Hypericin Photodynamic Therapy Induces Cytotoxicity and Modulates Cytokine Secretion in MCF-7 Breast Cancer Cells
Abstract
1. Introduction
1.1. Rationale
1.2. Objectives
2. Materials and Methods
2.1. Study Design
- Cell culture and preparation of the MCF-7 breast cancer cell line.
- Hypericin treatment at different concentrations (0–1.0 µM).
- Photodynamic therapy, using defined light doses (0, 1, 2, and 5 J/cm2).
- Outcome measurements, including:
- Cell viability using the MTT assay and Trypan Blue exclusion test.
- Quantification of cytokines (IL-6, IL-8, IL-10, TNF-α) in culture supernatants.
- Untreated cells (no hypericin, no light).
- Cells treated with hypericin but kept in the dark (dark controls).
- Cells exposed to light without hypericin (light-only controls).
2.2. Chemicals and Reagents
2.3. Cell Line and Culture Conditions
- 2 mM L-glutamine
- 1 mM sodium pyruvate
- 1500 mg/L sodium bicarbonate
- 0.01 mg/mL human recombinant insulin
- 10% FBS
2.4. Hypericin Preparation and Treatment
2.5. PDT Procedure
- Irradiance at the cell surface: 35 mW/cm2 (verified using Newport power meter).
- Light doses applied: 1, 2, and 5 J/cm2.
- Distance from light source to cells: 30 cm.
2.6. Cell Viability Assessment—MTT Assay
- A 150 µL aliquot from each well was transferred to a new 96-well plate.
