Beyond Fixed Thresholds: Cluster-Derived MRI Boundaries Improve Assessment of Crohn’s Disease Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. MR Acquisition
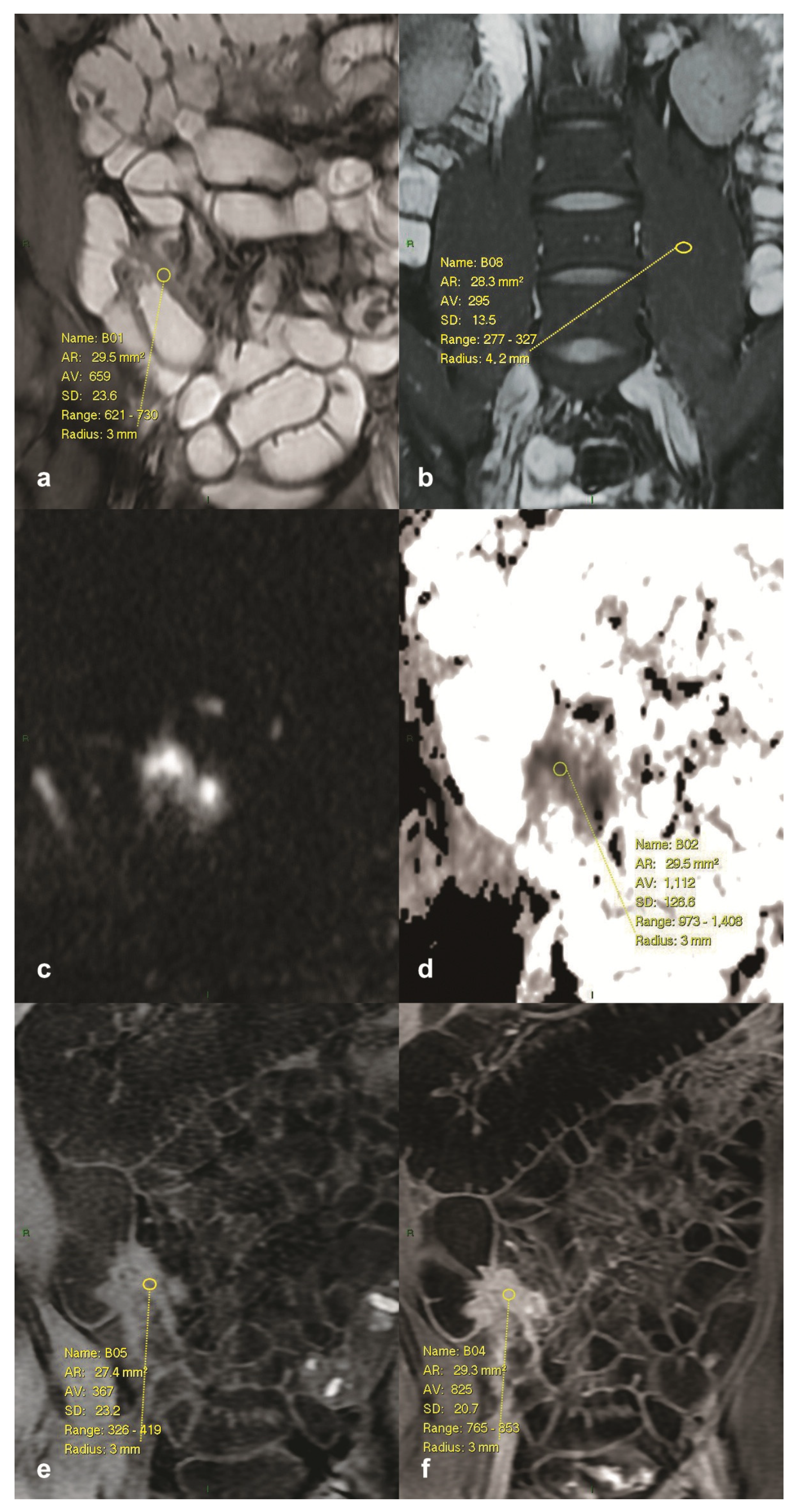
2.3. Image Analysis and Measurements
2.4. Statistical Analysis
3. Results
3.1. Literature-Based MaRIA Classification
3.2. Literature-Based DWI MaRIA (Clermont) Classification
3.3. Cluster-Derived MaRIA Classification
3.4. Cluster-Derived DWI MaRIA Classification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADC | Apparent Diffusion Coefficient |
| AI | Artificial Intelligence |
| ANOVA | Analysis of Variance |
| ASSET | Array Spatial Sensitivity Encoding Technique |
| CDAI | Crohn’s Disease Activity Index |
| CD | Crohn’s Disease |
| CI | Confidence Interval |
| CV | Coefficient of Variation |
| DA | Discriminant Analysis |
| DWI | Diffusion-Weighted Imaging |
| ECCO | European Crohn’s and Colitis Organisation |
| ESGAR | European Society of Gastrointestinal and Abdominal Radiology |
| ESP | European Society of Pathology |
| FIESTA | Fast Imaging Employing Steady-State Acquisition |
| FOV | Field of View |
| GBCA | Gadolinium-Based Contrast Agent |
| IBD | Inflammatory Bowel Disease |
| IBUS | International Bowel Ultrasound Group |
| LAVA | Liver Acquisition with Volume Acceleration |
| MaRIA | Magnetic Resonance Index of Activity |
| MANOVA | Multivariate Analysis of Variance |
| MRE | Magnetic Resonance Enterography |
| NEX | Number of Excitations |
| PICMI | Pediatric Inflammatory Crohn’s MRE Index |
| P-sMARIA | Pediatric simplified Magnetic Resonance Index of Activity |
| RCE | Relative Contrast Enhancement |
| ROI | Region of Interest |
| SD | Standard Deviation |
| SI | Signal Intensity |
| sMARIA | Simplified Magnetic Resonance Index of Activity |
| SPSS | Statistical Package for the Social Sciences |
| TE | Echo Time |
| TR | Repetition Time |
| WSI | Wall Signal Intensity |
References
- Panés, J.; Bouzas, R.; Chaparro, M.; García-Sánchez, V.; Gisbert, J.P.; Martínez de Guereñu, B.; Mendoza, J.L.; Paredes, J.M.; Quiroga, S.; Ripollés, T.; et al. Systematic review: The use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn’s disease. Aliment. Pharmacol. Ther. 2011, 34, 125–145. [Google Scholar] [CrossRef]
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Hudson, A.S.; Wahbeh, G.T.; Zheng, H.B. Imaging and endoscopic tools in pediatric inflammatory bowel disease: What’s new? World J. Clin. Pediatr. 2024, 13, 89091. [Google Scholar] [CrossRef]
- Durak, M.B. Increased Use of Magnetic Resonance Enterography in Crohn’s Disease. Dis. Colon. Rectum 2025, 68, e108. [Google Scholar] [CrossRef]
- Foti, P.V.; Travali, M.; Farina, R.; Palmucci, S.; Coronella, M.; Spatola, C.; Puzzo, L.; Garro, R.; Inserra, G.; Riguccio, G.; et al. Can Conventional and Diffusion-Weighted MR Enterography Biomarkers Differentiate Inflammatory from Fibrotic Strictures in Crohn’s Disease? Medicina 2021, 57, 265. [Google Scholar] [CrossRef] [PubMed]
- Thormann, M.; Melekh, B.; Bär, C.; Pech, M.; Omari, J.; Wienke, A.; Meyer, H.-J.; Surov, A. Apparent Diffusion Coefficient for Assessing Crohn’s Disease Activity: A Meta-Analysis. Eur. Radiol. 2023, 33, 1677–1686. [Google Scholar] [CrossRef]
