.4 | 4.89 ± 1.95 | 1.58 ± 0.63 | 0.66 ± 0.08 | 0.09 ± 0.03 | 0.39 ± 3.44 | <LOQ | |
| 60 | 0.57 ± 0.25 | 3.84 ± 2.7 | 1.27 ± 0.87 | 0.37 ± 0.43 | 0.08 ± 0.12 | 0.18 ± 0.17 | <LOQ | |
| 10% Suc + 2.5% Xyl | Control | 0.68 ± 0.56 | 3.19 ± 3.74 | 0.8 ± 0.54 | 0.72 ± 0.05 | 0.03 ± 0.04 | 0.24 ± 0.41 | <LOQ |
| 0 | 0.96 ± 0.42 | 0.62 ± 0.74 | 0.15 ± 0.14 | 0.09 ± 0.02 | 0.01 ± 0.01 | 0.19 ± 0.44 | <LOQ | |
| 1 | 3.28 ± 1.92 | 1.15 ± 0.83 | 0.46 ± 0.21 | 0.13 ± 0.03 | 0.01 ± 0.02 | 0.47 ± 0.7 | <LOQ | |
| 5 | 6.56 ± 5.33 | 2.08 ± 0.78 | 1.1 ± 0.47 | 0.17 ± 0.22 | 0.03 ± 0.03 | 0.51 ± 0.49 | <LOQ | |
| 10 | 6.81 ± 1.18 | 3.29 ± 2.78 | 1.87 ± 0.69 | 0.26 ± 0.29 | 0.1 ± 0.06 | 0.45 ± 0.35 | <LOQ | |
| 30 | 2.78 ± 3.68 | 5.02 ± 1.23 | 1.64 ± 0.54 | 0.91 ± 0.16 | 0.05 ± 0.03 | 0.29 ± 0.36 | <LOQ | |
| 60 | 1.4 ± 5.47 | 5.13 ± 1.49 | 1.8 ± 0.49 | 0.49 ± 0.18 | 0.2 ± 0.03 | 0.21 ± 0.1 | <LOQ | |
| 10% Suc + 5% Xyl | Control | 0.9 ± 0.53 | 2.71 ± 3.79 | 0.44 ± 1.18 | 0.07 ± 0.66 | 0.01 ± 0.07 | 0.36 ± 0.34 | <LOQ |
| 0 | 1.32 ± 0.65 | 0.67 ± 0.51 | 0.13 ± 0.18 | 0.06 ± 0.05 | 0.00 ± 0.01 | 0.18 ± 0.21 | <LOQ | |
| 1 | 6.23 ± 2.51 | 2.1 ± 0.65 | 0.78 ± 0.31 | 0.08 ± 0.1 | 0.02 ± 0.02 | 1.06 ± 0.42 | <LOQ | |
| 5 | 7.34 ± 6.88 | 2.26 ± 2.58 | 0.83 ± 2.24 | 0.19 ± 0.2 | 0.02 ± 0.13 | 1.09 ± 0.39 | <LOQ | |
| 10 | 7.81 ± 3.2 | 2.89 ± 5.24 | 1.45 ± 1.76 | 0.22 ± 1.33 | 0.02 ± 0.08 | 0.94 ± 0.33 | <LOQ | |
| 30 | 2.14 ± 7.16 | 3.17 ± 1.95 | 1.00 ± 1.09 | 0.1 ± 0.21 | 0.04 ± 0.08 | 1.11 ± 0.62 | <LOQ | |
| 60 | 0.73 ± 1.8 | 4.98 ± 5.36 | 0.95 ± 2.2 | 0.16 ± 0.65 | 0.11 ± 0.26 | 0.21 ± 0.26 | <LOQ | |
| 10% Suc + 7.5% Xyl | Control | 1.59 ± 1.1 | 3.27 ± 2.22 | 0.64 ± 0.3 | 1.83 ± 0.07 | 0.04 ± 0.03 | 3.24 ± 0.43 | <LOQ |
| 0 | 1.97 ± 0.62 | 1.43 ± 0.39 | 0.42 ± 0.09 | 0.1 ± 0.04 | <LOQ | 0.69 ± 0.17 | <LOQ | |
| 1 | 5.28 ± 4.98 | 2.49 ± 1.95 | 0.9 ± 0.8 | 0.14 ± 0.05 | 0.02 ± 0.03 | 0.86 ± 1.66 | <LOQ | |
| 5 | 8.32 ± 7.83 | 2.95 ± 1.78 | 1.13 ± 1.37 | 0.11 ± 0.27 | 0.01 ± 0.04 | 0.9 ± 1.00 | <LOQ | |
| 10 | 9.84 ± 2.38 | 4.2 ± 3.34 | 1.87 ± 1.14 | 0.14 ± 0.04 | 0.03 ± 0.05 | 1.38 ± 1.62 | <LOQ | |