- Absorbance was read at 550 nm using a microplate reader (ELx800, Bio-Tek Instruments Inc., Winooski, VT, USA).
2.7. Trypan Blue Exclusion Assay
2.8. Cytokine Quantification
2.9. Statistical Analysis
- Normality of data was assessed using the Shapiro–Wilk test.
- For MTT results, comparisons between groups and controls were made using Student’s t-test.
- Cytokine data were analyzed using Kruskal–Wallis ANOVA, followed by a multiple comparison post hoc test.
2.10. Summary of Experimental Workflow
- Seed MCF-7 cells → 24 h adherence.
- Incubate with hypericin for 2 h in the dark.
- Irradiate with the specified light dose or keep as dark/light control.
- Incubate 24 h post-PDT in dark.
- Collect supernatants → cytokine assay.
- Perform MTT and Trypan Blue viability assays.
- Analyze results statistically.
3. Results
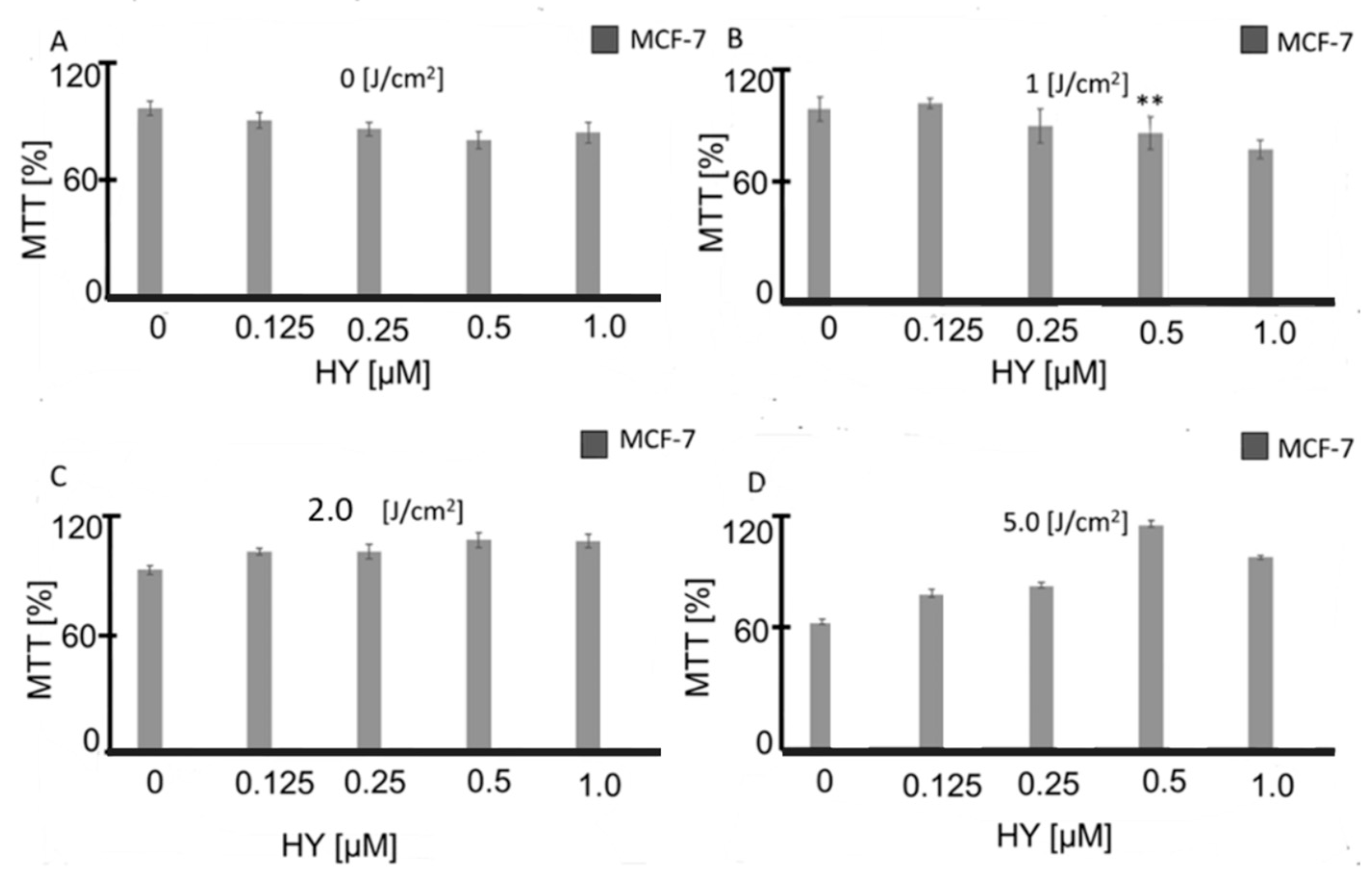
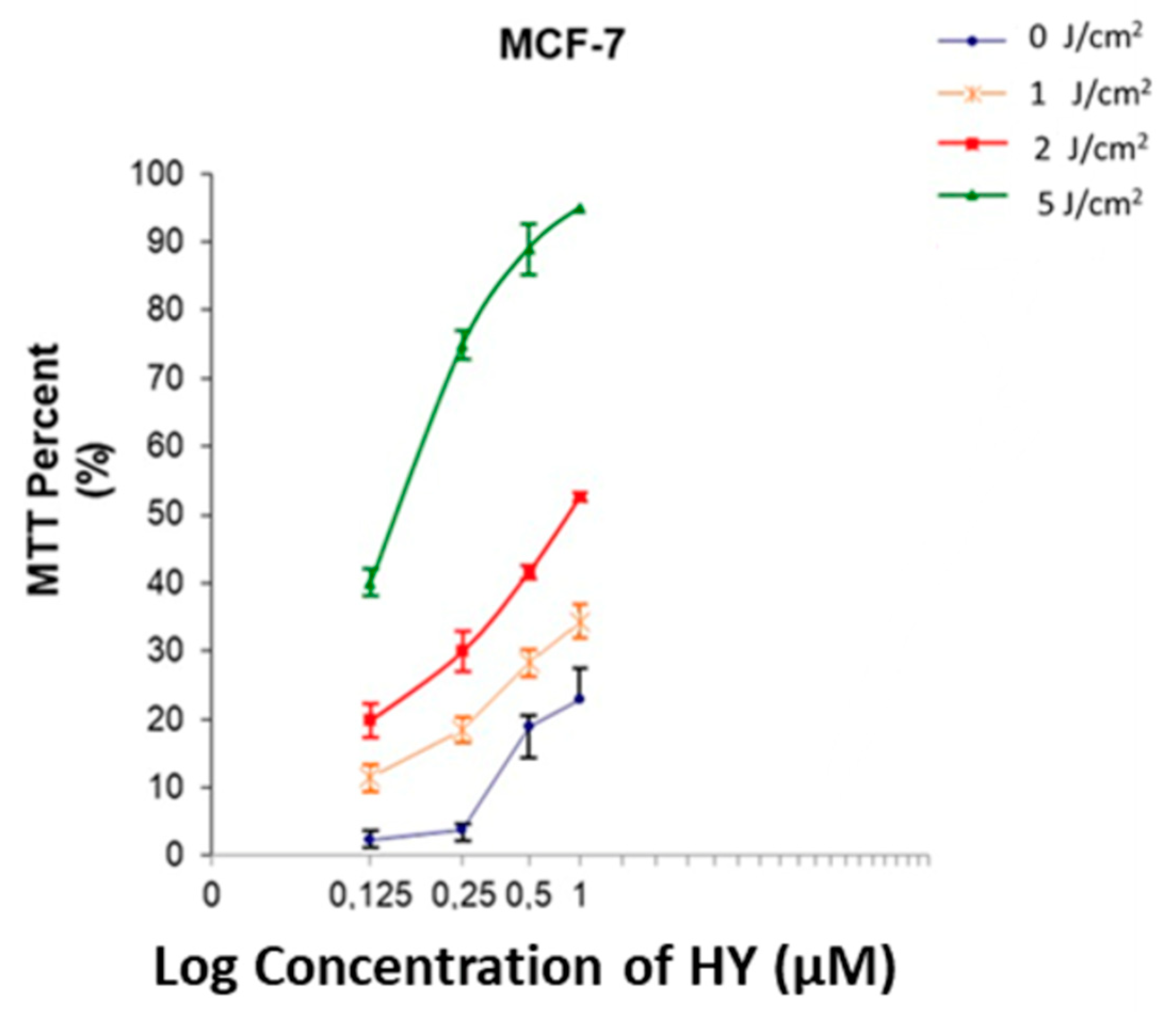
3.1. Effect of HYP-PDT on MCF-7 Cell Viability
3.2. Validation of Viability by Trypan Blue Exclusion
- At 0.125 µM HY, viability decreased slightly to 95 ± 2.3%,
- At 0.5 µM HY, viability dropped to 84 ± 15%,
- At 1.0 µM HY, viability was 87 ± 5% compared to 98.9 ± 0.3% in untreated controls (Table 1).
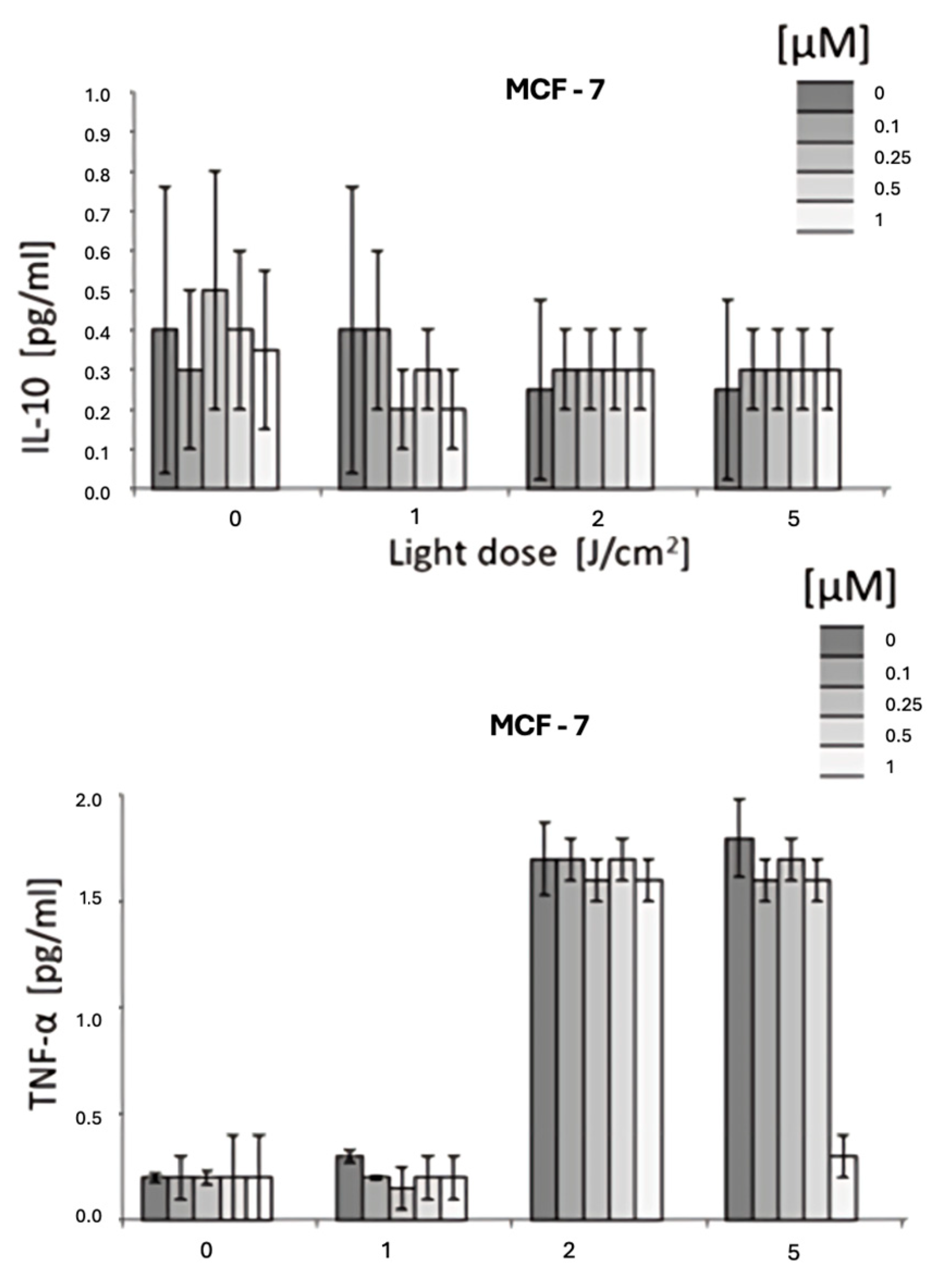
3.3. Cytokine Modulation by HY-PDT
- IL-6: A significant reduction in IL-6 levels was observed, with up to a 50% decrease at 1 µM hypericin combined with PDT (p < 0.05).