- Jannatdoust, P.; Valizadeh, P.; Razaghi, M.; Rouzbahani, M.; Abbasi, A.; Arian, A. Role of Abbreviated Non-Contrast-Enhanced MR-Enterography in the Evaluation of Crohn’s Disease Activity and Complications as an Alternative for Full-Protocol Contrast-Enhanced Study: A Systematic Review and Meta-Analysis. Res. Diagn. Interv. Imaging 2023, 6, 100030. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H. DWI at MR Enterography for Evaluating Bowel Inflammation in Crohn Disease. AJR Am. J. Roentgenol. 2016, 207, 40–48. [Google Scholar] [CrossRef]
- Vymazal, J.; Rulseh, A.M. MRI Contrast Agents and Retention in the Brain: Review of Contemporary Knowledge and Recommendations to the Future. Insights Imaging 2024, 15, 179. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, J.C.; Rodby, R.A.; Yee, J.; Wang, C.L.; Fine, D.; McDonald, R.J.; Perazella, M.A.; Dillman, J.R.; Davenport, M.S. Use of Intravenous Gadolinium-based Contrast Media in Patients with Kidney Disease: Consensus Statements from the American College of Radiology and the National Kidney Foundation. Radiology 2021, 298, 28–35. [Google Scholar] [CrossRef]
- Rimola, J.; Rodríguez, S.; García-Bosch, O.; Ordás, I.; Ayala, E.; Aceituno, M.; Pellisé, M.; Ayuso, C.; Ricart, E.; Donoso, L.; et al. Magnetic Resonance for Assessment of Disease Activity and Severity in Ileocolonic Crohn’s Disease. Gut 2009, 58, 1113–1120. [Google Scholar] [CrossRef]
- Buisson, A.; Joubert, A.; Montoriol, P.F.; Da Ines, D.; Hordonneau, C.; Pereira, B.; Garcier, J.M.; Bommelaer, G.; Petitcolin, V. Diffusion-Weighted Magnetic Resonance Imaging for Detecting and Assessing Ileal Inflammation in Crohn’s Disease. Aliment. Pharmacol. Ther. 2013, 37, 537–545. [Google Scholar] [CrossRef]
- Kumar, S.; De Kock, I.; Blad, W.; Hare, R.; Pollok, R.; Taylor, S.A. Magnetic Resonance Enterography and Intestinal Ultrasound for the Assessment and Monitoring of Crohn’s Disease. J. Crohn’s Colitis 2024, 18, 1450–1463. [Google Scholar] [CrossRef]
- Bonifacio, C.; Dal Buono, A.; Levi, R.; Gabbiadini, R.; Reca, C.; Bezzio, C.; Francone, M.; Armuzzi, A.; Balzarini, L. Reporting of Magnetic Resonance Enterography in Inflammatory Bowel Disease: Results of an Italian Survey. J. Clin. Med. 2024, 13, 3953. [Google Scholar] [CrossRef] [PubMed]
- Everitt, B.S.; Landau, S.; Leese, M.; Stahl, D. Cluster Analysis, 5th ed.; Wiley: Chichester, UK, 2011. [Google Scholar] [CrossRef]
- Qiu, J.; Hu, Y.; Li, L.; Erzurumluoglu, A.M.; Braenne, I.; Whitehurst, C.; Schmitz, J.; Arora, J.; Bartholdy, B.A.; Gandhi, S.; et al. Deep Representation Learning for Clustering Longitudinal Survival Data from Electronic Health Records. Nat. Commun. 2025, 16, 2534. [Google Scholar] [CrossRef] [PubMed]
- Riggott, C.; Fairbrass, K.M.; Black, C.J.; Gracie, D.J.; Ford, A.C. Novel symptom clusters predict disease impact and healthcare utilisation in inflammatory bowel disease: Prospective longitudinal follow-up study. Aliment. Pharmacol. Ther. 2023, 58, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Hordonneau, C.; Buisson, A.; Scanzi, J.; Goutorbe, F.; Pereira, B.; Borderon, C.; Da Ines, D.; Montoriol, P.F.; Garcier, J.M.; Boyer, L.; et al. Diffusion-Weighted Magnetic Resonance Imaging in Ileocolonic Crohn’s Disease: Validation of Quantitative Index of Activity. Am. J. Gastroenterol. 2014, 109, 89–98. [Google Scholar] [CrossRef]
- Li, Y.; Langhorst, J.; Koch, A.K.; Demircioglu, A.; Nensa, F.; Kirchner, J.; Beiderwellen, K.; Catalano, O.; Forsting, M.; Herrmann, K.; et al. Assessment of Ileocolonic Inflammation in Crohn’s Disease: Which Surrogate Marker Is Better—MaRIA, Clermont, or PET/MR Index? Initial Results of a Feasibility Trial. J. Nucl. Med. 2019, 60, 851–857. [Google Scholar] [CrossRef]
