Physiological Determinants of PR Interval in Healthy Fetuses: Insights from Correlation and Regression Modeling
Abstract
1. Introduction
2. Materials and Methods
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamela-Olkowska, A.; Dangel, J. Estimation of the atrioventricular time interval by pulse Doppler in the normal fetal heart. Ginekol. Pol. 2009, 80, 584–589. [Google Scholar] [PubMed]
- Phoon, C.K.L.; Kim, M.Y.; Buyon, J.P.; Friedman, D.M. Finding the “PR-fect” solution: What is the best tool to measure fetal cardiac PR intervals for the detection and possible treatment of early conduction disease? Congenit. Heart Dis. 2012, 7, 349–360. [Google Scholar] [CrossRef]
- McKinney, W. Data Structures for Statistical Computing in Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010; pp. 56–61. Available online: https://proceedings.scipy.org/articles/Majora-92bf1922-00a (accessed on 1 August 2025).
- Harris, C.R.; Millman, K.J.; Van Der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Seabold, S.; Perktold, J. Statsmodels: Econometric and Statistical Modeling with Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010; pp. 92–96. [Google Scholar]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Waskom, M.L. Seaborn: Statistical Data Visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Terpilowski, M. Scikit-posthocs: Pairwise Multiple Comparisons in Python. J. Open Source Softw. 2019, 4, 1169. [Google Scholar] [CrossRef]
- Wojakowski, A.; Izbizky, G.; Carcano, M.E.; Aiello, H.; Marantz, P.; Otaño, L. Fetal Doppler mechanical PR interval: Correlation with fetal heart rate, gestational age and fetal sex. Ultrasound Obstet. Gynecol. 2009, 34, 538–542. [Google Scholar] [CrossRef]
- Kato, Y.; Takahashi-Igari, M.; Inaba, T.; Sumazaki, R.; Horigome, H. Comparison of PR intervals determined by fetal magnetocardiography and pulsed Doppler echocardiography. Fetal Diagn. Ther. 2012, 32, 109–115. [Google Scholar] [CrossRef]
- Hansahiranwadee, W. Diagnosis and Management of Fetal Autoimmune Atrioventricular Block. Int. J. Women’s Health 2020, 12, 633–639. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bravo-Valenzuela, N.J.; Rocha, L.A.; Nardozza, L.M.M.; Júnior, E.A. Fetal cardiac arrhythmias: Current evidence. Ann. Pediatr. Cardiol. 2018, 11, 148–163, Erratum in Ann. Pediatr. Cardiol. 2018, 11, 332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clowse, M.E.; Eudy, A.M.; Kiernan, E.; Williams, M.R.; Bermas, B.; Chakravarty, E.; Sammaritano, L.R.; Chambers, C.D.; Buyon, J. The prevention, screening and treatment of congenital heart block from neonatal lupus: A survey of provider practices. Rheumatology 2018, 57 (Suppl. S5), v9–v17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smith, V.; Arunthavanathan, S.; Nair, A.; Ansermet, D.; da Silva Costa, F.; Wallace, E.M. A systematic review of cardiac time intervals utilising non-invasive fetal electrocardiogram in normal fetuses. BMC Pregnancy Childbirth 2018, 18, 370. [Google Scholar] [CrossRef]