- IL-8: Similarly, IL-8 levels were markedly reduced in response to HYP-PDT at higher light doses and hypericin concentrations.
- IL-10: No statistically significant differences were detected for IL-10 across treatment groups (p > 0.05).
3.4. IC50 Determination for HY-PDT
3.5. Cytokine Ratio Analysis
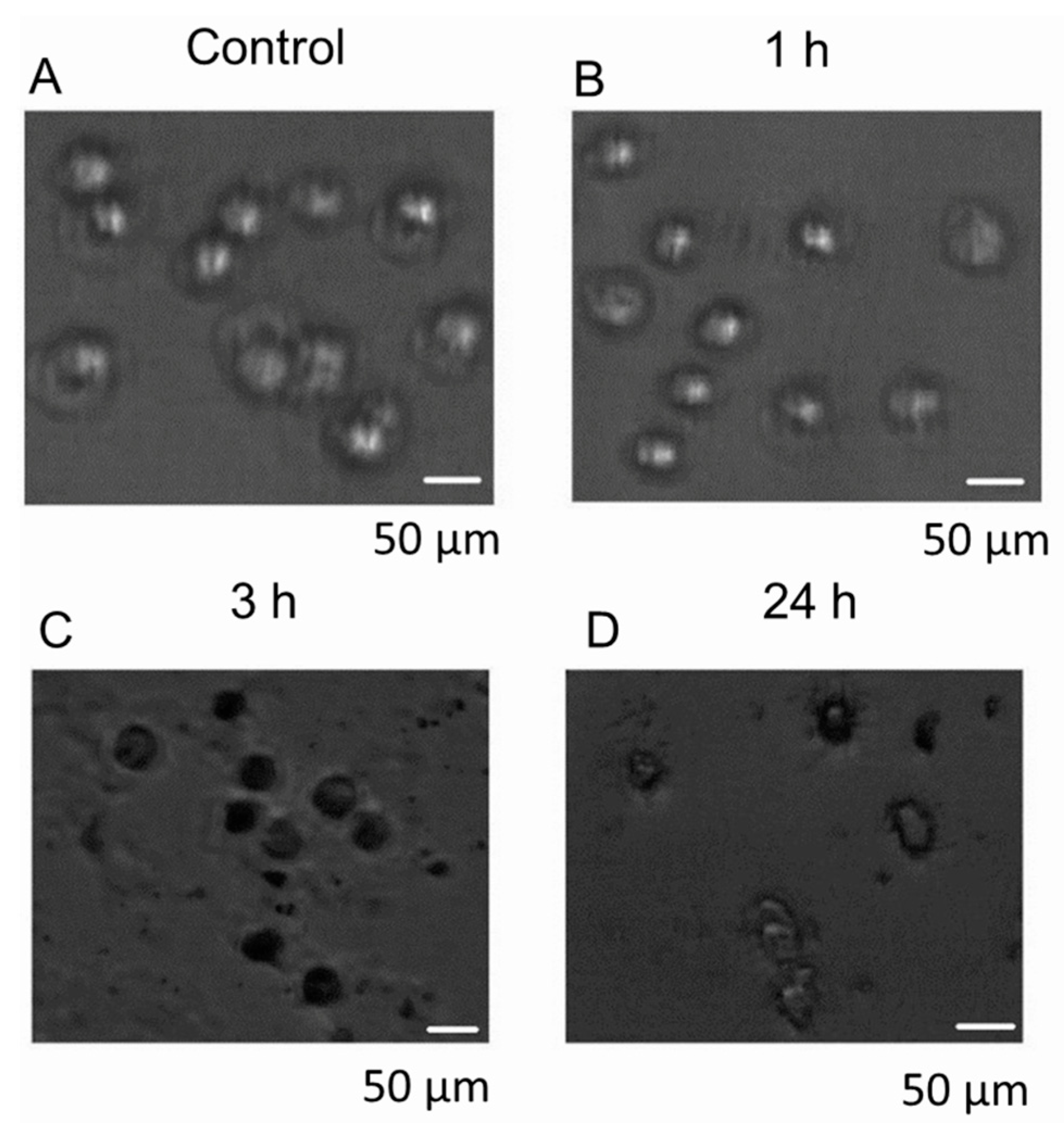
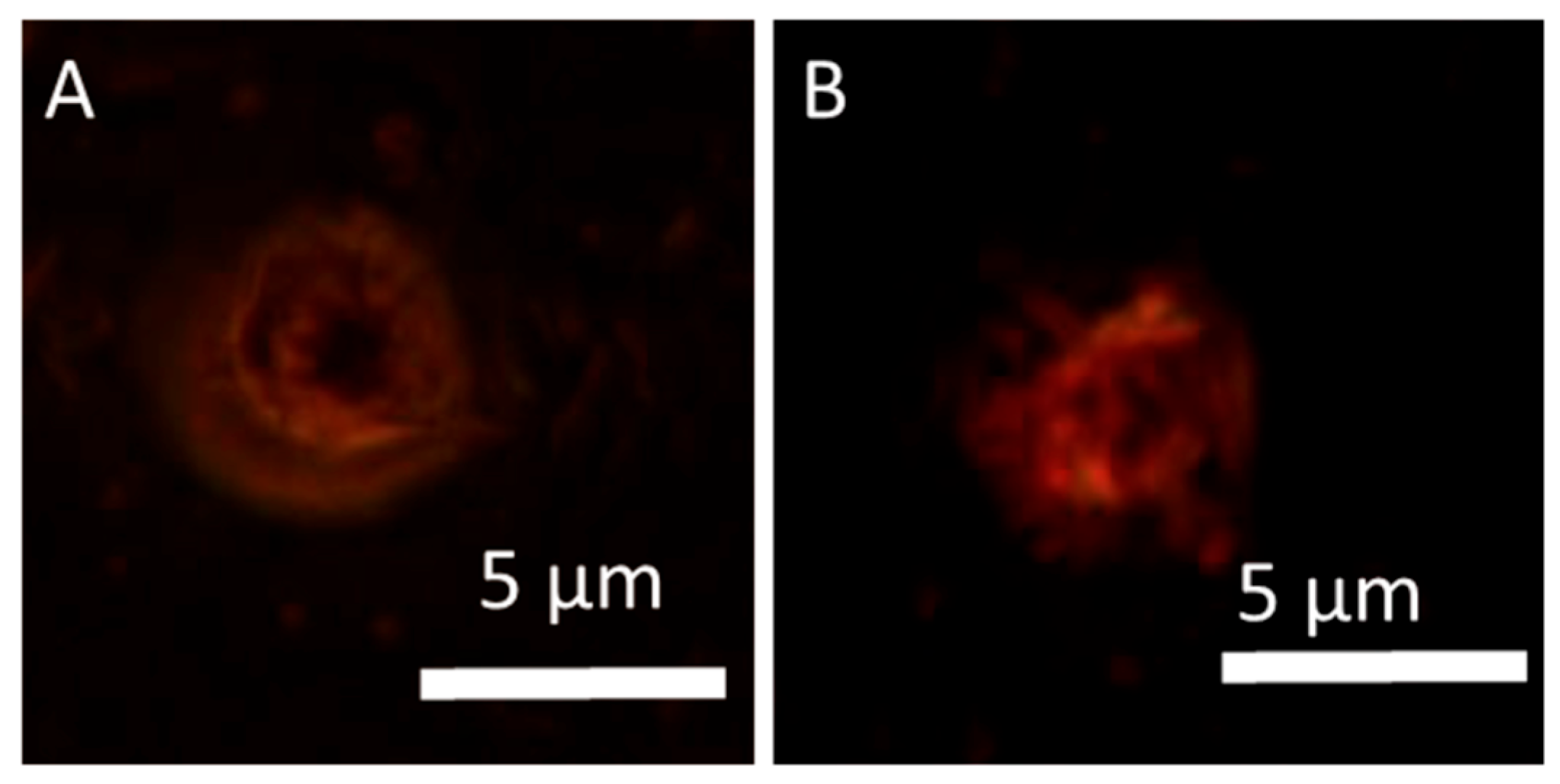
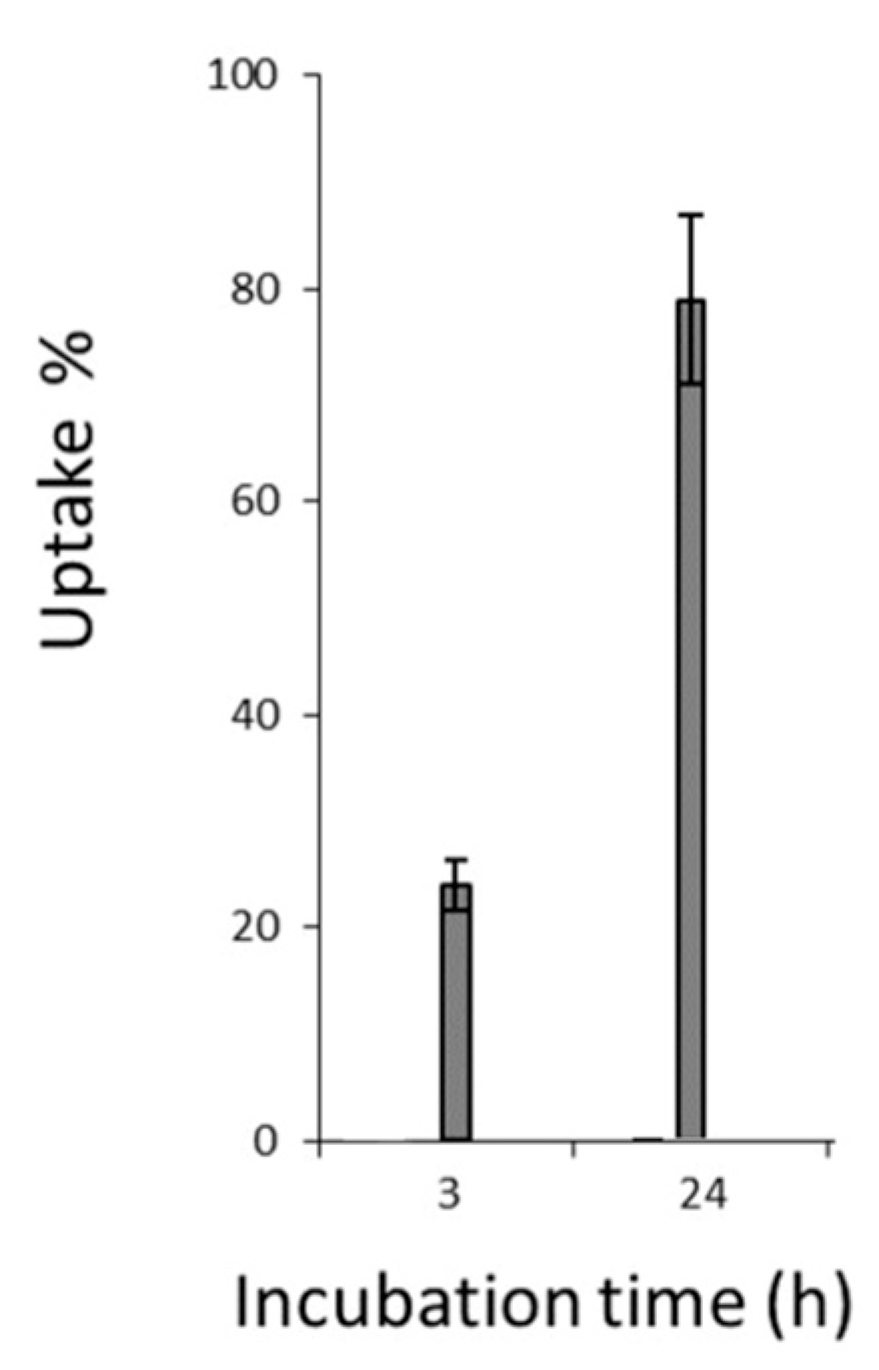
3.6. Cellular Uptake and Localization of Hypericin
3.7. Quantification of Hypericin Uptake
3.8. Summary of Findings
- HYP-PDT decreased cell viability only at higher hypericin concentrations and light doses.
- IL-6 and IL-8 decreased significantly, while TNF-α increased, indicating a pro-inflammatory shift.