| 30 | 3.04 ± 5.92 | 4.93 ± 1.09 | 1.62 ± 0.5 | 0.18 ± 0.21 | 0.04 ± 0.03 | 0.47 ± 1.41 | <LOQ | |
| 60 | 0.46 ± 0.54 | 5.78 ± 5.54 | 1.38 ± 0.97 | 0.29 ± 0.18 | 0.15 ± 0.14 | 0.15 ± 0.3 | <LOQ | |
| 10% Suc + 10% Xyl | Control | 0.6 ± 1.38 | 3.36 ± 3.11 | 0.42 ± 0.68 | 0.07 ± 3.28 | 0.02 ± 0.05 | 0.35 ± 6.56 | <LOQ |
| 0 | 1.26 ± 1.15 | 1.51 ± 0.83 | 0.24 ± 0.32 | 0.06 ± 0.12 | 0.01 ± 0.00 | 0.56 ± 0.73 | <LOQ | |
| 1 | 3.43 ± 2.64 | 1.66 ± 1.7 | 0.39 ± 0.79 | 0.08 ± 0.17 | 0.01 ± 0.06 | 0.91 ± 0.7 | <LOQ | |
| 5 | 5.77 ± 7.71 | 2.68 ± 1.28 | 0.99 ± 1.21 | 0.14 ± 0.12 | 0.02 ± 0.04 | 0.89 ± 0.73 | <LOQ | |
| 10 | 5.35 ± 2.44 | 2.92 ± 3.8 | 1.09 ± 1.83 | 0.19 ± 0.18 | 0.03 ± 0.07 | 0.71 ± 0.34 | <LOQ | |
| 30 | 1.12 ± 6.72 | 3.7 ± 1.12 | 0.86 ± 0.66 | 0.27 ± 0.08 | 0.06 ± 0.02 | 0.44 ± 0.74 | <LOQ | |
| 60 | 2.7 ± 0.28 | 2.12 ± 4.77 | 0.54 ± 1.43 | 0.13 ± 0.34 | 0.02 ± 0.16 | 0.15 ± 0.15 | <LOQ |
References
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) Fertility Estimates 1950–2019; Institute for Health Metrics and Evaluation (IHME): Seattle, WA, USA, 2020. [Google Scholar]
- Collaborators, G.O.D.; Bernabe, E.; Marcenes, W.; Hernandez, C.; Bailey, J.; Abreu, L.; Alipour, V.; Amini, S.; Arabloo, J.; Arefi, Z. Global, regional, and national levels and trends in burden of oral conditions from 1990 to 2017: A systematic analysis for the global burden of disease 2017 study. J. Dent. Res. 2020, 99, 362–373. [Google Scholar] [CrossRef]
- Park, Y.-D.; Jang, J.-H.; Oh, Y.-J.; Kwon, H.-J. Analyses of organic acids and inorganic anions and their relationship in human saliva before and after glucose intake. Arch. Oral Biol. 2014, 59, 1–11. [Google Scholar] [CrossRef]
- Scardina, G.; Messina, P. Good oral health and diet. J. Biomed. Biotechnol. 2012, 2012, 720692. [Google Scholar] [CrossRef]
- Rajasekaran, J.J.; Krishnamurthy, H.K.; Bosco, J.; Jayaraman, V.; Krishna, K.; Wang, T.; Bei, K. Oral Microbiome: A Review of Its Impact on Oral and Systemic Health. Microorganisms 2024, 12, 1797. [Google Scholar] [CrossRef]
- WHO. Global Oral Health Status Report: Towards Universal Health Coverage for Oral Health by 2030; WHO Publications: Geneva, Switzerland, 2022.