- Dalmaijer, E.S.; Nord, C.L.; Astle, D.E. Statistical power for cluster analysis. BMC Bioinform. 2022, 23, 205. [Google Scholar] [CrossRef]
- Chen, L.; Santos, J.B.W.; Gaza, J.; Perez, A.; Miranda-Quintana, R.A. Hierarchical Extended Linkage Method (HELM)’s Deep Dive into Hybrid Clustering Strategies. J. Chem. Inf. Model. 2025, 65, 6209–6220. [Google Scholar] [CrossRef]
- Kucharzik, T.; Taylor, S.; Allocca, M.; Burisch, J.; Ellul, P.; Iacucci, M.; Maaser, C.; Baldin, P.; Bhatnagar, G.; Ben-Horin, S.; et al. ECCO-ESGAR-ESP-IBUS Guideline on Diagnostics and Monitoring of Patients with Inflammatory Bowel Disease: Part 1. J. Crohn’s Colitis 2025, 19, jjaf106. [Google Scholar] [CrossRef]
- Dane, B.; Dillman, J.R.; Fidler, J.; Anupindi, S.A.; Fulmer, C.G.; Gordon, I.O.; Bruining, D.H.; Deepak, P.; Abualruz, A.-R.; Al-Hawary, M.; et al. SAR Consensus Recommendations for Defining Small Bowel Crohn Disease Strictures at CT and MR Enterography. Radiology 2025, 316, e243123. [Google Scholar] [CrossRef]
- Yanai, H.; Feakins, R.; Allocca, M.; Burisch, J.; Ellul, P.; Iacucci, M.; Maaser, C.; Zilli, A.; Zidar, N.; Wilkens, R.; et al. ECCO-ESGAR-ESP-IBUS Guideline on Diagnostics and Monitoring of Patients with Inflammatory Bowel Disease: Part 2. J. Crohn’s Colitis 2025, 19, jjaf107. [Google Scholar] [CrossRef]
- Amadu, M.; Soldera, J. Duodenal Crohn’s Disease: Case Report and Systematic Review. World J. Methodol. 2024, 14, 88619. [Google Scholar] [CrossRef]
- Seo, N. Comprehensive Review of Magnetic Resonance Enterography-Based Activity Scoring Systems for Crohn’s Disease. iMRI 2025, 29, 1–13. [Google Scholar] [CrossRef]
- Vitello, A.; Maida, M.; Shahini, E.; Macaluso, F.S.; Orlando, A.; Grova, M.; Ramai, D.; Serviddio, G.; Facciorusso, A. Current Approaches for Monitoring of Patients with Inflammatory Bowel Diseases: A Narrative Review. J. Clin. Med. 2024, 13, 1008. [Google Scholar] [CrossRef] [PubMed]
- Lo, R.W.; Bhatnagar, G.; Kutaiba, N.; Srinivasan, A.R. Evaluating luminal and post-operative Crohn’s disease activity on magnetic resonance enterography: A review of radiological disease activity scores. World J. Gastroenterol. 2025, 31, 107419. [Google Scholar] [CrossRef] [PubMed]
- Bohra, A.; Vasudevan, A.; Kutaiba, N.; Van Langenberg, D.R. Challenges and Strategies to Optimising the Quality of Small-Bowel Magnetic Resonance Enterography in Crohn’s Disease. Diagnostics 2022, 12, 2533. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.; Taylor, S.A. Small bowel imaging in inflammatory bowel disease: Updates for 2023. Expert Rev. Gastroenterol. Hepatol. 2023, 17, 1117–1134. [Google Scholar] [CrossRef]
- Maino, C.; Mariani, I.; Drago, S.G.; Franco, P.N.; Giandola, T.P.; Donati, F.; Boraschi, P.; Ippolito, D. Computed Tomography and Magnetic Resonance Enterography: From Protocols to Diagnosis. Diagnostics 2024, 14, 2584. [Google Scholar] [CrossRef]
- Bae, H.; Seo, N.; Kang, E.A.; Cheon, J.H.; Lim, J.S.; Kim, M.-J. Validation of the simplified magnetic resonance index of activity by using DWI without gadolinium enhancement to evaluate bowel inflammation in Crohn’s disease. Eur. Radiol. 2023, 33, 3266–3275. [Google Scholar] [CrossRef]