- Hypericin showed rapid cellular uptake and uniform intracellular distribution.
- Light diffraction analysis provided insights into how cellular morphology may affect PDT light penetration.
4. Discussion
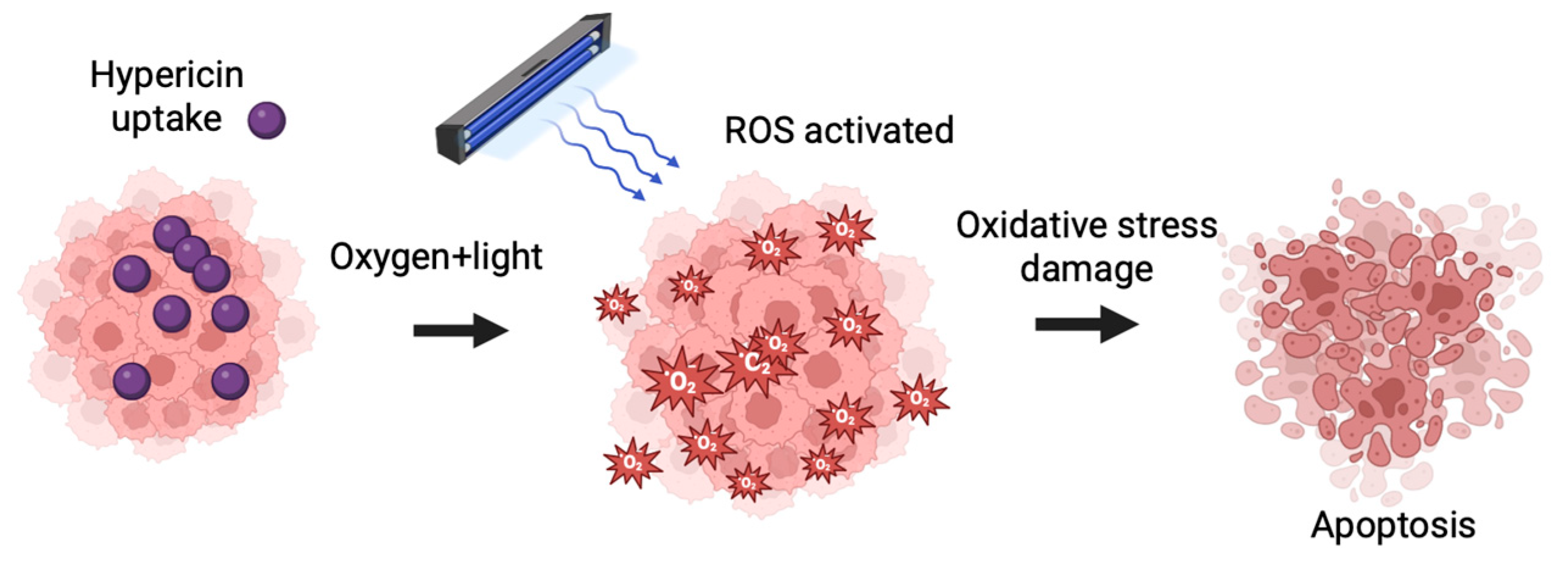
4.1. HYP-PDT and Advances in Targeted Cancer Therapy
4.2. Mitochondrial Targeting and Overcoming Tumor Hypoxia
4.3. Combination Therapies and Clinical Implications
4.4. Molecular Mechanisms: Apoptosis and Bcl-2 Family Regulation
4.5. Natural Compounds and Future Nanomedicine Applications
4.6. Cytokine Modulation and Immunological Implications
4.7. Immunogenic Cell Death and STAT3/NF-κB Pathway Modulation by HYP-PDT
4.8. Study Significance and Future Directions
- Validating these findings in in vivo breast cancer models.
