Predictors of Impaired Exercise Performance in Patients Qualified for Cardiac Rehabilitation: The Impact of Sex and Comorbidities
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Parameters
2.3. Cardiopulmonary Exercise Test
2.4. Six-Minute Walk Test
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Comparison Between Patients with and Without an Increased VE/VCO2 Slope and Between Patients with and Without Significantly Reduced Peak VO2
3.3. Predictors of Increased VE/VCO2 Slope
3.4. Predictors of Significantly Reduced Peak VO2
4. Discussion
4.1. Exercise Capacity Parameters and Sex
4.2. Comorbidities Burden as a Determinant of Exercise Capacity and Ventilation
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 6MWT | 6-minute walk test |
| 6MWTd | distance covered during 6-minute walk test |
| BMI | body mass index |
| CAD | coronary artery disease |
| CCI | Charlson Comorbidity Index |
| CI | confidence interval |
| CKD | Chronic kidney disease |
| CPET | cardiopulmonary exercise test |
| eGFR | estimated glomerular filtration rate |
| GLS | global longitudinal strain |
| HF | heart failure |
| IQR | interquartile range |
| LV | left ventricular |
| NYHA | New York Heart Association |
| OR | odds ratio |
| PAD | peripheral artery disease |
| PCI | percutaneous coronary intervention |
| T2DM | type 2 diabetes mellitus |
| VE/VCO2 | slope ventilation-to-carbon dioxide output slope |
| VO2 | oxygen consumption |
References
- Martens, P.; Augusto, S.N.; Finet, J.E., Jr.; Tang, W.H.W. Distinct Impact of Noncardiac Comorbidities on Exercise Capacity and Functional Status in Chronic Heart Failure. JACC Heart Fail. 2023, 11, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.C.; Lavie, C.J.; Sui, X.; Blair, S.N. Running and Mortality: Is More Actually Worse? Mayo Clin. Proc. 2016, 91, 534–536. [Google Scholar] [CrossRef] [PubMed]
- Hautamäki, M.; Lyytikäinen, L.P.; Mahdiani, S.; Eskola, M.; Lehtimäki, T.; Nikus, K.; Antila, K.; Oksala, N.; Hernesniemi, J. The association between charlson comorbidity index and mortality in acute coronary syndrome—The MADDEC study. Scand. Cardiovasc. J. 2020, 54, 146–152. [Google Scholar] [CrossRef]
- Karabağ, T.; Altuntaş, E.; Kalaycı, B.; Şahin, B.; Somuncu, M.U.; Çakır, M.O. The relationship of Charlson comorbidity index with stent restenosis and extent of coronary artery disease. Interv. Med. Appl. Sci. 2018, 10, 70–75. [Google Scholar] [CrossRef]
- Ho, T.A.; Lio, K.U.; Patel, P.; Wang, Y.; Arshad, H.; Li, S.; Rali, P. Comorbidity profiles and pulmonary embolism risk assessment: Leveraging the Charlson Comorbidity Index for improved prognostication in a national data set. Pulm. Circ. 2024, 14, e70010. [Google Scholar] [CrossRef]