- Hassan, N.S.M.S.; Moghazy, K.M.M.; Salem, O.E.; Afifi, A.H.; Emara, D.M.; Mohamed, M.M.R. Simplified Magnetic Resonance Index of Activity Score versus Simple Endoscopic Score in Crohn’s Disease: A Prospective Study. Egypt. J. Radiol. Nucl. Med. 2024, 55, 208. [Google Scholar] [CrossRef]
- Rieder, F.; Baker, M.E.; Bruining, D.H.; Fidler, J.L.; Ehman, E.C.; Sheedy, S.P.; Heiken, J.P.; Ream, J.M.; Holmes, D.R., III; Inoue, A.; et al. Reliability of MR Enterography Features for Describing Fibrostenosing Crohn Disease. Radiology 2024, 312, e233039. [Google Scholar] [CrossRef]
- Ripollés, T.; Poza, J.; Martínez-Pérez, M.J.; Suarez Ferrer, C.; Blanc, E.; Paredes, J.M. Evaluation of Crohn’s Disease Activity: Validation of a Segmental Simple Ultrasound Score in a Multicenter Study. Inflamm. Bowel Dis. 2025, 31, 2081–2087. [Google Scholar] [CrossRef]
- Bhatnagar, G.; Mallett, S.; Quinn, L.; Beable, R.; Bungay, H.; Betts, M.; Greenhalgh, R.; Gupta, A.; Higginson, A.; Hyland, R.; et al. Interobserver Variation in the Interpretation of Magnetic Resonance Enterography in Crohn’s Disease. Br. J. Radiol. 2022, 95, 20210995. [Google Scholar] [CrossRef]
- Focht, G.; Cytter-Kuint, R.; Greer, M.-L.C.; Pratt, L.-T.; Castro, D.A.; Church, P.C.; Walters, T.D.; Hyams, J.; Navon, D.; Martin de Carpi, J.; et al. Development, Validation, and Evaluation of the Pediatric Inflammatory Crohn’s Magnetic Resonance Enterography Index From the ImageKids Study. Gastroenterology 2022, 163, 1306–1320. [Google Scholar] [CrossRef] [PubMed]
- Dillman, J.R.; Abu Ata, N.; Towbin, A.J.; Anton, C.G.; Smith, E.A.; Zhang, B.; Imbus, R.; Tkach, J.A.; Denson, L.A. The Simplified MR Index of Activity Score in Pediatric Small-Bowel Crohn Disease: An Interreader Agreement and Responsiveness Study. AJR Am. J. Roentgenol. 2023, 220, 126–133. [Google Scholar] [CrossRef]
- Lovett, G.C.; Schulberg, J.D.; Hamilton, A.L.; Wilding, H.E.; Kamm, M.A.; Wright, E.K. Intestinal Ultrasound and MRI for Monitoring Therapeutic Response in Luminal Crohn’s Disease: A Systematic Review. J. Am. Coll. Radiol. 2024, 21, 441–463. [Google Scholar] [CrossRef]
- Srinivasan, A.R. Treat to Target in Crohn’s Disease: A Practical Guide for Clinicians. World J. Gastroenterol. 2024, 30, 50–69. [Google Scholar] [CrossRef]
- Caron, B.; Jairath, V.; Laurent, V.; Stoker, J.; Laghi, A.; D’Haens, G.R.; Danese, S.; Peyrin-Biroulet, L. Defining Magnetic Resonance Imaging Treatment Response and Remission in Crohn’s Disease: A Systematic Review. J. Crohn’s Colitis 2024, 18, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Revés, J.; Fernández-Clotet, A.; Ordás, I.; Buisson, A.; Bazoge, M.; Hordonneau, C.; Ellul, P.; D’Anastasi, M.; Elorza, A.; Aduna, M.; et al. Early Biological Therapy Within 12 Months of Diagnosis Leads to Higher Transmural Healing Rates in Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2025, 23, 1194–1203.e2. [Google Scholar] [CrossRef] [PubMed]
- Chirra, P.V.; Sleiman, J.; Gandhi, N.S.; Gordon, I.O.; Hariri, M.; Baker, M.E.; Ottichilo, R.; Bruining, D.H.; Kurowski, J.A.; Viswanath, S.E.; et al. Radiomics to Detect Inflammation and Fibrosis on Magnetic Resonance Enterography in Stricturing Crohn’s Disease. J. Crohn’s Colitis 2024, 18, 1660–1671. [Google Scholar] [CrossRef]
- Brem, O.; Elisha, D.; Konen, E.; Amitai, M.; Klang, E. Deep Learning in Magnetic Resonance Enterography for Crohn’s Disease Assessment: A Systematic Review. Abdom. Radiol. 2024, 49, 3183–3189. [Google Scholar] [CrossRef]