- Exploring combination therapies with chemotherapy, immunotherapy, or targeted drugs.
- Investigating cytokine regulation as a biomarker for treatment response.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full form |
| BC | Breast cancer |
| PDT | Photodynamic therapy |
| HYP | Hypericin |
| HYP-PDT | Hypericin-mediated photodynamic therapy |
| PS | Photosensitizer |
| 1O2 | Singlet oxygen |
| ROS | Reactive oxygen species |
| H2O2 | Hydrogen peroxide |
| OH· | Hydroxyl radical |
| O2·− | Superoxide anion |
| MCF-7 | Human breast adenocarcinoma cell line MCF-7 |
| IL | Interleukin |
| IL-1 | Interleukin 1 |
| IL-2 | Interleukin 2 |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| IL-10 | Interleukin 10 |
| IL-11 | Interleukin 11 |
| IL-12 | Interleukin 12 |
| IL-17A | Interleukin 17A |
| IL-18 | Interleukin 18 |
| IL-19 | Interleukin 19 |
| IL-20 | Interleukin 20 |
| IL-22 | Interleukin 22 |
| IL-23 | Interleukin 23 |
| IFN-γ | Interferon gamma |
| TGF-β | Transforming growth factor beta |
| IRF-1 | Interferon regulatory factor 1 |
| IRF-2 | Interferon regulatory factor 2 |
| gp130 or GP130 | Glycoprotein 130 |
| MMP-1 | Matrix metalloproteinase 1 |
| TNF-α | Tumor necrosis factor alpha |
| DAMPs | Danger-associated molecular patterns |
| IC50 | Half-maximal inhibitory concentration |
| ATCC | American Type Culture Collection |
| DMEM/F12 | Dulbecco’s Modified Eagle’s Medium: F-12 |
| EMEM | Eagle’s Minimum Essential Medium |
| FBS | Fetal bovine serum |
| EDTA | Ethylenediaminetetraacetic acid |
| PBS | Phosphate-buffered saline |
| DMSO | Dimethyl sulfoxide |
| IR | Infrared |
| CO2 | Carbon dioxide |
| OD | Optical density |
| SD | Standard deviation |
| ANOVA | Analysis of variance |
| TNBC | Triple negative breast cancer |
| AuNPs | Gold nanoparticles |
| BCRP | Breast cancer resistance protein |
| MRP1 | Multidrug resistance-associated protein 1 |
| NFATc1 | Nuclear factor of activated T cells c1 |
| MAPK | Mitogen-activated protein kinase |
| PI3K | Phosphoinositide 3-kinase |
| STAT3 | Signal transducer and activator of transcription 3 |
| ADAMTS9 | A disintegrin and metalloproteinase with thrombospondin motifs 9 |
| HPPH | 2-(1-hexyloxyethyl)-2-devinyl pyropheophorbide a |
| SnEt2 | Tin ethyl etiopurpurin |
| LuTex | Lutetium texaphyrin |
| ISO | International Organization for Standardization |
| CBD | Cannabidiol |
References
- Wang, J.; Wu, S.G. Breast Cancer: An Overview of Current Therapeutic Strategies, Challenge, and Perspectives. Breast Cancer 2023, 15, 721–730. [Google Scholar] [CrossRef]
- Mokoena, D.R.; George, B.P.; Abrahamse, H. Photodynamic Therapy Induced Cell Death Mechanisms in Breast Cancer. Int. J. Mol. Sci. 2021, 22, 10506. [Google Scholar] [CrossRef]
- Nicolini, A.; Carpi, A.; Rossi, G. Cytokines in Breast Cancer. Cytokine Growth Factor Rev. 2006, 17, 325–337. [Google Scholar] [CrossRef]
- Esquivel-Velázquez, M.; Ostoa-Saloma, P.; Palacios-Arreola, M.I.; Nava-Castro, K.E.; Castro, J.I.; Morales-Montor, J. The Role of Cytokines in Breast Cancer Development and Progression. J. Interferon Cytokine Res. 2015, 35, 1–16. [Google Scholar] [CrossRef]
- Habanjar, O.; Bingula, R.; Decombat, C.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. Crosstalk of Inflammatory Cytokines within the Breast Tumor Microenvironment. Int. J. Mol. Sci. 2023, 24, 4002. [Google Scholar] [CrossRef]
- Alvarez, N.; Sevilla, A. Current Advances in Photodynamic Therapy (PDT) and the Future Potential of PDT-Combinatorial Cancer Therapies. Int. J. Mol. Sci. 2024, 25, 1023. [Google Scholar] [CrossRef]
- Teng, K.X.; Niu, L.Y.; Yang, Q.Z. A Host-Guest Strategy for Converting the Photodynamic Agents from a Singlet Oxygen Generator to a Superoxide Radical Generator. Chem. Sci. 2022, 13, 5951–5956. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Xu, H.; Meyers, A.D.; Musani, A.I.; Wang, L.; Tagg, R.; Barqawi, A.B.; Chen, Y.K. Photodynamic Therapy for Treatment of Solid Tumors: Potential and Technical Challenges. Technol. Cancer Res. Treat. 2008, 7, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Mang, T.S.; Allison, R.; Hewson, G.; Snider, W.; Moskowitz, R. A Phase II/III Clinical Study of Tin Ethyl Etiopurpurin (Purlytin)-Induced Photodynamic Therapy for the Treatment of Recurrent Cutaneous Metastatic Breast Cancer. Cancer J. Sci. Am. 1998, 4, 378–384. [Google Scholar]
- Anand, S.; Ortel, B.J.; Pereira, S.P.; Hasan, T.; Maytin, E.V. Biomodulatory Approaches to Photodynamic Therapy for Solid Tumors. Cancer Lett. 2012, 326, 8–16. [Google Scholar] [CrossRef]
- Baskaran, R.; Lee, J.; Yang, S.G. Clinical Development of Photodynamic Agents and Therapeutic Applications. Biomater. Res. 2018, 22, 25. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New Photosensitizers for Photodynamic Therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zeng, Y.; Zhang, Z.; Fu, J.; You, L.; He, Y.; Hao, Y.; Gu, Z.; Yu, Z.; Qu, C.; et al. Hypericin-Mediated Photodynamic Therapy for the Treatment of Cancer: A Review. J. Pharm. Pharmacol. 2021, 73, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Sobaci, G.; Bayraktar, M.Z.; Karslioglu, Y.; Durukan, A.H.; Hurmeric, V.; Aykas, S. Hypericin-Enhanced Argon Laser Photocoagulation for Subfoveal Choroidal Neovascular Membrane in Age-Related Macular Degeneration: A Pilot Study. Eur. J. Ophthalmol. 2006, 16, 119–128. [Google Scholar] [CrossRef]