- Strehlow, S.L.; Pathak, B.; Goodwin, T.M.; Perez, B.M.; Ebrahimi, M.; Lee, R.H. The mechanical PR interval in fetuses of women with intrahepatic cholestasis of pregnancy. Am. J. Obstet. Gynecol. 2010, 203, 455.e1–455.e5. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, P.V.; Nielsen, J.B.; Skov, M.W.; Pietersen, A.; Graff, C.; Lind, B.; Struijk, J.J.; Olesen, M.S.; Haunsø, S.; Køber, L.; et al. Electrocardiographic PR interval duration and cardiovascular risk: Results from the Copenhagen ECG Study. Can. J. Cardiol. 2017, 33, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Lantto, J.; Johnson, J.; Huhta, H.; Haapsamo, M.; Kiviranta, P.; Räsänen, K.; Voipio, H.M.; Sonesson, S.E.; Voipio, J.; Räsänen, J.; et al. Atrioventricular conduction abnormalities are associated with poor outcome following intermittent umbilical cord occlusions in fetal sheep. Acta Obstet. Gynecol. Scand. 2025, 104, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Glickstein, J.; Buyon, J.; Kim, M.; Friedman, D.; PRIDE Investigators. The fetal Doppler mechanical PR interval: A validation study. Fetal Diagn. Ther. 2004, 19, 31–34. [Google Scholar] [CrossRef]
- Glickstein, J.S.; Buyon, J.; Friedman, D. Pulsed Doppler echocardiographic assessment of the fetal PR interval. Am. J. Cardiol. 2000, 86, 236–239. [Google Scholar] [CrossRef]
- Bolnick, A.D.; Borgida, A.F.; Egan, J.F.X.; Zelop, C.M. Influence of gestational age and fetal heart rate on the fetal mechanical PR interval. J. Matern. Fetal Neonatal Med. 2004, 15, 303–305. [Google Scholar] [CrossRef]
- Tomek, V.; Janousek, J.; Reich, O.; Gilík, J.; Gebauer, R.A.; Skovránek, J. Atrioventricular conduction time in fetuses assessed by Doppler echocardiography. Physiol. Res. 2011, 60, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Bergman, G.; Jacobsson, L.A.; Wahren-Herlenius, M.; Sonesson, S.E. Doppler echocardiographic and electrocardiographic atrioventricular time intervals in newborn infants: Evaluation of techniques for surveillance of fetuses at risk for congenital heart block. Ultrasound Obstet. Gynecol. 