- Lin, H.; Xi, Y.B.; Yang, Z.C.; Tong, Z.J.; Jiang, G.; Gao, J.; Kang, B.; Ma, Y.; Zhang, W.; Wang, Z.H. Optimizing Prediction of In-Hospital Mortality in Elderly Patients with Acute Myocardial Infarction: A Nomogram Approach Using the Age-Adjusted Charlson Comorbidity Index Score. J. Am. Heart Assoc. 2024, 13, e032589. [Google Scholar] [CrossRef]
- Górski, J. Fizjologiczne Podstawy Wysiłku Fizycznego; PWN: Warsaw, Poland, 2023. [Google Scholar]
- Keteyian, S.J.; Brawner, C.A.; Savage, P.D.; Ehrman, J.K.; Schairer, J.; Divine, G.; Aldred, H.; Ophaug, K.; Ades, P.A. Peak aerobic capacity predicts prognosis in patients with coronary heart disease. Am. Heart J. 2008, 156, 292–300. [Google Scholar] [CrossRef]
- Guazzi, M.; Arena, R.; Halle, M.; Piepoli, M.F.; Myers, J.; Lavie, C.J. 2016 Focused Update: Clinical Recommendations for Cardiopulmonary Exercise Testing Data Assessment in Specific Patient Populations. Circulation 2016, 133, e694–e711. [Google Scholar] [CrossRef]
- Francis, D.P.; Shamim, W.; Davies, L.C.; Piepoli, M.F.; Ponikowski, P.; Anker, S.D.; Coats, A.J. Cardiopulmonary exercise testing for prognosis in chronic heart failure: Continuous and independent prognostic value from VE/VCO2slope and peak VO2. Eur. Heart J. 2000, 21, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Agostoni, P.; Arena, R.; Belardinelli, R.; Dumitrescu, D.; Hager, A.; Myers, J.; Rauramaa, R.; Riley, M.; Takken, T.; et al. The Role of Gas Exchange Variables in Cardiopulmonary Exercise Testing for Risk Stratification and Management of Heart Failure with Reduced Ejection Fraction. Am. Heart J. 2018, 202, 116–126. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, X.; Ma, W.; Song, H.; Gong, Z.; Wang, Q.; Che, L.; Xu, W.; Jiang, J.; Xu, J.; et al. VE/VCO2 slope and its prognostic value in patients with chronic heart failure. Exp. Ther. Med. 2015, 9, 1407–1412. [Google Scholar] [CrossRef]
- Malhotra, R.; Bakken, K.; D’Elia, E.; Lewis, G.D. Cardiopulmonary Exercise Testing in Heart Failure. JACC Heart Fail. 2016, 4, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Kosinski, C.; Besson, C.; Amati, F. Exercise Testing in Individuals with Diabetes, Practical Considerations for Exercise Physiologists. Front. Physiol. 2019, 10, 1257. [Google Scholar] [CrossRef]
- Hayden, C.M.; Begue, G.; Gamboa, J.L.; Baar, K.; Roshanravan, B. Review of Exercise Interventions to Improve Clinical Outcomes in Nondialysis CKD. Kidney Int. Rep. 2024, 9, 3097–3115. [Google Scholar] [CrossRef]
- Cersosimo, A.; Longo Elia, R.; Condello, F.; Colombo, F.; Pierucci, N.; Arabia, G.; Matteucci, A.; Metra, M.; Adamo, M.; Vizzardi, E.; et al. Cardiac rehabilitation in patients with atrial fibrillation. Minerva Cardiol. Angiol. 2025. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/publications/i/item/9789240088542 (accessed on 8 February 2025).