- Ostańska, E.; Barnaś, E.; Bartusik-Aebisher, D.; Dynarowicz, K.; Szpunar, M.; Skręt-Magierło, J.; Aebisher, D. Histopathological Analysis of the Effect of Photodynamic Action on Post-Chemotherapy Excised Breast Cancer Tissue. Medicina 2022, 58, 700. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, G.P.; de Souza, T.F.M.; Cerchiaro, G.; Pinhal, M.A.D.S.; Ribeiro, A.O.; Girão, M.J.B.C. Hypericin in Photobiological Assays: An Overview. Photodiagn. Photodyn. Ther. 2021, 35, 102343. [Google Scholar] [CrossRef]
- Krupka-Olek, M.; Bożek, A.; Czuba, Z.P.; Kłósek, M.; Cieślar, G.; Kawczyk-Krupka, A. Cytotoxic and Immunomodulatory Effects of Hypericin as a Photosensitizer in Photodynamic Therapy Used on Skin Cell Cultures. Pharmaceutics 2024, 16, 696. [Google Scholar] [CrossRef]
- Xia, Y.; Gupta, G.; Castano, A.; Mroz, P.; Avci, P.; Hamblin, M. CpG Oligodeoxynucleotide as Immune Adjuvant Enhances Photodynamic Therapy Response in Murine Metastatic Breast Cancer. J. Biophotonics 2014, 7, 897–905. [Google Scholar] [CrossRef]
- Mirmalek, S.A.; Azizi, M.A.; Jangholi, E.; Yadollah-Damavandi, S.; Javidi, M.A.; Parsa, Y.; Parsa, T.; Salimi-Tabatabaee, S.A.; Kolagar, H.G.; Alizadeh-Navaei, R. Cytotoxic and Apoptogenic Effect of Hypericin, the Bioactive Component of Hypericum perforatum, on the MCF-7 Human Breast Cancer Cell Line. Cancer Cell Int. 2016, 16, 62. [Google Scholar] [CrossRef] [PubMed]
- Acar, M.; Ocak, Z.; Erdogan, K.; Cetin, E.N.; Hatipoglu, O.F.; Uyeturk, U.; Gunduz, E.; Gunduz, M. The Effects of Hypericin on ADAMTS and p53 Gene Expression in MCF-7 Breast Cancer Cells. Cancer Biol. Ther. 2014, 15, 81–89. [Google Scholar]
- Mikeš, J.; Jendželovský, R.; Sačková, V.; Uhrinová, I.; Kello, M.; Kuliková, L.; Fedorocko, P. The Role of p53 in the Efficiency of Photodynamic Therapy with Hypericin and Subsequent Long-Term Survival of Colon Cancer Cells. Photochem. Photobiol. Sci. 2009, 8, 1558–1567. [Google Scholar] [CrossRef]
- Candido, N.M.; de Melo, M.T.; Franchi, L.P.; Primo, F.L.; Tedesco, A.C.; Rahal, P.; Calmon, M.F. Combining Photodynamic Therapy and Chemotherapy: Improving Breast Cancer Treatment with Nanotechnology. J. Biomed. Nanotechnol. 2018, 14, 994–1008. [Google Scholar] [CrossRef]
- Mokoena, D.; George, B.P.; Abrahamse, H. Conjugation of Hypericin to Gold Nanoparticles for Enhancement of Photodynamic Therapy in MCF-7 Breast Cancer Cells. Pharmaceutics 2022, 14, 2212. [Google Scholar] [CrossRef] [PubMed]
- Roser, M.; Ritchie, H. Cancer. Our World in Data. 2015. Available online: https://ourworldindata.org/cancer (accessed on 23 April 2023).
- Labi, V.; Erlacher, M. How Cell Death Shapes Cancer. Cell Death Dis. 2015, 6, e1675. [Google Scholar] [CrossRef] [PubMed]
- Delbridge, A.R.; Grabow, S.; Strasser, A.; Vaux, D.L. Thirty Years of BCL-2: Translating Cell Death Discoveries into Novel Cancer Therapies. Nat. Rev. Cancer 2016, 16, 99–109. [Google Scholar] [CrossRef]
- Ouyang, Z.; Guo, X.; Chen, X.; Liu, B.; Zhang, Q.; Yin, Z.; Zhai, Z.; Qu, X.; Liu, X.; Peng, D.; et al. Hypericin Targets Osteoclast and Prevents Breast Cancer-Induced Bone Metastasis via NFATc1 Signaling Pathway. Oncotarget 2017, 9, 1868–1884. [Google Scholar] [CrossRef]
- Comșa, Ș.; Cîmpean, A.M.; Raica, M. The Story of MCF-7 Breast Cancer Cell Line: 40 Years of Experience in Research. Anticancer Res. 2015, 35, 3147–3154. [Google Scholar]
- Jendželovský, R.; Jendželovská, Z.; Kuchárová, B.; Fedoročko, P. Breast Cancer Resistance Protein Is the Enemy of Hypericin Accumulation and Toxicity of Hypericin-Mediated Photodynamic Therapy. Biomed. Pharmacother. 2019, 109, 2173–2181. [Google Scholar] [CrossRef]
- Jendzelovský, R.; Mikeš, J.; Koval, J.; Soucek, K.; Procházková, J.; Kello, M.; Sacková, V.; Hofmanová, J.; Kozubík, A.; Fedorocko, P. Drug Efflux Transporters, MRP1 and BCRP, Affect the Outcome of Hypericin-Mediated Photodynamic Therapy in HT-29 Adenocarcinoma Cells. Photochem. Photobiol. Sci. 2009, 8, 1716–1723. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Gu, X.; Li, G.; Bao, Z.; Li, L. Mitochondrial Mechanisms of Necroptosis in Liver Diseases. Int. J. Mol. Sci. 2021, 22, 66. [Google Scholar] [CrossRef] [PubMed]
- Vasan, K.; Werner, M.; Chandel, N.S. Mitochondrial Metabolism as a Target for Cancer Therapy. Cell Metab. 2020, 32, 341–352. [Google Scholar] [CrossRef]
- Luo, X.; Gong, X.; Su, L.; Lin, H.; Yang, Z.; Yan, X.; Gao, J. Activatable Mitochondria-Targeting Organoarsenic Prodrugs for Bioenergetic Cancer Therapy. Angew. Chem. Int. Ed. 2021, 60, 1403–1410. [Google Scholar] [CrossRef]
- Cui, L.; Gouw, A.M.; LaGory, E.L.; Guo, S.; Attarwala, N.; Tang, Y.; Qi, J.; Chen, Y.-S.; Gao, Z.; Casey, K.M.; et al. Mitochondrial Copper Depletion Suppresses Triple-Negative Breast Cancer in Mice. Nat. Biotechnol. 2021, 39, 357–367. [Google Scholar] [CrossRef]
