Psychometric Evaluation of the Serbian Version of the Southampton Dupuytren’s Scoring Scheme in Patients with Dupuytren’s Contracture
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khaliq, F.; Orji, C. Dupuytren’s Contracture: A Review of the Literature. Cureus 2024, 16, e74945. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hindocha, S. Risk Factors, Disease Associations, and Dupuytren Diathesis. Hand Clin. 2018, 34, 307–314. [Google Scholar] [CrossRef]
- Ross, D.C. Epidemiology of Dupuytren’s Disease. Hand Clin. 1999, 15, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Finsen, V.; Dalen, H.; Nesheim, J. The Prevalence of Dupuytren’s Disease among Two Different Ethnic Groups in Northern Norway. J. Hand Surg. Am. 2002, 27, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Anthony, S.G.; Lozano-Calderon, S.A.; Simmons, B.P.; Jupiter, J.B. Gender Ratio of Dupuytren’s Disease in the Modern U.S. Population. Hand 2008, 3, 87–90. [Google Scholar] [CrossRef]
- Geoghegan, J.M.; Forbes, J.; Clark, D.I.; Smith, C.; Hubbard, R. Dupuytren’s Disease Risk Factors. J. Hand Surg. Br. 2004, 29, 423–426. [Google Scholar] [CrossRef]
- van Straalen, R.J.M.; de Boer, M.R.; Vos, F.; Werker, P.M.N.; Broekstra, D.C. The incidence and prevalence of Dupuytren’s disease in primary care: Results from a text mining approach on registration data. Scand. J. Prim. Health Care 2025, 43, 173–180. [Google Scholar] [CrossRef]
- Karkampouna, S.; Kreulen, M.; Obdeijn, M.C.; Kloen, P.; Dorjée, A.L.; Rivellese, F.; Chojnowski, A.; Clark, I.; Kruithof-de Julio, M. Connective Tissue Degeneration: Mechanisms of Palmar Fascia Degeneration (Dupuytren’s Disease). Curr. Mol. Biol. Rep. 2016, 2, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Plusch, K.; Carfagno, J.; Givner, D.; Fletcher, D.; Aita, D.; Gallant, G.G.; Abboudi, J.; Beredjiklian, P. An Evaluation of the Source and Content of Dupuytren’s Disease Information Available on the Internet. Cureus 2021, 13, e19356. [Google Scholar] [CrossRef]
- Simón-Pérez, C.; Rodríguez-Mateos, J.I.; Maestro, I.A.; Alvarez-Quiñones, M.; Simon-Perez, E.; Martín-Ferrero, M.A. Long-Term Recurrence of Dupuytren’s Disease Treated with Clostridium Histolyticum Collagenase. Surgical Treatment and Anatomopathological Study. Arch. Orthop. Trauma Surg. 2024, 144, 2085–2091. [Google Scholar]
- Nanchahal, J.; Chan, J.K. Treatments for Early-Stage Dupuytren’s Disease: An Evidence-Based Approach. J. Hand Surg. Eur. Vol. 2023, 48, 191–198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mafi, R.; Hindocha, S.; Khan, W. Recent Surgical and Medical Advances in the Treatment of Dupuytren’s Disease—A Systematic Review of the Literature. Open Orthop. J. 2012, 6, 77–82. [Google Scholar] [PubMed] [PubMed Central]