- Moynihan, P. Sugars and dental caries: Evidence for setting a recommended threshold for intake. Adv. Nutr. 2016, 7, 149–156. [Google Scholar] [CrossRef]
- Park, Y.-N. The inhibitive effect of erythritol on growth and acidogenic ability of Streptococcus mutans. J. Digit. Converg. 2013, 11, 515–522. [Google Scholar] [CrossRef]
- Mäkinen, K.K. Sugar alcohols, caries incidence, and remineralization of caries lesions: A literature review. Int. J. Dent. 2010, 2010, 981072. [Google Scholar] [CrossRef]
- Milgrom, P.; Söderling, E.; Nelson, S.; Chi, D.; Nakai, Y. Clinical evidence for polyol efficacy. Adv. Dent. Res. 2012, 24, 112–116. [Google Scholar] [CrossRef]
- Zhan, L.; Featherstone, J.; Lo, J.; Krupansky, C.; Hoang, N.; DenBesten, P.; Huynh, T. Clinical efficacy and effects of xylitol wipes on bacterial virulence. Adv. Dent. Res. 2012, 24, 117–122. [Google Scholar] [CrossRef]
- de Cock, P.; Mäkinen, K.; Honkala, E.; Saag, M.; Kennepohl, E.; Eapen, A. Erythritol is more effective than xylitol and sorbitol in managing oral health endpoints. Int. J. Dent. 2016, 2016, 9868421. [Google Scholar] [CrossRef]
- Thabuis, C.; Cheng, C.; Wang, X.; Pochat, M.; Han, A.; Miller, L.; Wils, D.; Guerin-Deremaux, L. Effects of maltitol and xylitol chewing-gums on parameters involved in dental caries development. Eur. J. Paediatr. Dent. 2013, 14, 303–308. [Google Scholar]
- Falony, G.; Honkala, S.; Runnel, R.; Olak, J.; Nõmmela, R.; Russak, S.; Saag, M.; Mäkinen, P.-L.; Mäkinen, K.; Vahlberg, T. Long-term effect of erythritol on dental caries development during childhood: A posttreatment survival analysis. Caries Res. 2016, 50, 579–588. [Google Scholar] [CrossRef]
- Janket, S.-J.; Benwait, J.; Isaac, P.; Ackerson, L.K.; Meurman, J.H. Oral and systemic effects of xylitol consumption. Caries Res. 2019, 53, 491–501. [Google Scholar] [CrossRef]
- Dawood, I.-M.; Samarrai, S.K. Saliva and oral health. Int. J. Adv. Res. Biol. Sci. 2018, 5, 1–45. [Google Scholar] [CrossRef]
- Layden, B.T.; Angueira, A.R.; Brodsky, M.; Durai, V.; Lowe, W.L., Jr. Short chain fatty acids and their receptors: New metabolic targets. Transl. Res. 2013, 161, 131–140. [Google Scholar] [CrossRef]
- Cai, G.; Jin, B.; Saint, C.; Monis, P. Genetic manipulation of butyrate formation pathways in Clostridium butyricum. J. Biotechnol. 2011, 155, 269–274. [Google Scholar] [CrossRef]
- Weimer, P.; Moen, G. Quantitative analysis of growth and volatile fatty acid production by the anaerobic ruminal bacterium Megasphaera elsdenii T81. Appl. Microbiol. Biotechnol. 2013, 97, 4075–4081. [Google Scholar] [CrossRef]