- Theodossiou, T.A.; Olsen, C.E.; Jonsson, M.; Kubin, A.; Hothersall, J.S.; Berg, K. The Diverse Roles of Glutathione-Associated Cell Resistance Against Hypericin Photodynamic Therapy. Redox Biol. 2017, 12, 191–197. [Google Scholar] [CrossRef]
- Mokoena, D.; George, B.P.; Abrahamse, H. Cannabidiol Combination Enhances Photodynamic Therapy Effects on MCF-7 Breast Cancer Cells. Cells 2024, 13, 187. [Google Scholar] [CrossRef]
- Blank, M.; Lavie, G.; Mandel, M.; Keisari, Y. Effects of Photodynamic Therapy with Hypericin in Mice Bearing Highly Invasive Solid Tumors. Oncol. Res. 2000, 12, 409–418. [Google Scholar] [CrossRef]
- Oleinick, N.L.; Morris, R.L.; Belichenko, I. The Role of Apoptosis in Response to Photodynamic Therapy: What, Where, Why, and How. Photochem. Photobiol. Sci. 2002, 1, 1–21. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.V.F.; Shinobu-Mesquita, C.S.; Meirelles, L.E.F.; Mari, N.L.; César, G.B.; Gonçalves, R.S.; Caetano, W.; Damke, E.; Silva, V.R.; Damke, G.M. Effects of Hypericin Encapsulated on Pluronic F127 Photodynamic Therapy Against Triple Negative Breast Cancer. Photodiagnosis Photodyn. Ther. 2021, 34, 102206. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, X.; Cheng, W.; Wang, Y.; Yi, K.; Wang, Z.; Zhang, Y.; Shao, L.; Zhao, T. Hypericin-Photodynamic Therapy Inhibits the Growth of Adult T-Cell Leukemia Cells through Induction of Apoptosis and Suppression of Viral Transcription. Retrovirology 2019, 16, 5. [Google Scholar] [CrossRef]
- Theodossiou, T.A.; Ali, M.; Grigalavicius, M.; Grallert, B.; Dillard, P.; Schink, K.O.; Olsen, C.E.; Wälchli, S.; Inderberg, E.M.; Kubin, A.; et al. Simultaneous Defeat of MCF7 and MDA-MB-231 Resistances by a Hypericin PDT–Tamoxifen Hybrid Therapy. NPJ Breast Cancer 2019, 5, 13. [Google Scholar] [CrossRef]
- Lange, C.; Lehmann, C.; Mahler, M.; Bednarski, P.J. Comparison of Cellular Death Pathways after mTHPC-Mediated Photodynamic Therapy (PDT) in Five Human Cancer Cell Lines. Cancers 2019, 11, 702. [Google Scholar] [CrossRef] [PubMed]
- Haake, L.R.; El Menuawy, A.; Rennau, H.; Marthe, F.; Hähnel, U.; Bock, F.; Hildebrandt, G.; Manda, K. Viability and Radiosensitivity of Human Tumor Cells from Breast and Colon Are Influenced by Hypericum perforatum Extract HP01. Int. J. Mol. Sci. 2025, 26, 622. [Google Scholar] [CrossRef]
- Woźniak, M.; Nowak-Perlak, M. Hypericin-Based Photodynamic Therapy Displays Higher Selectivity and Phototoxicity Towards Melanoma and Squamous Cell Cancer Compared to Normal Keratinocytes In Vitro. Int. J. Mol. Sci. 2023, 24, 16897. [Google Scholar] [CrossRef]
- Koval, J.; Mikeš, J.; Jendzelovský, R.; Kello, M.; Solár, P.; Fedorocko, P. Degradation of HER2 Receptor through Hypericin-Mediated Photodynamic Therapy. Photochem. Photobiol. Sci. 2010, 9, 200–205. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Agawal, M.L.; Larkin, H.E.; Friedman, L.R.; Xue, L.Y.; Oleinick, N.L. The Induction of Partial Resistance to Photodynamic Therapy by the Proto-Oncogene BCL-2. Photochem. Photobiol. 1996, 64, 845–852. [Google Scholar] [CrossRef]
- Kim, H.R.; Luo, Y.; Li, G.; Kessel, D. Enhanced Apoptotic Response to Photodynamic Therapy after BCL-2 Transfection. Cancer Res. 1999, 59, 3429–3432. [Google Scholar] [PubMed]
- Oltvai, Z.N.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 Heterodimerizes In Vivo with a Conserved Homolog, Bax, that Accelerates Programmed Cell Death. Cell 1993, 74, 609–617. [Google Scholar] [CrossRef]
- Srivastava, M.; Ahmad, N.; Gupta, S.; Mukhtar, H. Involvement of Bcl-2 and Bax in Photodynamic Therapy-Mediated Apoptosis. Antisense Bcl-2 Oligonucleotide Sensitizes RIF 1 Cells to Photodynamic Therapy Apoptosis. J. Biol. Chem. 2001, 276, 15481–15488. [Google Scholar] [CrossRef]
- Yuan, H.; Jiang, A.; Fang, H.; Chen, Y.; Guo, Z. Optical Properties of Natural Small Molecules and Their Applications in Imaging and Nanomedicine. Adv. Drug Deliv. Rev. 2021, 179, 113917. [Google Scholar] [CrossRef]
- Liu, Y.; Long, K.; Wang, T.; Kang, W.; Wang, W. Carrier-Free Nanodrugs for Stemness Inhibition-Enhanced Photodynamic Therapy. Aggregate 2023, 4, e284. [Google Scholar] [CrossRef]
- Lippitz, B.E.; Harris, R.A. Cytokine Patterns in Cancer Patients: A Review of the Correlation Between Interleukin 6 and Prognosis. Oncoimmunology 2016, 5, e1093722. [Google Scholar] [CrossRef]
- Wang, H.; Yang, X. Association between Serum Cytokines and Progression of Breast Cancer in Chinese Population. Medicine (Baltimore) 2017, 96, e8840. [Google Scholar] [CrossRef] [PubMed]
- Badache, A.; Hynes, N.E. Interleukin-6 Inhibits Proliferation and, in Cooperation with an Epidermal Growth Factor Receptor Autocrine Loop, Increases Migration of T47D Breast Cancer Cells. Cancer Res. 2001, 61, 383–391. [Google Scholar]
- Danforth, D.N., Jr.; Sgagias, M.K. Interleukin-1 Alpha and Interleukin-6 Act Additively to Inhibit Growth of MCF-7 Breast Cancer Cells In Vitro. Cancer Res. 1993, 53, 1538–1545. [Google Scholar]
- Honma, S.; Shimodaira, K.; Shimizu, Y.; Tsuchiya, N.; Saito, H.; Yanaihara, T.; Okai, T. The Influence of Inflammatory Cytokines on Estrogen Production and Cell Proliferation in Human Breast Cancer Cells. Endocr. J. 2002, 49, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Conze, D.; Weiss, L.; Regan, P.S.; Bhushan, A.; Weaver, D.; Johnson, P.; Rincon, M. Autocrine Production of Interleukin-6 Causes Multidrug Resistance in Breast Cancer Cells. Cancer Res. 2001, 61, 8851–8858. [Google Scholar] [PubMed]