- Available online: https://www.mdcalc.com/calc/3917/charlson-comorbidity-index-cci#evidence (accessed on 8 February 2025).
- Wasserman, K.; Hansen, J.E.; Sue, D.Y.; Stringer, W.W.; Whipp, B.J. Clinical exercise testing. Principles of exercise testing and interpretation including pathophysiology and clinical applications. Med. Sci. Sports. Exerc. 2005, 37, 1249. [Google Scholar]
- Hansen, J.E.; Sue, D.Y.; Wasserman, K. Predicted values for clinical exercise testing. Am. Rev. Respir. Dis. 1984, 129, S49–S55. [Google Scholar] [CrossRef] [PubMed]
- Glaab, T.; Taube, C. Practical guide to cardiopulmonary exercise testing in adults. Respir. Res. 2022, 23, 9. [Google Scholar] [CrossRef]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Enright, P.L.; Sherrill, D.L. Reference equations for the six-minute walk in healthy adults. Am. J. Respir. Crit. Care Med. 1998, 158, 1384–1387, Erratum in Am. J. Respir. Crit. Care Med. 2020, 201, 393. [Google Scholar] [CrossRef]
- Giannitsi, S.; Bougiakli, M.; Bechlioulis, A.; Kotsia, A.; Michalis, L.K.; Naka, K.K. 6-minute walking test: A useful tool in the management of heart failure patients. Ther. Adv. Cardiovasc. Dis. 2019, 13, 1753944719870084. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salvioni, E.; Corrà, U.; Piepoli, M.; Rovai, S.; Correale, M.; Paolillo, S.; Pasquali, M.; Magrì, D.; Vitale, G.; MECKI Score Research Group; et al. Gender and age normalization and ventilation efficiency during exercise in heart failure with reduced ejection fraction. ESC Heart Fail. 2020, 7, 371–380. [Google Scholar] [CrossRef]
- Neder, J.A.; Nery, L.E.; Peres, C.; Whipp, B.J. Reference values for dynamic responses to incremental cycle ergometry in males and females aged 20 to 80. Am. J. Respir. Crit. Care Med. 2001, 164, 1481–1486. [Google Scholar] [CrossRef]
- Sun, X.G.; Hansen, J.E.; Garatachea, N.; Storer, T.W.; Wasserman, K. Ventilatory efficiency during exercise in healthy subjects. Am. J. Respir. Crit. Care Med. 2002, 166, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Arena, R.; Myers, J.; Hsu, L.; Peberdy, M.A.; Pinkstaff, S.; Bensimhon, D.; Chase, P.; Vicenzi, M.; Guazzi, M. The minute ventilation/carbon dioxide production slope is prognostically superior to the oxygen uptake efficiency slope. J. Card. Fail. 2007, 13, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.B.; Collins, S.É.; Stickland, M.K. Measurement and Interpretation of Exercise Ventilatory Efficiency. Front. Physiol. 2020, 11, 659. [Google Scholar] [CrossRef]