- Zhang, M.; Zeng, Y.; Fang, Z.; Wang, Y.; Zhang, R.; Ye, Z.; Cao, Q.; Mao, R.; Sun, C.; Chen, Z.; et al. MRI Radiomics Enhances Radiologists’ Ability for Characterizing Intestinal Fibrosis in Patients with Crohn’s Disease. Insights Imaging 2024, 15, 165. [Google Scholar] [CrossRef]
- Chirra, P.; Sharma, A.; Bera, K.; Cohn, H.M.; Kurowski, J.A.; Amann, K.; Rivero, M.J.; Madabhushi, A.; Lu, C.; Paspulati, R.; et al. Integrating Radiomics with Clinicoradiological Scoring Can Predict High-Risk Patients Who Need Surgery in Crohn’s Disease: A Pilot Study. Inflamm. Bowel Dis. 2023, 29, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.X.; Li, H.; Towbin, A.J.; Ata, N.A.; Smith, E.A.; Tkach, J.A.; Denson, L.A.; He, L.; Dillman, J.R. Machine Learning Diagnosis of Small-Bowel Crohn Disease Using T2-Weighted MRI Radiomic and Clinical Data. AJR Am. J. Roentgenol. 2024, 222, e2329812. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Hao, Y.; Liu, H.; Liu, X.; Yan, W.; Mao, J.; Chen, B.T. Computed Tomography Enterography Radiomics and Machine Learning for Identification of Crohn’s Disease. BMC Med. Imaging 2024, 24, 302. [Google Scholar] [CrossRef]
- Huang, S.C.; Pareek, A.; Jensen, M.; Lungren, M.P.; Yeung, S.; Chaudhari, A.S. Self-Supervised Learning for Medical Image Classification: A Systematic Review and Implementation Guidelines. NPJ Digit. Med. 2023, 6, 74. [Google Scholar] [CrossRef]
- Rubin, D.T. Transmural Healing in Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2023, 19, 101–103. [Google Scholar] [PubMed] [PubMed Central]
- Sands, B.E.; Danese, S.; Chapman, J.C.; Gurjar, K.; Grieve, S.; Thakur, D.; Griffith, J.; Joshi, N.; Kligys, K.; Dignass, A. Mucosal and Transmural Healing and Long-Term Outcomes in Crohn’s Disease. Inflamm. Bowel Dis. 2025, 31, 857–877. [Google Scholar] [CrossRef]
- Yalon, M.; Mohammadinejad, P.; Inoue, A.; Takahashi, H.; Ehman, E.C.; Esquivel, A.; Fletcher, E.C.; Behnke, C.J.; Lee, Y.S.; Fidler, J.L.; et al. Discordance between MR enterography and endoscopic detection of Crohn’s disease ileal strictures: Evidence to inform recommendations. Abdom. Radiol. 2025, 50, 2806–2816. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Value |
|---|---|
| Female gender | 22 (44%) |
| Male gender | 28 (56%) |
| Median age of the patients | 31.82 (18–66) |
| Previous intestinal resection | 0 |
| Abdominal pain | 17 (34%) |
| Joint pain | 5 (10%) |
| Vision problems | 1 (2%) |
| Skin changes | 2 (4%) |
| Active perianal fistulas | 4 (8%) |
| Chronic perianal fistulas | 3 (6%) |
| Temperature above 37.8 °C | 5 (10%) |
| Loose stools | 12 (24%) |
| Clinical activity (CDAI > 150) | 35 (70%) |
| Initial MRE examination | 15 (30%) |
| Monitoring of disease | 35 (70%) |
| Suspicion of extraintestinal complications | 7 (14%) |
| One pathological segment | 23 (46%) |
| Two pathological segments | 13 (26%) |
| Three or more pathological segments | 14 (28%) |
| Comb sign | 40 (80%) |
| Free intraperitoneal fluid | 24 (48%) |
| Ulcerations of the bowel wall | 22 (44%) |
| Increased density of locoregional fat | 22 (44%) |
| Entero-enteral fistulas | 4 (8%) |
| Abscess | 1 (2%) |
| Parameter | Mean | SD | Min | Max | CV | CI | Skewness | Kurtosis | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| T2 SI wall/T2 SI m. psoas | 1.28 | 0.27 | 1.1 | 1.8 | 21.25 | 0.94–1.62 | 1.44 | 0.17 | 0.321 |