- Fidalgo, T.K.; Freitas-Fernandes, L.B.; Angeli, R.; Muniz, A.M.; Gonsalves, E.; Santos, R.; Nadal, J.; Almeida, F.C.; Valente, A.P.; Souza, I.P. Salivary metabolite signatures of children with and without dental caries lesions. Metabolomics 2013, 9, 657–666. [Google Scholar] [CrossRef]
- Kolumban, A.; Moldovan, M.; Tig, I.A.; Chifor, I.; Cuc, S.; Bud, M.; Badea, M.E. An Evaluation of the Demineralizing Effects of Various Acidic Solutions. Appl. Sci. 2021, 11, 8270. [Google Scholar] [CrossRef]
- Schulz, A.; Lang, R.; Behr, J.; Hertel, S.; Reich, M.; Kümmerer, K.; Hannig, M.; Hannig, C.; Hofmann, T. Targeted metabolomics of pellicle and saliva in children with different caries activity. Sci. Rep. 2020, 10, 697. [Google Scholar] [CrossRef]
- Kashyap, B.; Kullaa, A. Salivary Metabolites Produced by Oral Microbes in Oral Diseases and Oral Squamous Cell Carcinoma: A Review. Metabolites 2024, 14, 277. [Google Scholar] [CrossRef]
- Crable, B.R.; Plugge, C.M.; McInerney, M.J.; Stams, A.J. Formate formation and formate conversion in biological fuels production. Enzym. Res. 2011, 2011, 532536. [Google Scholar] [CrossRef]
- Huang, C.B.; Alimova, Y.; Myers, T.M.; Ebersole, J.L. Short-and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch. Oral Biol. 2011, 56, 650–654. [Google Scholar] [CrossRef]
- Mazi, T.A.; Stanhope, K.L. Erythritol: An In-Depth Discussion of Its Potential to Be a Beneficial Dietary Component. Nutrients 2023, 15, 204. [Google Scholar] [CrossRef]
- Honkala, S.; Runnel, R.; Saag, M.; Olak, J.; Nõmmela, R.; Russak, S.; Mäkinen, P.-L.; Vahlberg, T.; Falony, G.; Mäkinen, K. Effect of erythritol and xylitol on dental caries prevention in children. Caries Res. 2014, 48, 482–490. [Google Scholar] [CrossRef]
- Park, Y.-N.; Jeong, S.-S.; Zeng, J.; Kim, S.-H.; Hong, S.-J.; Ohk, S.-H.; Choi, C.-H. Anti-cariogenic effects of erythritol on growth and adhesion of Streptococcus mutans. Food Sci. Biotechnol. 2014, 23, 1587–1591. [Google Scholar] [CrossRef]
- da Costa Rosa, T.; de Almeida Neves, A.; Azcarate-Peril, M.A.; Divaris, K.; Wu, D.; Cho, H.; Moss, K.; Paster, B.J.; Chen, T.; B Freitas-Fernandes, L.; et al. The bacterial microbiome and metabolome in caries progression and arrest. J. Oral Microbiol. 2021, 13, 1886748. [Google Scholar] [CrossRef]
- Shimazu, T.; Hirschey, M.D.; Huang, J.-Y.; Ho, L.T.; Verdin, E. Acetate metabolism and aging: An emerging connection. Mech. Ageing Dev. 2010, 131, 511–516. [Google Scholar] [CrossRef]
- Mäkinen, K.K. Sugar alcohol sweeteners as alternatives to sugar with special consideration of xylitol. Med. Princ. Pract. 2011, 20, 303–320. [Google Scholar] [CrossRef]