- Nanchahal, J.; Ball, C.; Rombach, I.; Williams, L.; Kenealy, N.; Dakin, H.; O’Connor, H.; Davidson, D.; Werker, P.; Dutton, S.J.; et al. Anti-Tumour Necrosis Factor Therapy for Early-Stage Dupuytren’s Disease (RIDD): A Phase 2b, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Rheumatol. 2022, 4, e407–e416. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Izadi, D.; Layton, T.B.; Williams, L.; McCann, F.; Cabrita, M.; Espirito Santo, A.I.; Xie, W.; Fritzsche, M.; Colin-York, H.; Feldmann, M.; et al. Identification of TNFR2 and IL-33 as Therapeutic Targets in Localized Fibrosis. Sci. Adv. 2019, 5, eaay0370. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beaudreuil, J.; Allard, A.; Zerkak, D.; Gerber, R.A.; Cappelleri, J.C.; Quintero, N.; Lasbleiz, S.; Bernabé, B.; Orcel, P.; Bardin, P.; et al. Unité Rhumatologique des Affections de la Main (URAM) Scale: Development and Validation of a Tool to Assess Dupuytren’s Disease–Specific Disability. Arthritis Care Res. 2011, 63, 1448–1455. [Google Scholar] [CrossRef]
- Mohan, A.; Vadher, J.; Ismail, H.; Warwick, D. The Southampton Dupuytren’s Scoring Scheme. J. Plast. Surg. Hand Surg. 2014, 48, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Bradet-Levesque, I.; Audet, J.; Roy, J.S.; Flamand, V.H. Measuring Functional Outcome in Dupuytren’s Disease: A Systematic Review of Patient-Reported Outcome Measures. J. Hand Ther. 2022, 35, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.; Zhang, W.; Scammell, B. Validity of the Disabilities of the Arm, Shoulder and Hand patient-reported outcome measure (DASH) and the Quickdash when used in Dupuytren’s disease. J. Hand Surg. Eur. Vol. 2016, 41, 589–599. [Google Scholar] [CrossRef]
- Gómez-Herrero, D.; Carrera-Hueso, F.J.; Sanjuan-Cerveró, R.; Montaner-Alonso, D.; Aguilella-Fernandez, L.; Vazquez-Ferreiro, P.; Poquet-Jornet, J.E.; García-Jiménez, E. Validation of a spanish version of the ‘Unité Rhumatologique Des Affections De La Main’ (URAM) scale. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.; Clelland, A.D.; Davis, T.R.C.; Scammell, B.E.; Zhang, W.; Russell, P.; Fullilove, S.; Chakrabarti, I.; Davidson, D.; Rodrigues, J. A Comparative Analysis of Multidimensional Computerized Adaptive Testing for the DASH and QuickDASH Scores in Dupuytren’s Disease. J. Hand Surg. Eur. Vol. 2022, 47, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Bendixen, L.L.; Jørgensen, R.W.; Jensen, C.H. A Patient-Reported Outcome Measure for Patients with Dupuytren’s Disease. Dan. Med. J. 2020, 67, A03190190. [Google Scholar] [PubMed]
- Bindra, R.R.; Dias, J.J.; Heras-Palau, C.; Amadio, P.C.; Chung, K.C.; Burke, F.D. Assessing Outcome after Hand Surgery: The Current State. J. Hand Surg. Br. 2003, 28, 289–294. [Google Scholar] [CrossRef]