| Wall thickness (mm) | 3.38 | 0.37 | 3.1 | 4.0 | 10.95 | 2.92–3.84 | 1.07 | −0.33 | 0.639 |
| MaRIA | 6.27 | 0.36 | 5.8 | 6.7 | 5.75 | 5.82–6.71 | −0.14 | −1.54 | 0.999 |
| DWI MaRIA | 3.38 | 0.75 | 2.7 | 4.5 | 22.09 | 2.40–4.35 | 0.72 | −0.93 | 0.869 |
| ADC | 1.66 | 0.26 | 1.4 | 2.1 | 15.52 | 1.34–1.98 | 1.21 | 0.10 | 0.656 |
| Parameter | Mean | SD | Min | Max | CV | CI | Skewness | Kurtosis | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| T2 SI wall/T2 SI m. psoas | 1.27 | 0.37 | 0.8 | 2.2 | 29.06 | 1.08–1.47 | 0.89 | 0.50 | 0.850 |
| Wall thickness (mm) | 3.93 | 0.71 | 3.1 | 5.4 | 18.08 | 3.55–4.31 | 0.91 | −0.27 | 0.698 |
| MaRIA | 8.58 | 1.13 | 7.1 | 10.9 | 13.18 | 7.98–9.18 | 0.30 | −0.74 | 0.941 |
| DWI MaRIA | 4.65 | 1.21 | 3.2 | 6.9 | 25.97 | 4.01–5.30 | 0.88 | −0.35 | 0.508 |
| ADC | 1.39 | 0.16 | 1.0 | 1.7 | 11.55 | 1.31–1.48 | −0.21 | 0.53 | 0.795 |
| Parameter | Mean | SD | Min | Max | CV | CI | Skewness | Kurtosis | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| T2 SI wall/T2 SI m. psoas | 1.89 | 0.44 | 1.1 | 3.0 | 23.58 | 1.77–2.00 | 0.44 | −0.34 | 0.556 |
| Wall thickness (mm) | 5.68 | 1.20 | 3.5 | 8.8 | 21.17 | 5.38–5.99 | 0.46 | 0.09 | 0.927 |
| MaRIA | 20.11 | 6.05 | 11.4 | 33.7 | 30.08 | 18.58–21.63 | 0.44 | −1.26 | 0.019 |
| DWI MaRIA | 16.77 | 4.99 | 9.9 | 27.1 | 29.75 | 15.51–18.03 | 0.37 | −1.24 | 0.063 |
| ADC | 1.13 | 0.10 | 0.9 | 1.4 | 8.79 | 1.11–1.16 | 0.50 | −0.07 | 0.310 |
| Active | Inactive | Severe Disease | Contribution % | |
|---|---|---|---|---|
| DWI MaRIA | Lower | Moderate *1 | Higher *2 | 38.047 |
| MaRIA | Lower | Moderate *1 | Higher *2 | 28.282 |
| ADC | Higher *2 | Moderate *1 | Lower | 22.023 |
| Wall thickness | Lower | Moderate | Higher *2 | 8.954 |
| T2 SI wall/T2 SI m. psoas | Moderate | Lower | Higher *2 | 2.695 |
| n/m | 5/5 | 16/16 | 61/63 | |
| % | 100.00 | 100.00 | 96.83 |
| Inactive | Active | Severe Disease | |
|---|---|---|---|
| Inactive | 0.00 | 2.60 | 4.95 |
| Active | 2.60 | 0.00 | 4.12 |
| Severe disease | 4.95 | 4.12 | 0.00 |
| Parameter | Mean | SD | Min | Max | CV | CI | Skewness | Kurtosis | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| T2 SI wall/T2 SI m. psoas | 1.27 | 0.34 | 0.8 | 2.2 | 26.89 | 1.12–1.43 | 0.96 | 0.72 | 0.275 |
| Wall thickness (mm) | 3.80 | 0.68 | 3.1 | 5.4 | 17.93 | 3.49–4.11 | 1.12 | 0.32 | 0.732 |
| MaRIA | 8.03 | 1.42 | 5.8 | 10.9 | 17.64 | 7.39–8.67 | 0.19 | −0.90 | 0.799 |
| DWI MaRIA | 4.35 | 1.23 | 2.7 | 6.9 | 28.32 | 3.79–4.91 | 0.91 | 0.06 | 0.691 |
| ADC | 1.46 | 0.22 | 1.0 | 2.1 | 14.73 | 1.36–1.55 | 0.96 | 2.43 | 0.608 |
| Parameter | Mean | SD | Min | Max | CV | CI | Skewness | Kurtosis | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| T2 SI wall/T2 SI m. psoas | 1.55 | 0.44 | 1.1 | 2.6 | 28.71 | 1.29–1.81 | 0.94 | 0.02 | 0.544 |
| Wall thickness (mm) | 4.24 | 0.44 | 3.5 | 4.9 | 10.28 | 3.99–4.49 | −0.09 | −0.99 | 0.996 |
| MaRIA | 13.76 | 0.88 | 11.4 | 15.1 | 6.41 | 13.25–14.27 | −1.14 | 1.71 | 0.993 |
| DWI MaRIA | 11.01 | 0.72 | 9.2 | 12.1 | 6.51 | 10.60–11.43 | 0.01 | −1.19 | 0.896 |
| ADC | 1.20 | 0.09 | 1.1 | 1.4 | 7.16 | 1.15–1.25 | 0.33 | −1.18 | 0.895 |
| Parameter | Mean | SD | Min | Max | CV | CI | Skewness | Kurtosis | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| T2 SI wall/T2 SI m. psoas | 1.98 | 0.40 | 1.4 | 3.0 | 20.15 | 1.87–2.10 | 0.72 | −0.38 | 0.465 |