- Lim, J.H.; Jeong, Y.; Song, S.H.; Ahn, J.H.; Lee, J.R.; Lee, S.M. Penetration of an antimicrobial zinc-sugar alcohol complex into Streptococcus mutans biofilms. Sci. Rep. 2018, 8, 16154. [Google Scholar] [CrossRef]
- Hashino, E.; Kuboniwa, M.; Alghamdi, S.A.; Yamaguchi, M.; Yamamoto, R.; Cho, H.; Amano, A. Erythritol alters microstructure and metabolomic profiles of biofilm composed of S treptococcus gordonii and P orphyromonas gingivalis. Mol. Oral. Microbiol. 2013, 28, 435–451. [Google Scholar] [CrossRef] [PubMed]

| Compounds | Linear Range (mM) | Linear Equation | r2 | LOD (µM) | LOQ (µM) |
|---|---|---|---|---|---|
| Lactate | 0.01–5 | y = 0.3481x + 7.6541 | 0.9988 | 0.62 | 1.84 |
| Acetate | 0.01–5 | y = 0.5411x + 15.29 | 0.9998 | 1.05 | 3.18 |
| Propionate | 0.01–5 | y = 0.272x + 5.9467 | 0.9999 | 1.32 | 3.95 |
| Formate | 0.01–5 | y = 0.4988x + 7.1799 | 0.9995 | 0.58 | 1.76 |
| Butyrate | 0.01–5 | y = 0.3084x + 3.3577 | 0.9995 | 0.53 | 1.61 |
| Pyruvate | 0.01–5 | y = 0.1361x + 1.53 | 0.9998 | 0.37 | 1.12 |
| Valerate | 0.01–5 | y = 0.1701x + 2.1809 | 0.9999 | 0.51 | 1.56 |
| Lactate | Acetate | Propionate | Formate | Butyrate | Pyruvate | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Statistic | p | p_corr | Statistic | p | p_corr | Statistic | p | p_corr | Statistic | p | p_corr | Statistic | p | p_corr | Statistic | p | p_corr | ||
| 10% Suc + 1% Ery | S1 | −0.429 | 0.669 | 1 | −1.988 | 0.051 | 1 | −1.38 | 0.172 | 1 | −2 | 0.05 | 1 | −1.406 | 0.164 | 1 | −2.4 | 0.019 | 1 |
| E2.5 | 3.099 | 0.003 | 0.155 | 2.276 | 0.026 | 1 | 2.634 | 0.01 | 0.573 | 2.706 | 0.009 | 0.473 | −2.06 | 0.043 | 1 | 0.696 | 0.489 | 1 | |
| E5 | 0.45 | 0.654 | 1 | −0.212 | 0.833 | 1 | 0.168 | 0.867 | 1 | 2.157 | 0.035 | 1 | −1.062 | 0.292 | 1 | −0.741 | 0.461 | 1 | |
| E7.5 | 3.102 | 0.003 | 0.154 | 1.908 | 0.061 | 1 | 2.195 | 0.032 | 1 | 2.643 | 0.01 | 0.561 | −1.54 | 0.128 | 1 | 1.741 | 0.086 | 1 | |
| E10 | 2.209 | 0.031 | 1 | −0.135 | 0.893 | 1 | 0.413 | 0.681 | 1 | −1.182 | 0.241 | 1 | −2.337 | 0.022 | 1 | 0.548 | 0.586 | 1 | |
| X1 | 0.011 | 0.991 | 1 | −2.594 | 0.012 | 0.639 | −3.298 | 0.002 | 0.085 | −1.771 | 0.081 | 1 | −3.843 | 0 | 0.014 | −2.053 | 0.044 | 1 | |
| X2.5 | 0.772 | 0.443 | 1 | −1.696 | 0.094 | 1 | −2.149 | 0.035 | 1 | −2.419 | 0.018 | 1 | −2.888 | 0.005 | 0.285 | −0.22 | 0.826 | 1 | |
| X5 | 0.171 | 0.865 | 1 | −1.581 | 0.118 | 1 | −1.508 | 0.136 | 1 | 0.003 | 0.998 | 1 | −2.999 | 0.004 | 0.208 | −1.923 | 0.059 | 1 | |
| X7.5 | −0.381 | 0.705 | 1 | −3.171 | 0.002 | 0.125 | −2.863 | 0.006 | 0.307 | −1.244 | 0.218 | 1 | −3.172 | 0.002 | 0.125 | −1.819 | 0.073 | 1 | |
| X10 | 1.24 | 0.219 | 1 | −1.746 | 0.085 | 1 | −0.879 | 0.383 | 1 | −0.184 | 0.855 | 1 | −3.908 | 0 | 0.011 | −2.114 | 0.038 | 1 | |