2006, 28, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, L.; Seale, A.N.; Belmar, C.; Oseku-Afful, S.; Thomas, M.J.; Taylor, M.J.; Roughton, M.; Gardiner, H.M. PR interval: A comparison of electrical and mechanical methods in the fetus. Early Hum. Dev. 2007, 83, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Bolin, E.H.; Siegel, E.R.; Eswaran, H.; Lowery, C.L.; Zakaria, D.; Best, T.H. Cardiac time intervals derived by magnetocardiography in fetuses exposed to pregnancy hypertension syndromes. J. Perinatol. 2016, 36, 643–648. [Google Scholar] [CrossRef]
- Van Leeuwen, P.; Schiermeier, S.; Lange, S.; Klein, A.; Geue, D.; Hatzmann, W.; Grönemeyer, D.H. Gender-related changes in magnetocardiographically determined fetal cardiac time intervals in intrauterine growth retardation. Pediatr. Res. 2006, 59, 820–824. [Google Scholar] [CrossRef][Green Version]
- Cuneo, B.F.; Bitant, S.; Strasburger, J.F.; Kaizer, A.M.; Wakai, R.T. Assessment of atrioventricular conduction by echocardiography and magnetocardiography in normal and anti-Ro/SSA-antibody-positive pregnancies. Ultrasound Obstet. Gynecol. 2019, 54, 625–633. [Google Scholar] [CrossRef]
- Sirico, A.; Diemert, A.; Glosemeyer, P.; Hecher, K. Third Trimester Umbilical Artery Doppler in Low-Risk Pregnancies and its Correlation to Estimated Fetal Weight and Birthweight. Ultraschall Med. 2021, 42, 285–290. Erratum in Ultraschall Med. 2021, 42, e55. (In English) [Google Scholar] [CrossRef]

| Gestational Age (Weeks) | Count | % of Total | Cumulative % |
|---|---|---|---|
| 16–19 | 19 | 2.054054 | 2.054054 |
| 20–23 | 101 | 10.91892 | 12.97297 |
| 24–27 | 461 | 49.83784 | 62.81081 |
| 28+ | 344 | 37.18919 | 100 |
| (a) | ||||
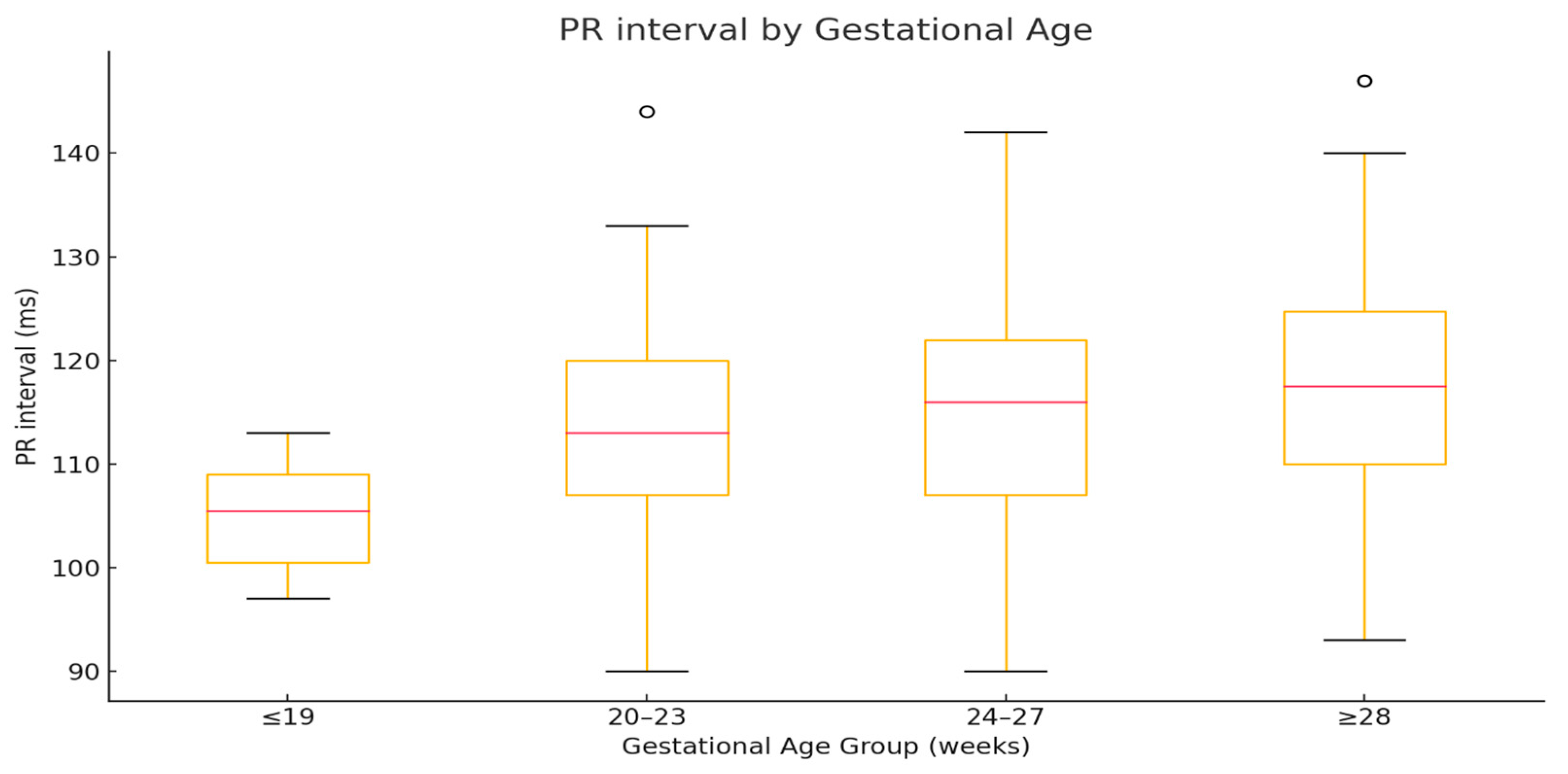
| Group | Median | Q1 | Q3 | IQR |
| ≤19 | 105.5 | 100.5 | 109 | 8.5 |
| 20–23 | 113 | 107 | 120 | 13 |
| 24–27 | 116 | 107 | 122 | 15 |
| ≥28 | 117.5 | 110 | 124.75 | 14.75 |
| (b) | ||||
| Comparison | Significance | |||
| ≤19 vs. 20–23 | p < 0.01 | |||
| ≤19 vs. 24–27 | p < 0.01 | |||
| ≤19 vs. ≥28 | p < 0.01 | |||
| 20–23 vs. 24–27 | ns | |||
| 20–23 vs. ≥28 | p < 0.05 | |||
| 24–27 vs. ≥28 | ns | |||
| Variable | Spearman’s ρ | p-Value |
|---|---|---|
| Fetal Heart Rate (FHR) | −0.256 | <0.01 |
| Femur Length (FL) | 0.174 | <0.01 |
| Pulmonary Valve Diameter (PVD) | 0.169 | <0.01 |
| Biparietal Diameter (BPD) | 0.168 | <0.01 |
| Head Circumference (HC) | 0.168 | <0.01 |
| Fronto-Occipital Diameter (FOD) | 0.167 | <0.01 |
| Abdominal Circumference (AC) | 0.163 | <0.01 |
| Gestational Age | 0.159 | <0.01 |
| Estimated Fetal Weight (EFW) | 0.159 | <0.01 |
| Aortic Valve Diameter (AVD) | 0.155 | <0.01 |
| Femur Length (FL) | 0.151 | <0.01 |
| Middle Cerebral Artery Peak Systolic Velocity (MCA PSV) | 0.103 | <0.05 |