| Wall thickness (mm) | 6.10 | 1.02 | 4.1 | 8.8 | 16.73 | 5.80–6.39 | 0.74 | 0.71 | 0.606 |
| MaRIA | 21.92 | 5.65 | 13.7 | 33.7 | 25.79 | 20.30–23.55 | 1.13 | −1.37 | 0.174 |
| DWI MaRIA | 18.41 | 4.43 | 12.6 | 27.1 | 24.04 | 17.14–19.68 | 0.19 | −1.37 | 0.095 |
| ADC | 1.11 | 0.10 | 0.9 | 1.4 | 8.61 | 1.09–1.14 | 0.73 | 0.60 | 0.396 |
| Inactive | Active | Severe Disease | Contribution % | |
|---|---|---|---|---|
| DWI MaRIA | Lower | Moderate *1 | Higher *2 | 41.499 |
| MaRIA | Lower | Moderate *1 | Higher *2 | 26.636 |
| ADC | Higher *2 | Moderate *1 | Lower | 13.730 |
| T2 SI wall/T2 SI m. psoas | Lower | Moderate *1 | Higher *2 | 13.524 |
| Wall thickness | Lower | Moderate *1 | Higher *2 | 4.611 |
| n/m | 21/21 | 12/14 | 48/49 | |
| Homogeneity % | 100 | 85.71 | 97.96 |
| Inactive | Active | Severe Disease | |
|---|---|---|---|
| Inactive | 0.00 | 3.59 | 5.72 |
| Active | 3.59 | 0.00 | 2.85 |
| Severe disease | 5.72 | 2.85 | 0.00 |
| Parameter | Mean | SD | Min | Max | CV | CI | Skewness | Kurtosis | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| T2 SI wall/T2 SI m. psoas | 1.27 | 0.34 | 0.8 | 2.2 | 26.31 | 1.12–1.42 | 1.00 | 0.92 | 0.263 |
| Wall thickness (mm) | 3.81 | 0.67 | 3.1 | 5.4 | 17.51 | 3.52–4.11 | 1.07 | 0.33 | 0.688 |
| MaRIA | 8.18 | 1.56 | 5.8 | 11.4 | 19.07 | 7.49–8.88 | 0.33 | −0.72 | 0.924 |
| DWI MaRIA | 4.64 | 1.81 | 2.7 | 10.7 | 38.98 | 3.83–5.44 | 1.88 | 0.76 | 0.075 |
| ADC | 1.45 | 0.21 | 1.0 | 2.1 | 14.72 | 1.35–1.54 | 1.02 | 2.49 | 0.477 |
| Parameter | Mean | SD | Min | Max | CV | CI | Skewness | Kurtosis | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| T2 SI wall/T2 SI m. psoas | 1.81 | 0.44 | 1.1 | 2.7 | 24.17 | 1.67–1.96 | 0.34 | −0.68 | 0.508 |
| Wall thickness (mm) | 5.49 | 1.15 | 3.5 | 8.4 | 20.95 | 5.10–5.87 | 0.25 | −0.38 | 0.997 |
| MaRIA | 15.66 | 1.90 | 13.1 | 20.0 | 12.13 | 15.03–16.30 | 0.63 | −0.54 | 0.734 |
| DWI MaRIA | 13.14 | 1.92 | 9.9 | 17.9 | 14.62 | 12.50–13.78 | 0.18 | −0.51 | 0.955 |
| ADC | 1.14 | 0.09 | 0.9 | 1.4 | 8.15 | 1.11–1.17 | 0.15 | 0.06 | 0.505 |
| Parameter | Mean | SD | Min | Max | CV | CI | Skewness | Kurtosis | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| T2 SI wall/T2 SI m. psoas | 2.03 | 0.42 | 1.4 | 3.0 | 20.77 | 1.85–2.20 | 0.81 | −0.26 | 0.303 |
| Wall thickness (mm) | 6.04 | 1.21 | 4.1 | 8.8 | 20.02 | 5.54–6.54 | 0.72 | 0.21 | 0.835 |
| MaRIA | 27.04 | 2.35 | 23.9 | 33.7 | 8.68 | 26.07–28.01 | 1.16 | 1.29 | 0.427 |
| DWI MaRIA | 22.38 | 2.04 | 18.9 | 27.1 | 9.12 | 21.54–23.22 | 0.16 | 0.24 | 0.713 |
| ADC | 1.12 | 0.11 | 1.0 | 1.4 | 9.69 | 1.08–1.17 | 0.98 | 0.19 | 0.311 |
| Active | Inactive | Severe Disease | Contribution % | |
|---|---|---|---|---|
| Wall thickness | Lower | Moderate *1 | Higher *2 | 49.248 |
| DWI MaRIA | Lower | Moderate *1 | Higher *2 | 35.993 |
| MaRIA | Lower | Moderate *1 | Higher *2 | 8.133 |
| ADC | Higher *2 | Moderate *1 | Lower | 6.009 |
| T2 SI wall/T2 SI m. psoas | Lower | Moderate *1 | Higher *2 | 0.617 |
| n/m | 21/22 | 31/37 | 25/25 | |
| Homogeneity % | 95.45 | 83.78 | 100 |
| Inactive | Active | Severe Disease | |
|---|---|---|---|
| Inactive | 0.00 | 9.26 | 24.22 |
| Active | 9.26 | 0.00 | 15.27 |
| Severe disease | 24.22 | 15.27 | 0.00 |
| Parameter | Mean | SD | Min | Max | CV | CI | Skewness | Kurtosis | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| T2 SI wall/T2 SI m. psoas | 1.27 | 0.34 | 0.8 | 2.2 | 26.89 | 1.12–1.43 | 0.96 | 0.72 | 0.275 |