- Hudak, P.L.; Amadio, P.C.; Bombardier, C. Development of an Upper Extremity Outcome Measure: The DASH (Disabilities of the Arm, Shoulder and Hand). Am. J. Ind. Med. 1996, 29, 602–608. [Google Scholar] [CrossRef]
- Ware, J., Jr.; Kosinski, M.; Keller, S.D. A 12-Item Short-Form Health Survey: Construction of Scales and Preliminary Tests of Reliability and Validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef]
- Huskisson, E.C. Measurement of Pain. Lancet 1974, 2, 1127–1131. [Google Scholar] [CrossRef]
- Wild, D.; Grove, A.; Martin, M.; Eremenco, S.; McElroy, S.; Verjee-Lorenz, A.; Erikson, P. Principles of Good Practice for the Translation and Cultural Adaptation Process for Patient-Reported Outcomes (PRO) Measures: Report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health 2005, 8, 94–104. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Cronbach’s Alpha. BMJ 1997, 314, 572. [Google Scholar] [CrossRef]
- Evans, J.D. Straightforward Statistics for the Behavioral Sciences; Brooks/Cole Publishing: Pacific Grove, CA, USA, 1996. [Google Scholar]
- Wilburn, J.; McKenna, S.P.; Perry-Hinsley, D.; Bayat, A. The Impact of Dupuytren Disease on Patient Activity and Quality of Life. J. Hand Surg. Am. 2013, 38, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Harryson, M.; Eklund, M.; Arner, M.; Wilbrand, S. Patient-Reported Outcome in Dupuytren’s Disease Treated with Fasciectomy, Collagenase or Needle Fasciotomy: A Swedish Registry Study. J. Hand Surg. Glob. Online 2023, 5, 733–739. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jørgsholm, J.; Wejnold Jørgensen, R. The Minimal Clinically Important Difference of the Southampton Dupuytren’s Scoring Scheme. J. Plast. Surg. Hand Surg. 2023, 57, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.; Tan, E.S.L.; Thomas, M.; Taylor, F.; Elliott, D.; Bindra, R. Collagenase Injections for Dupuytren’s Contracture: Prospective Cohort Study in a Public Health Setting. ANZ J. Surg. 2019, 89, 573–577. [Google Scholar] [CrossRef] [PubMed]

| Variables | n = 68 |
|---|---|
| Age, mean ± sd | 64.6 ± 9.5 |
| Gender-male, n (%) | |
| Male | 55 (80.9) |
| Female | 13 (19.1) |
| SDSS total score, mean (95% CI) | 7.1 (5.7–8.5) |
| DASH, mean (95% CI) | 26.5 (20.2–32.9) |
| SF-12, mean (95% CI) | |
| PCS | 47.0 (44.4–49.7) |
| MCS | 42.1 (40.3–43.9) |
| VAS, median (25th–75th percentile) | 2 (0.9–4.3) |
| No Problem | Minor Inconvenience | Modest Inconvenience | Definitely Troublesome | Severe Problem | |
|---|---|---|---|---|---|
| How much trouble do you have with: | |||||
| Discomfort | 17 (25.0) | 25 (36.8) | 11 (16.2) | 10 (14.7) | 5 (7.4) |
| Personal activities, e.g., washing face, dressing, washing hands, washing hair, putting on gloves | 18 (26.5) | 15 (22.1) | 16 (23.5) | 9 (13.2) | 10 (14.7) |
| Domestic activities, e.g., holding a glass/cup, opening jars, eating, cooking. | 23 (33.8) | 18 (26.5) | 15 (22.1) | 6 (8.8) | 6 (8.8) |