- Fukada, T.; Hibi, M.; Yamanaka, Y.; Takahishi-Tezuka, M.; Fujitani, Y.; Yamaguchi, T.; Nakajima, K.; Hirano, T. Two Signals Are Necessary for Cell Proliferation Induced by a Cytokine Receptor GP130: Involvement of STAT3 in Anti-Apoptosis. Immunity 1996, 5, 449–460. [Google Scholar] [CrossRef]
- Fukada, T.; Ohtani, T.; Yoshida, Y.; Shirogane, T.; Nishida, K.; Nakajima, K.; Hibi, M.; Hirano, T. STAT3 Orchestrates Contradictory Signals in Cytokine-Induced G1 to S Cell-Cycle Transition. EMBO J. 1998, 17, 6670–6677. [Google Scholar] [CrossRef]
- Horiguchi, A.; Oya, M.; Marumo, K.; Murai, M. STAT3, but Not ERKs, Mediates the IL-Induced Proliferation of Renal Cancer Cells, ACHN and 769P. Kidney Int. 2002, 61, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Bromberg, J.F.; Wrzeszczynska, M.H.; Devgan, G.; Zhao, Y.; Pestell, R.G.; Albanese, C.; Darnell, J.E. STAT3 as an Oncogene. Cell 1999, 98, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T. Molecular Basis Underlying Functional Pleiotropy of Cytokines and Growth Factors. Biochem. Biophys. Res. Commun. 1999, 260, 303–308. [Google Scholar] [CrossRef]
- Minniti De Sanctis, V.; Agolli, L.; Visco, V.; Monaco, F.; Muni, R.; Spagnoli, A.; Campanella, B.; Valeriani, M.; Osti, M.F.; Amanti, C. Cytokines, Fatigue, and Cutaneous Erythema in Early Stage Breast Cancer Patients Receiving Adjuvant Radiation Therapy. Biomed. Res. Int. 2014, 2014, 523568. [Google Scholar] [CrossRef]
- Muraro, E.; Furlan, C.; Avanzo, M.; Martorelli, D.; Comaro, E.; Rizzo, A.; Fae, D.A.; Berretta, M.; Militello, L.; Del Conte, A.; et al. Local High-Dose Radiotherapy Induces Systemic Immunomodulating Effects of Potential Therapeutic Relevance in Oligometastatic Breast Cancer. Front. Immunol. 2017, 8, 1476. [Google Scholar] [CrossRef]
- Li, L.; Chen, L.; Zhang, W.; Liao, Y.; Chen, J.; Shi, Y.; Luo, S. Serum Cytokine Profile in Patients with Breast Cancer. Cytokine 2017, 89, 173–178. [Google Scholar] [CrossRef]
- Ahmad, N.; Ammar, A.; Storr, S.J.; Green, A.R.; Rakha, E.; Ellis, I.O.; Martin, S.G. IL-6 and IL-10 Are Associated with Good Prognosis in Early Stage Invasive Breast Cancer Patients. Cancer Immunol. Immunother. 2018, 67, 537–549. [Google Scholar] [CrossRef]
- Conroy, S.M.; Courneya, K.S.; Brenner, D.R.; Shaw, E.; O’Reilly, R.; Yasui, Y.; Woolcott, C.G.; Friedenreich, C.M. Impact of Aerobic Exercise on Levels of IL-4 and IL-10: Results from Two Randomized Intervention Trials. Cancer Med. 2016, 5, 2385–2397. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, R.; Kundu, A.; Kundu, C.N. The Cytokines in Tumor Microenvironment: From Cancer Initiation-Elongation-Progression to Metastatic Outgrowth. Crit. Rev. Oncol. Hematol. 2024, 196, 104311. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 9001:2015 Quality Management Systems—Requirements. Available online: https://www.iso.org/standard/44929.html (accessed on 20 February 2025).
- Gaona-Esquivel, A.; Hernandez-M, D.S.; Hernández-Rodríguez, Y.M.; Cigarroa-Mayorga, O.E. The Role of Nd as a Dopant in Mn3O4NPs on the Heat Induction of Artificial Breast Tissue Due to the Irradiation of Microwaves. Mater. Chem. Phys. 2022, 292, 126822. [Google Scholar] [CrossRef]

| Hypericin (µM) | Trypan Blue Viability (%) | MTT Metabolic Activity (%) |
|---|---|---|
| 0 (control) | 98.9 ± 0.3 | 95 ± 3 |
| 0.125 | 95 ± 2.3 | 98 ± 5 |
| 0.25 | 93 ± 2 | 93 ± 4 |
| 0.5 | 84 ± 15 | 86 ± 6 |
| 1.0 | 87 ± 5 | 84 ± 2 |
| Ratio | Value |
|---|---|
| IL-6:TNF-α | 0.1:1.7 |
| IL-8:TNF-α | 3.6:1.7 |
| IL-10:TNF-α | 0.1:1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czarnecka-Czapczyńska, M.; Czuba, Z.; Aebisher, D.; Mytych, W.; Fiegler-Rudol, J.; Wiench, R.; Kawczyk-Krupka, A. Hypericin Photodynamic Therapy Induces Cytotoxicity and Modulates Cytokine Secretion in MCF-7 Breast Cancer Cells. J. Clin. Med. 2025, 14, 7514. https://doi.org/10.3390/jcm14217514
Czarnecka-Czapczyńska M, Czuba Z, Aebisher D, Mytych W, Fiegler-Rudol J, Wiench R, Kawczyk-Krupka A. Hypericin Photodynamic Therapy Induces Cytotoxicity and Modulates Cytokine Secretion in MCF-7 Breast Cancer Cells. Journal of Clinical Medicine. 2025; 14(21):7514. https://doi.org/10.3390/jcm14217514
Chicago/Turabian StyleCzarnecka-Czapczyńska, Magdalena, Zenon Czuba, David Aebisher, Wiktoria Mytych, Jakub Fiegler-Rudol, Rafał Wiench, and Aleksandra Kawczyk-Krupka. 2025. "Hypericin Photodynamic Therapy Induces Cytotoxicity and Modulates Cytokine Secretion in MCF-7 Breast Cancer Cells" Journal of Clinical Medicine 14, no. 21: 7514. https://doi.org/10.3390/jcm14217514
APA StyleCzarnecka-Czapczyńska, M., Czuba, Z., Aebisher, D., Mytych, W., Fiegler-Rudol, J., Wiench, R., & Kawczyk-Krupka, A. (2025). Hypericin Photodynamic Therapy Induces Cytotoxicity and Modulates Cytokine Secretion in MCF-7 Breast Cancer Cells. Journal of Clinical Medicine, 14(21), 7514. https://doi.org/10.3390/jcm14217514