| Umbilical Artery Pulsatility Index (UA PI) | −0.084 | <0.05 |
| Estimated Weight (Percentile) | 0.071 | <0.05 |
| Aortic-to-Pulmonary Valve Ratio (Ao/TP) | −0.07 | <0.05 |
| Head-to-Abdominal Circumference Ratio (HC/AC) | −0.093 | ns |
| Pulsatility Index of Ductus Venosus (PIV) | −0.065 | ns |
| Cerebroplacental Ratio (CPR) | 0.052 | ns |
| Middle Cerebral Artery Pulsatility Index (MCA) | 0.038 | ns |
| Biparietal-to-Fronto-Occipital Ratio (BPD/FOD) | 0.032 | ns |
| Biparietal-to-Femur Length Ratio (BPD/FL) | −0.032 | ns |
| (a) | ||||
| Group | Median | Q1 | Q3 | IQR |
| ≤19 | 149 | 144.25 | 152.75 | 8.5 |
| 20–23 | 147 | 141 | 153 | 12 |
| 24–27 | 143 | 138 | 149.75 | 11.75 |
| ≥28 | 141 | 135 | 148 | 13 |
| (b) | ||||
| Comparison | z | p | ||
| ≤19 vs. 20–23 | 1.118602 | ns | ||
| ≤19 vs. 24–27 | 2.603423 | ns | ||
| ≤19 vs. ≥28 | 3.692449 | p < 0.01 | ||
| 20–23 vs. 24–27 | 3.087323 | p < 0.05 | ||
| 20–23 vs. ≥28 | 5.356437 | p < 0.01 | ||
| 24–27 vs. ≥28 | 3.738765 | p < 0.01 | ||
| Variable | Standardized Coefficient | Direction |
|---|---|---|
| Fetal Heart Rate (FHR) | −2.011 | Negative |
| Biparietal Diameter (BPD) | 0.644 | Positive |
| Pulmonary Valve Diameter (PVD) | 0.392 | Positive |
| Umbilical Artery Pulsatility Index (UA PI) | −0.382 | Negative |
| Fronto-Occipital Diameter (FOD) | 0.033 | Positive |
| Model R2 | 0.099 | Explains ~9.9% of PR interval variability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swiercz, G.; Janiak, K.; Pawlik, L.; Mlodawska, M.; Kaczmarek, P.; Mlodawski, J. Physiological Determinants of PR Interval in Healthy Fetuses: Insights from Correlation and Regression Modeling. J. Clin. Med. 2025, 14, 7522. https://doi.org/10.3390/jcm14217522
Swiercz G, Janiak K, Pawlik L, Mlodawska M, Kaczmarek P, Mlodawski J. Physiological Determinants of PR Interval in Healthy Fetuses: Insights from Correlation and Regression Modeling. Journal of Clinical Medicine. 2025; 14(21):7522. https://doi.org/10.3390/jcm14217522
Chicago/Turabian StyleSwiercz, Grzegorz, Katarzyna Janiak, Lukasz Pawlik, Marta Mlodawska, Piotr Kaczmarek, and Jakub Mlodawski. 2025. "Physiological Determinants of PR Interval in Healthy Fetuses: Insights from Correlation and Regression Modeling" Journal of Clinical Medicine 14, no. 21: 7522. https://doi.org/10.3390/jcm14217522
APA StyleSwiercz, G., Janiak, K., Pawlik, L., Mlodawska, M., Kaczmarek, P., & Mlodawski, J. (2025). Physiological Determinants of PR Interval in Healthy Fetuses: Insights from Correlation and Regression Modeling. Journal of Clinical Medicine, 14(21), 7522. https://doi.org/10.3390/jcm14217522