| Work/Social interaction, e.g., using the computer, writing, shaking hands, cosmetic appearance. | 26 (38.2) | 18 (26.5) | 14 (20.6) | 2 (2.9) | 8 (11.8) |
| Hobbies, e.g., driving/cycling, racket sports, DIY, playing musical instruments, gardening. | 20 (29.4) | 26 (38.2) | 4 (5.9) | 8 (11.8) | 10 (14.7) |
| Personal activities, e.g.,: washing face, dressing, washing hands, washing hair, putting on gloves | 18 (26.5) | 15 (22.1) | 16 (23.5) | 9 (13.2) | 10 (14.7) |
| Domains | Nº Items | Cronbach’s Alpha | Internal Consistency | |
|---|---|---|---|---|
| SDSS | Single factor | 5 | 0.914 | Excellent |
| Chi-Squared Goodness of Fit | df | p | RMSEA (90% CI) | IFI | CFI | TLI | |
|---|---|---|---|---|---|---|---|
| SDSS | 10.094 | 5 | 0.073 | 0.123 (0.000–0.234) | 0.979 | 0.978 | 0.956 |
| DASH | SF-12 | VAS | |||
|---|---|---|---|---|---|
| PCS | MCS | ||||
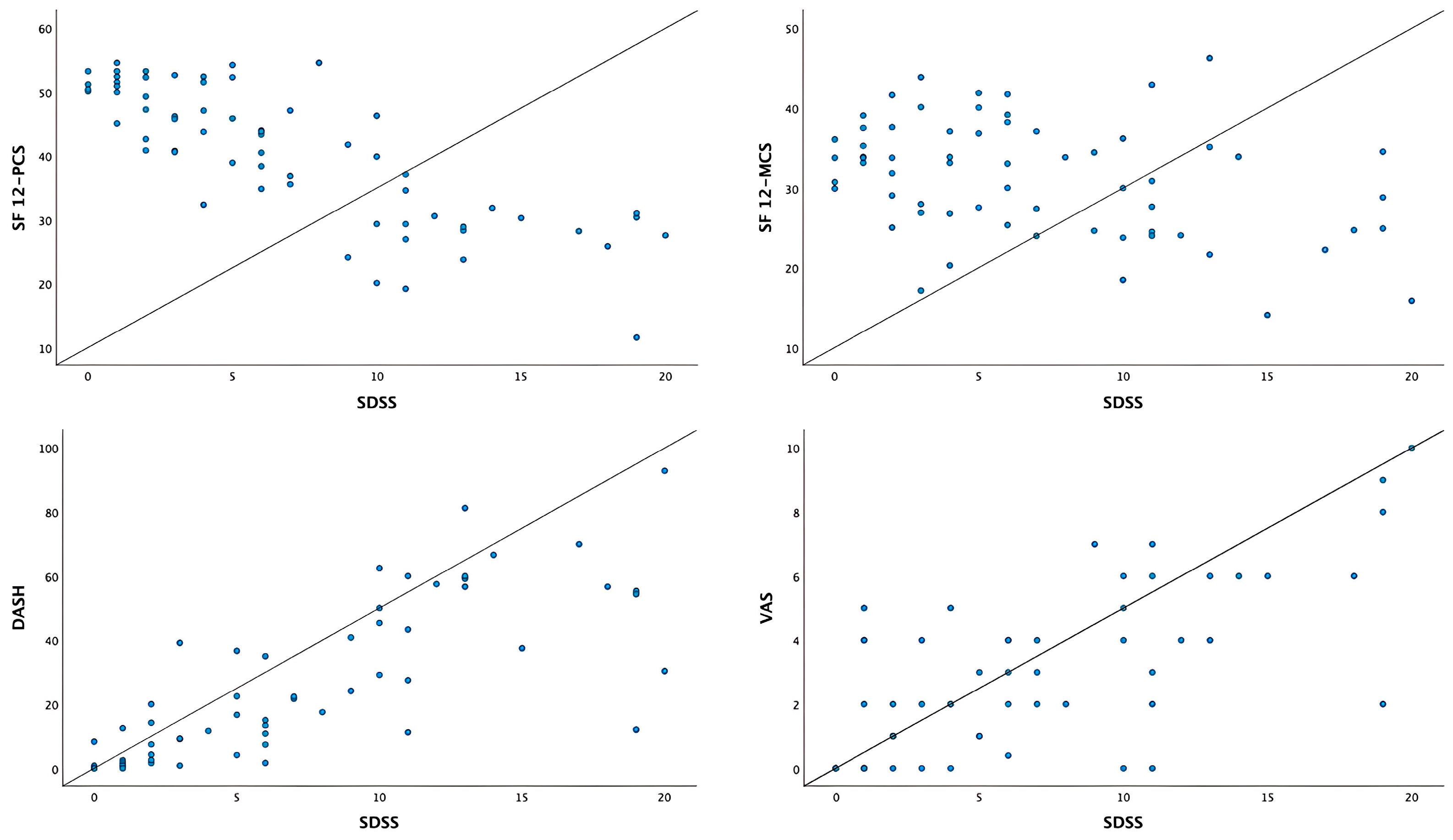
| SDSS | 0.779 * | −0.802 * | −0.363 * | 0.702 * | |
| DASH | −0.768 * | −0.349 * | 0.819 * | ||
| SF-12 | PCS | 0.223 | −0.730 * | ||
| MCS | −0.231 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vucetic, M.; Pavlovic, V.; Markovic, K.; Milutinovic, S.; Stanimirovic, N.; Joksimovic, L.; Matejic, A.; Petrovic, B.; Jovanovic, N.; Bogosavljevic, N.; et al. Psychometric Evaluation of the Serbian Version of the Southampton Dupuytren’s Scoring Scheme in Patients with Dupuytren’s Contracture. J. Clin. Med. 2025, 14, 7528. https://doi.org/10.3390/jcm14217528
Vucetic M, Pavlovic V, Markovic K, Milutinovic S, Stanimirovic N, Joksimovic L, Matejic A, Petrovic B, Jovanovic N, Bogosavljevic N, et al. Psychometric Evaluation of the Serbian Version of the Southampton Dupuytren’s Scoring Scheme in Patients with Dupuytren’s Contracture. Journal of Clinical Medicine. 2025; 14(21):7528. https://doi.org/10.3390/jcm14217528
Chicago/Turabian StyleVucetic, Milos, Vedrana Pavlovic, Ksenija Markovic, Suzana Milutinovic, Nikolina Stanimirovic, Luka Joksimovic, Aleksandar Matejic, Bojan Petrovic, Nemanja Jovanovic, Nikola Bogosavljevic, and et al. 2025. "Psychometric Evaluation of the Serbian Version of the Southampton Dupuytren’s Scoring Scheme in Patients with Dupuytren’s Contracture" Journal of Clinical Medicine 14, no. 21: 7528. https://doi.org/10.3390/jcm14217528
APA StyleVucetic, M., Pavlovic, V., Markovic, K., Milutinovic, S., Stanimirovic, N., Joksimovic, L., Matejic, A., Petrovic, B., Jovanovic, N., Bogosavljevic, N., Aleksandric, D., Vasovic, D., Pilipovic, F., Radulovic, D., Stojcic, M., & Milic, N. (2025). Psychometric Evaluation of the Serbian Version of the Southampton Dupuytren’s Scoring Scheme in Patients with Dupuytren’s Contracture. Journal of Clinical Medicine, 14(21), 7528. https://doi.org/10.3390/jcm14217528