- Corrà, U.; Agostoni, P.; Giordano, A.; Cattadori, G.; Battaia, E.; La Gioia, R.; Scardovi, A.B.; Emdin, M.; Metra, M.; MECKI Score Research Group; et al. Sex Profile and Risk Assessment with Cardiopulmonary Exercise Testing in Heart Failure: Propensity Score Matching for Sex Selection Bias. Can. J. Cardiol. 2016, 32, 754–759. [Google Scholar] [CrossRef]
- Hsich, E.; Chadalavada, S.; Krishnaswamy, G.; Starling, R.C.; Pothier, C.E.; Blackstone, E.H.; Lauer, M.S. Long-term prognostic value of peak oxygen consumption in women versus men with heart failure and severely impaired left ventricular systolic function. Am. J. Cardiol. 2007, 100, 291–295. [Google Scholar] [CrossRef]
- Guazzi, M.; Arena, R.; Myers, J. Comparison of the prognostic value of cardiopulmonary exercise testing between male and female patients with heart failure. Int. J. Cardiol. 2006, 113, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.J.; Barros-Murphy, J.F.; Burns, A.T.; MacIsaac, R.J.; MacIsaac, A.I.; Prior, D.L.; La Gerche, A. Reduced Exercise Capacity in Diabetes Mellitus Is Not Associated with Impaired Deformation or Twist. J. Am. Soc. Echocardiogr. 2020, 33, 481–489. [Google Scholar] [CrossRef]
- Gojevic, T.; Van Ryckeghem, L.; Jogani, S.; Frederix, I.; Bakelants, E.; Petit, T.; Stroobants, S.; Dendale, P.; Bito, V.; Herbots, L.; et al. Pulmonary hypertension during exercise underlies unexplained exertional dyspnoea in patients with Type 2 diabetes. Eur. J. Prev. Cardiol. 2023, 30, 37–45. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; ESC Scientific Document Group; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Nayor, M.; Xanthakis, V.; Tanguay, M.; Blodgett, J.B.; Shah, R.V.; Schoenike, M.; Sbarbaro, J.; Farrell, R.; Malhotra, R.; Houstis, N.E.; et al. Clinical and Hemodynamic Associations and Prognostic Implications of Ventilatory Efficiency in Patients with Preserved Left Ventricular Systolic Function. Circ. Heart Fail. 2020, 13, e006729. [Google Scholar] [CrossRef] [PubMed]
- Ojima, S.; Kubozono, T.; Kawasoe, S.; Kawabata, T.; Salim, A.A.; Ikeda, Y.; Ohishi, M. VE/VCO2 slope in cardiopulmonary exercise testing was associated with left ventricular diastolic dysfunction in patients with reduced ejection fraction. Eur. Heart J. 2023, 44, ehad655.2585. [Google Scholar] [CrossRef]
- Coisne, A.; Aghezzaf, S.; Galli, E.; Mouton, S.; Richardson, M.; Dubois, D.; Delsart, P.; Domanski, O.; Bauters, C.; Charton, M.; et al. Prognostic values of exercise echocardiography and cardiopulmonary exercise testing in patients with primary mitral regurgitation. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 1552–1561. [Google Scholar] [CrossRef]
- Van Ryckeghem, L.; Keytsman, C.; Verboven, K.; Verbaanderd, E.; Frederix, I.; Bakelants, E.; Petit, T.; Jogani, S.; Stroobants, S.; Dendale, P.; et al. Exercise capacity is related to attenuated responses in oxygen extraction and left ventricular longitudinal strain in asymptomatic type 2 diabetes patients. Eur. J. Prev. Cardiol. 2022, 28, 1756–1766. [Google Scholar] [CrossRef]