| Wall thickness (mm) | 3.80 | 0.68 | 3.1 | 5.4 | 17.93 | 3.49–4.11 | 1.12 | 0.32 | 0.732 |
| MaRIA | 8.03 | 1.42 | 5.8 | 10.9 | 17.64 | 7.39–8.67 | −0.19 | −0.90 | 0.799 |
| DWI MaRIA | 4.35 | 1.23 | 2.7 | 6.9 | 28.32 | 3.79–4.91 | 0.91 | 0.06 | 0.691 |
| ADC | 1.46 | 0.22 | 1.0 | 2.1 | 14.73 | 1.36–1.55 | 0.96 | 2.43 | 0.608 |
| Parameter | Mean | SD | Min | Max | CV | CI | Skewness | Kurtosis | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| T2 SI wall/T2 SI m. psoas | 1.80 | 0.45 | 1.1 | 2.7 | 24.92 | 1.65–1.95 | 0.34 | −0.77 | 0.658 |
| Wall thickness (mm) | 5.37 | 1.06 | 3.5 | 7.4 | 19.75 | 5.02–5.72 | 0.01 | −1.00 | 0.509 |
| MaRIA | 15.43 | 1.88 | 11.4 | 19.5 | 12.17 | 14.80–16.06 | 0.36 | −0.42 | 0.509 |
| DWI MaRIA | 12.95 | 1.79 | 9.9 | 16.4 | 13.83 | 12.35–13.55 | −0.04 | −0.75 | 0.509 |
| ADC | 1.14 | 0.09 | 0.9 | 1.4 | 8.33 | 1.11–1.17 | 0.15 | −0.12 | 0.683 |
| Parameter | Mean | SD | Min | Max | CV | CI | Skewness | Kurtosis | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| T2 SI wall/T2 SI m. psoas | 2.01 | 0.42 | 1.4 | 3.0 | 20.72 | 1.84–2.18 | 0.87 | −0.17 | 0.330 |
| Wall thickness (mm) | 6.13 | 1.27 | 4.1 | 8.8 | 20.75 | 5.62–6.64 | 0.64 | −0.21 | 0.809 |
| MaRIA | 26.77 | 2.68 | 20.0 | 33.7 | 10.26 | 25.68–27.85 | 0.32 | 1.45 | 0.681 |
| DWI MaRIA | 22.21 | 2.19 | 17.9 | 27.1 | 9.85 | 21.23–23.09 | 0.40 | 0.20 | 0.833 |
| ADC | 1.13 | 0.11 | 1.0 | 1.4 | 9.55 | 1.08–1.17 | 0.91 | 0.13 | 0.412 |
| Inactive | Active | Severe Disease | Contribution % | |
|---|---|---|---|---|
| Wall thickness | Lower | Moderate *1 | Higher *2 | 48.544 |
| DWI MaRIA | Lower | Moderate *1 | Higher *2 | 44.890 |
| ADC | Higher *2 | Moderate *1 | Lower | 4.262 |
| MaRIA | Lower | Moderate *1 | Higher *2 | 2.152 |
| T2 SI wall/T2 SI m. psoas | Lower | Moderate *1 | Higher *2 | 0.152 |
| n/m | 21/21 | 31/37 | 25/26 | |
| homogeneity % | 100 | 83.78 | 96.15 |
| Inactive | Active | Severe Disease | |
|---|---|---|---|
| Inactive | 0.00 | 7.40 | 16.35 |
| Active | 7.40 | 0.00 | 9.41 |
| Severe disease | 16.35 | 9.41 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilipovic Grubor, J.; Stojanovic, S.; Niciforovic, D.; Basta Nikolic, M.; Jelicic, Z.D.; Radovic, M.N.; Ostojic, J. Beyond Fixed Thresholds: Cluster-Derived MRI Boundaries Improve Assessment of Crohn’s Disease Activity. J. Clin. Med. 2025, 14, 7523. https://doi.org/10.3390/jcm14217523
Pilipovic Grubor J, Stojanovic S, Niciforovic D, Basta Nikolic M, Jelicic ZD, Radovic MN, Ostojic J. Beyond Fixed Thresholds: Cluster-Derived MRI Boundaries Improve Assessment of Crohn’s Disease Activity. Journal of Clinical Medicine. 2025; 14(21):7523. https://doi.org/10.3390/jcm14217523
Chicago/Turabian StylePilipovic Grubor, Jelena, Sanja Stojanovic, Dijana Niciforovic, Marijana Basta Nikolic, Zoran D. Jelicic, Mirna N. Radovic, and Jelena Ostojic. 2025. "Beyond Fixed Thresholds: Cluster-Derived MRI Boundaries Improve Assessment of Crohn’s Disease Activity" Journal of Clinical Medicine 14, no. 21: 7523. https://doi.org/10.3390/jcm14217523
APA StylePilipovic Grubor, J., Stojanovic, S., Niciforovic, D., Basta Nikolic, M., Jelicic, Z. D., Radovic, M. N., & Ostojic, J. (2025). Beyond Fixed Thresholds: Cluster-Derived MRI Boundaries Improve Assessment of Crohn’s Disease Activity. Journal of Clinical Medicine, 14(21), 7523. https://doi.org/10.3390/jcm14217523