| 10% Suc + 2.5% Ery | S1 | −2.867 | 0.006 | 0.302 | −3.104 | 0.003 | 0.152 | −2.512 | 0.014 | 0.791 | −5.232 | 0 | 0 | 1.773 | 0.081 | 1 | −2.711 | 0.009 | 0.467 |
| E5 | −2.216 | 0.03 | 1 | −2.029 | 0.046 | 1 | −1.788 | 0.078 | 1 | −0.491 | 0.625 | 1 | −0.633 | 0.529 | 1 | −1.344 | 0.183 | 1 | |
| E7.5 | −0.114 | 0.909 | 1 | −0.584 | 0.561 | 1 | −0.714 | 0.478 | 1 | −0.263 | 0.794 | 1 | −0.567 | 0.573 | 1 | 1.159 | 0.251 | 1 | |
| X1 | −2.528 | 0.014 | 0.758 | −4.28 | 0 | 0.003 | −5.52 | 0 | 0 | −2.966 | 0.004 | 0.228 | −3.191 | 0.002 | 0.118 | −2.335 | 0.023 | 1 | |
| X2.5 | −1.69 | 0.096 | 1 | −3.017 | 0.004 | 0.197 | −3.324 | 0.001 | 0.078 | −3.222 | 0.002 | 0.107 | −2.509 | 0.015 | 0.796 | −1.122 | 0.266 | 1 | |
| X5 | −2.195 | 0.032 | 1 | −3.171 | 0.002 | 0.125 | −3.478 | 0 | 0.048 | −2.89 | 0.005 | 0.284 | −2.19 | 0.032 | 1 | −2.425 | 0.018 | 0.987 | |
| X7.5 | −2.777 | 0.007 | 0.389 | −4.784 | 0 | 0 | −4.513 | 0 | 0.001 | −1.623 | 0.109 | 1 | −2.537 | 0.014 | 0.741 | −2.027 | 0.047 | 1 | |
| X10 | −1.546 | 0.127 | 1 | −3.85 | 0 | 0.014 | −3.799 | 0 | 0.017 | −2.869 | 0.006 | 0.301 | −2.23 | 0.029 | 1 | −3.186 | 0.002 | 0.12 | |
| 10% Suc + 5% Ery | S1 | −0.79 | 0.433 | 1 | −1.748 | 0.085 | 1 | −1.399 | 0.166 | 1 | −4.489 | 0 | 0.001 | 1.01 | 0.316 | 1 | −1.934 | 0.057 | 1 |
| E7.5 | 2.195 | 0.032 | 1 | 1.705 | 0.093 | 1 | 1.445 | 0.153 | 1 | 0.319 | 0.751 | 1 | 0.332 | 0.741 | 1 | 2.145 | 0.036 | 1 | |
| X1 | −0.39 | 0.698 | 1 | −2.13 | 0.037 | 1 | −3.037 | 0.003 | 0.186 | −2.78 | 0.007 | 0.386 | −1.496 | 0.139 | 1 | −1.633 | 0.107 | 1 | |
| X2.5 | 0.319 | 0.751 | 1 | −1.42 | 0.16 | 1 | −2.126 | 0.037 | 1 | −3.104 | 0.003 | 0.153 | −1.551 | 0.126 | 1 | 0.65 | 0.518 | 1 | |
| X5 | −0.223 | 0.824 | 1 | −1.249 | 0.216 | 1 | −1.467 | 0.147 | 1 | −2.277 | 0.026 | 1 | −0.642 | 0.523 | 1 | −1.224 | 0.225 | 1 | |
| X7.5 | −0.74 | 0.462 | 1 | −2.677 | 0.009 | 0.511 | −2.734 | 0.008 | 0.438 | −1.572 | 0.121 | 1 | −1.077 | 0.285 | 1 | −1.507 | 0.136 | 1 | |
| X10 | 0.699 | 0.487 | 1 | −1.269 | 0.209 | 1 | −0.883 | 0.38 | 1 | −2.326 | 0.023 | 1 | −0.198 | 0.844 | 1 | −0.912 | 0.365 | 1 | |
| 10% Suc + 7.5% Ery | S1 | −2.853 | 0.006 | 0.315 | −2.91 | 0.005 | 0.268 | −2.325 | 0.023 | 1 | −5.236 | 0 | 0 | 1.42 | 0.16 | 1 | −3.068 | 0.003 | 0.17 |
| X1 | −2.511 | 0.014 | 0.793 | −4.066 | 0 | 0.007 | −5.231 | 0 | 0 | −2.924 | 0.005 | 0.257 | −2.526 | 0.014 | 0.763 | −2.656 | 0.01 | 0.54 | |
| X2.5 | −1.66 | 0.102 | 1 | −2.794 | 0.007 | 0.371 | −3.138 | 0.003 | 0.138 | −3.192 | 0.002 | 0.117 | −2.166 | 0.034 | 1 | −2.791 | 0.007 | 0.374 | |