- Hansen, D.; Abreu, A.; Ambrosetti, M.; Cornelissen, V.; Gevaert, A.; Kemps, H.; Laukkanen, J.A.; Pedretti, R.; Simonenko, M.; Wilhelm, M.; et al. Exercise intensity assessment and prescription in cardiovascular rehabilitation and beyond: Why and how: A position statement from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur. J. Prev. Cardiol. 2022, 29, 230–245. [Google Scholar] [CrossRef]
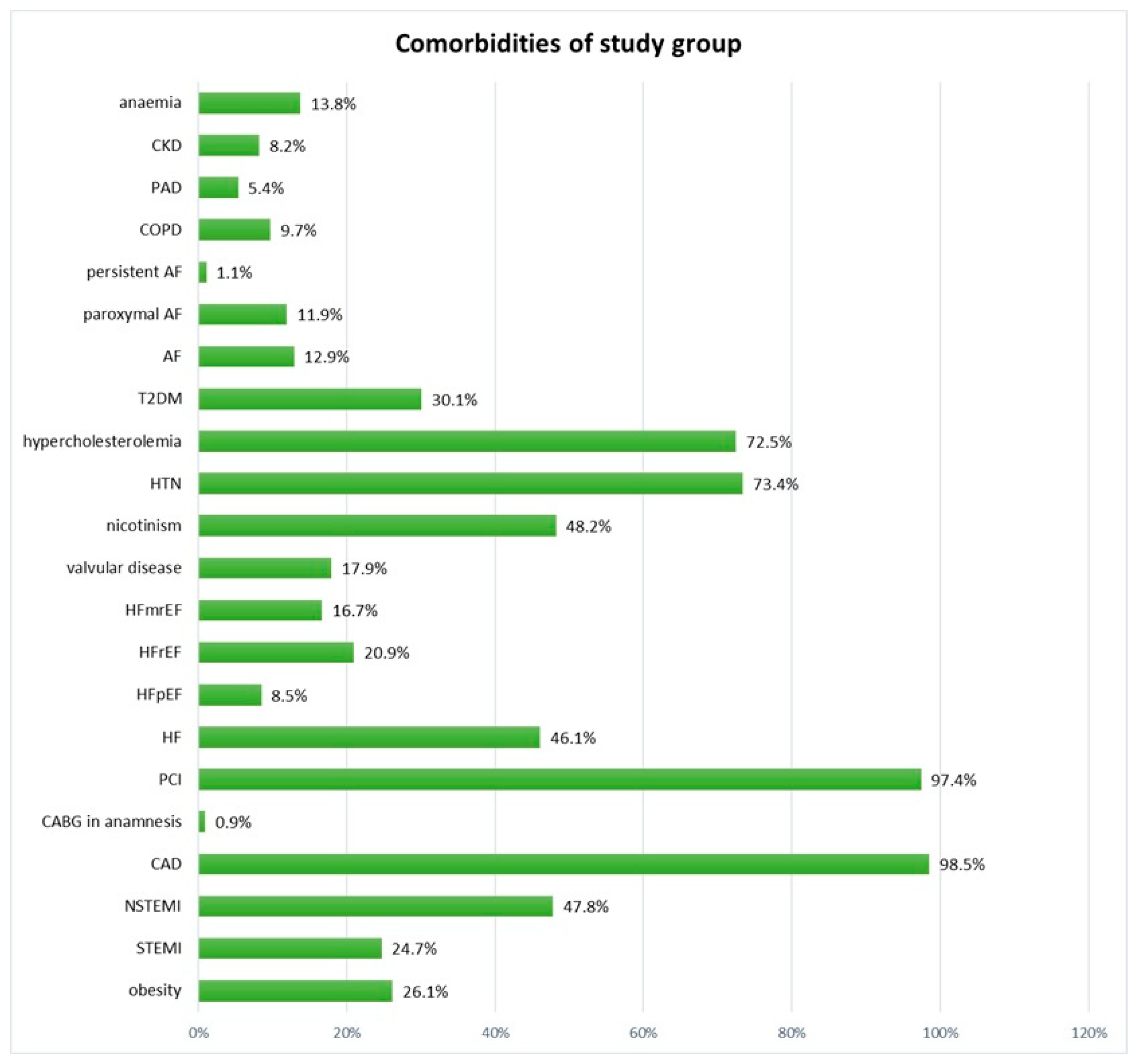
| Study Group (n = 425) Median (Q1–Q3); n (%) | |
|---|---|
| Age [years] | 63 (56–70) |
| Sex [male] | 316 (74.3) |
| Systolic blood pressure [mmHg] | 130 (120–142) |
| Diastolic blood pressure [mmHg] | 77 (70–84) |
| Heart rate [bpm] | 69 (63–75) |
| BMI [kg/m2] | 27.5 (25.3–30) |
| Charlson Comorbidity Index score [points] | 4 (3–5) |
| Symptoms and signs | |
| Edema | 23 (5.4) |
| Pulmonary circulatory congestion | 33 (7.8) |
| Dyspnea on exertion | 78 (18.4) |
| Dyspnea at rest | 10 (2.4) |
| rapid fatigue during exercise | 225 (52.9) |
| NYHA class 0 | 350 (82.4) |
| NYHA class I | 3 (0.7) |
| NYHA class II | 49 (11.5) |
| NYHA class III | 23 (5.4) |
| Chest pain | 45 (10.6) |
| CCS grade 0 | 401 (94.4) |
| CCS grade 1 | 2 (0.5) |
| CCS grade 2 | 19 (4.5) |
| CCS grade 3 | 3 (0.7) |
| Palpitations | 32 (7.5) |
| Echocardiography parameters | |
| LVEF [%] | 50 (42–60) |
| LVEF < 50% | 175 (41.2) |
| LVDD grade 0 | 276 (65.4) |
| LVDD grade 1 | 117 (27.5) |
| LVDD grade 2 | 18 (4.2) |
| LVDD grade 3 | 4 (0.9) |
| LVDD grade 4 | 8 (1.9) |
| LVDD | 147 (34.6) |
| Treatment at admission | |
| Statin | 324 (76.2) |
| Statin with ezetimibe | 93 (21.9) |
| ACE inhibitor/ARB/ARNI | 393 (92.5) |
| Beta-blocker | 374 (88.0) |
| CCB | 128 (30.1) |
| Diuretic | 194 (45.7) |
| MRA | 123 (28.9) |
| Metformin | 104 (24.5) |
| SGLT2 inhibitor | 128 (30.1) |
| Amiodarone | 28 (6.6) |
| Exercise tests parameters | |
| VE/VCO2 slope | 31.2 (27.6–35.3) |
| Patients with VE/VCO2 slope ≥ 36 | 87 (20.8) |
| Peak VO2 [mL/min/kg] | 17 (14–22) |
| Predicted peak VO2 [%] | 78 (67–89) |
| Patients with peak VO2 < 70% of predicted | 124 (29.4) |
| Patients with peak VO2 ≥ 85% | 281 (66.6) |
| 6MWD [m] | 555 (488–611) |
| Predicted 6MWD [%] | 99.8 (89.3–107.3) |
| Patients with 6MWD ≥ 100% of predicted | 208 (50.4) |
| Laboratory parameters | |
| HGB [g/dL] | 13.5 (12.6–14.4) |
| Creatinine [mg/dL] | 1.0 (0.8–1.1) |
| eGFR [mL/min/1.73 m2] | 87.1 (69.0–105.5) |
| eGFR > 90 mL/min/1.73 m2 | 191 (44.9) |
| eGFR ≥ 60 and <90 mL/min/1.73 m2 | 166 (39.1) |
| eGFR ≥ 45 and <60 mL/min/1.73 m2 | 41 (9.6) |
| eGFR ≥ 30 and <45 mL/min/1.73 m2 | 16 (3.8) |