| X5 | −2.172 | 0.033 | 1 | −2.923 | 0.005 | 0.258 | −3.178 | 0.002 | 0.122 | −2.833 | 0.006 | 0.333 | −1.475 | 0.145 | 1 | −3.042 | 0.003 | 0.183 | |
| X7.5 | −2.761 | 0.007 | 0.407 | −4.589 | 0 | 0.001 | −4.275 | 0 | 0.003 | −1.605 | 0.113 | 1 | −1.939 | 0.057 | 1 | −2.261 | 0.027 | 1 | |
| X10 | −1.513 | 0.135 | 1 | −3.602 | 6E−04 | 0.032 | −3.369 | 0.001 | 0.068 | −2.81 | 0.007 | 0.355 | −1.027 | 0.308 | 1 | −4.987 | 0 | 0 | |
| 10% Suc + 10% Ery | S1 | −2.221 | 0.03 | 1 | −1.89 | 0.063 | 1 | −1.602 | 0.114 | 1 | −0.371 | 0.712 | 1 | 2.273 | 0.026 | 1 | −2.658 | 0.01 | 0.538 |
| E2.5 | 0.898 | 0.373 | 1 | 2.284 | 0.026 | 1 | 2.915 | 0.005 | 0.264 | 3.196 | 0.002 | 0.116 | 1.791 | 0.078 | 1 | 0.195 | 0.846 | 1 | |
| E5 | −1.493 | 0.14 | 1 | −0.093 | 0.926 | 1 | −0.13 | 0.897 | 1 | 2.835 | 0.006 | 0.331 | 0.693 | 0.491 | 1 | −1.236 | 0.221 | 1 | |
| E7.5 | 0.842 | 0.403 | 1 | 1.931 | 0.058 | 1 | 2.352 | 0.022 | 1 | 3.144 | 0.003 | 0.135 | 1.328 | 0.189 | 1 | 1.486 | 0.142 | 1 | |
| xy1 | −1.849 | 0.069 | 1 | −2.418 | 0.018 | 1 | −3.901 | 0 | 0.012 | −0.927 | 0.357 | 1 | −0.859 | 0.393 | 1 | −2.284 | 0.026 | 1 | |
| xy2.5 | −1.041 | 0.302 | 1 | −1.581 | 0.119 | 1 | −2.401 | 0.019 | 1 | −1.811 | 0.075 | 1 | −1.037 | 0.303 | 1 | −0.958 | 0.342 | 1 | |
| xy5 | −1.576 | 0.12 | 1 | −1.441 | 0.154 | 1 | −1.934 | 0.057 | 1 | 1.212 | 0.23 | 1 | 0.151 | 0.881 | 1 | −2.349 | 0.022 | 1 | |
| xy7.5 | −2.145 | 0.036 | 1 | −2.991 | 0.004 | 0.212 | −3.267 | 0.002 | 0.093 | −0.961 | 0.34 | 1 | −0.394 | 0.695 | 1 | −1.987 | 0.051 | 1 | |
| xy10 | −0.781 | 0.438 | 1 | −1.548 | 0.126 | 1 | −1.466 | 0.147 | 1 | 1.029 | 0.307 | 1 | 0.773 | 0.442 | 1 | −3.098 | 0.003 | 0.156 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, S.-Y.; Park, J.-E. Comparison of Cariogenic Organic Acid Concentrations According to Combined Use of Sucrose and Sugar Alcohols. J. Clin. Med. 2025, 14, 7535. https://doi.org/10.3390/jcm14217535
Hwang S-Y, Park J-E. Comparison of Cariogenic Organic Acid Concentrations According to Combined Use of Sucrose and Sugar Alcohols. Journal of Clinical Medicine. 2025; 14(21):7535. https://doi.org/10.3390/jcm14217535
Chicago/Turabian StyleHwang, Su-Yeon, and Jung-Eun Park. 2025. "Comparison of Cariogenic Organic Acid Concentrations According to Combined Use of Sucrose and Sugar Alcohols" Journal of Clinical Medicine 14, no. 21: 7535. https://doi.org/10.3390/jcm14217535
APA StyleHwang, S.-Y., & Park, J.-E. (2025). Comparison of Cariogenic Organic Acid Concentrations According to Combined Use of Sucrose and Sugar Alcohols. Journal of Clinical Medicine, 14(21), 7535. https://doi.org/10.3390/jcm14217535