| eGFR ≥ 15 and <30 mL/min/1.73 m2 | 3 (0.7) |
| Urea [mg/dL] | 37 (30–44) |
| Total cholesterol [mg/dL] | 120 (106–145) |
| Low-density lipoproteins [mg/dL] | 63 (48–79) |
| High-density lipoproteins [mg/dL] | 41 (36–50) |
| Triglycerides [mg/dL] | 106 (83–139) |
| Glucose [mg/dL] | 98 (91–110) |
| VE/VCO2 Slope < 36 n = 331 | VE/VCO2 Slope ≥ 36 n = 87 | p-Value | Peak VO2 ≥ 70% of Predicted n = 298 | Peak VO2 < 70% of Predicted n = 124 | p-Value | |
|---|---|---|---|---|---|---|
| Age [years] | 62 (54–69) | 69 (63–74) | 0.210 | 64 (56–70) | 63 (54–70) | 0.0638 |
| Sex [male] | 256 (77.3) | 55 (63.2) | 0.007 | 211 (70.8) | 102 (82.3) | 0.014 |
| Obesity [BMI > 30 kg/m2] | 87 (26.6) | 21 (24.1) | 0.641 | 79 (26.7) | 29 (23.8) | 0.535 |
| SBP [mmHg] | 131 (120–143) | 126 (114–137) | 0.015 | 131 (120–144) | 127 (113–138) | 0.008 |
| DBP [mmHg] | 78 (70–84) | 76 (69–84) | 0.186 | 78 (70–84) | 77 (68–84) | 0.124 |
| HR [bpm] | 68 (62–72) | 72 (67–80) | <0.0001 | 69 (63–74) | 70 (63–78) | 0.320 |
| NYHA class 0 | 286 (86.4) | 58 (66.7) | <0.0001 | 251 (84.2) | 96 (77.4) | 0.019 |
| NYHA class I | 3 (0.9) | 0 (0.0) | 3 (1.0) | 0 (0.0) | ||
| NYHA class II | 30 (9.1) | 18 (20.7) | 34 (11.4) | 15 (12.1) | ||
| NYHA class III | 12 (3.6) | 11 (12.6) | 10 (3.4) | 13 (10.5) | ||
| LVEF [%] | 55 (45–60) | 45 (36–52) | <0.0001 | 55 (45–60) | 45 (38–56) | <0.0001 |
| Patients with LVEF < 50% | 113 (34.4) | 57 (65.5) | <0.0001 | 103 (34.6) | 72 (58.1) | <0.0001 |
| HGB [g/dL] | 13.7 (12.8–14.5) | 12.9 (12.0–14.4) | 0.002 | 13.6 (12.7–14.6) | 13.3 (12.3–14.2) | 0.042 |
| eGFR [mL/min/1.73 m2] | 92 (76–110) | 71 (57–86) | 0.0002 | 90 (71–106) | 81 (64–103) | 0.0003 |
| Charlson Comorbidity Index score [points] | 3 (2–4) | 5 (4–6) | <0.0001 | 3 (2–3) | 4 (3–6) | 0.0004 |
| Coronary artery disease | 324 (97.9) | 86 (98.9) | 0.559 | 294 (98.7) | 120 (96.8) | 0.196 |
| Heart failure | 123 (37.2) | 66 (75.9) | <0.0001 | 113 (37.9) | 82 (66.1) | <0.0001 |
| Hypertension | 238 (77.5) | 69 (79.3) | 0.164 | 216 (72.5) | 94 (75.8) | 0.481 |
| Type 2 diabetes mellitus | 83 (25.1) | 42 (48.3) | <0.0001 | 76 (25.5) | 52 (41.9) | 0.0008 |
| Atrial fibrillation | 42 (12.7) | 13 (14.9) | 0.580 | 35 (11.7) | 20 (16.1) | 0.223 |
| Chronic obstructive pulmonary disease | 30 (9.1) | 11 (12.6) | 0.318 | 26 (8.7) | 15 (12.1) | 0.287 |
| Peripheral artery disease | 12 (3.6) | 10 (11.5) | 0.003 | 10 (3.4) | 13 (10.5) | 0.003 |
| Chronic kidney disease | 14 (4.2) | 20 (23.0) | <0.0001 | 12 (4.0) | 23 (18.6) | <0.0001 |
| Valvular disease | 43 (13.0) | 31 (35.6) | <0.0001 | 39 (13.1) | 37 (29.9) | <0.0001 |
| LVDD | 97 (20.3) | 45 (51.7) | <0.0001 | 90 (30.2) | 57 (46.0) | 0.002 |
| Anemia | 35 (10.8) | 19 (23.2) | 0.003 | 33 (11.3) | 24 (20.2) | 0.019 |
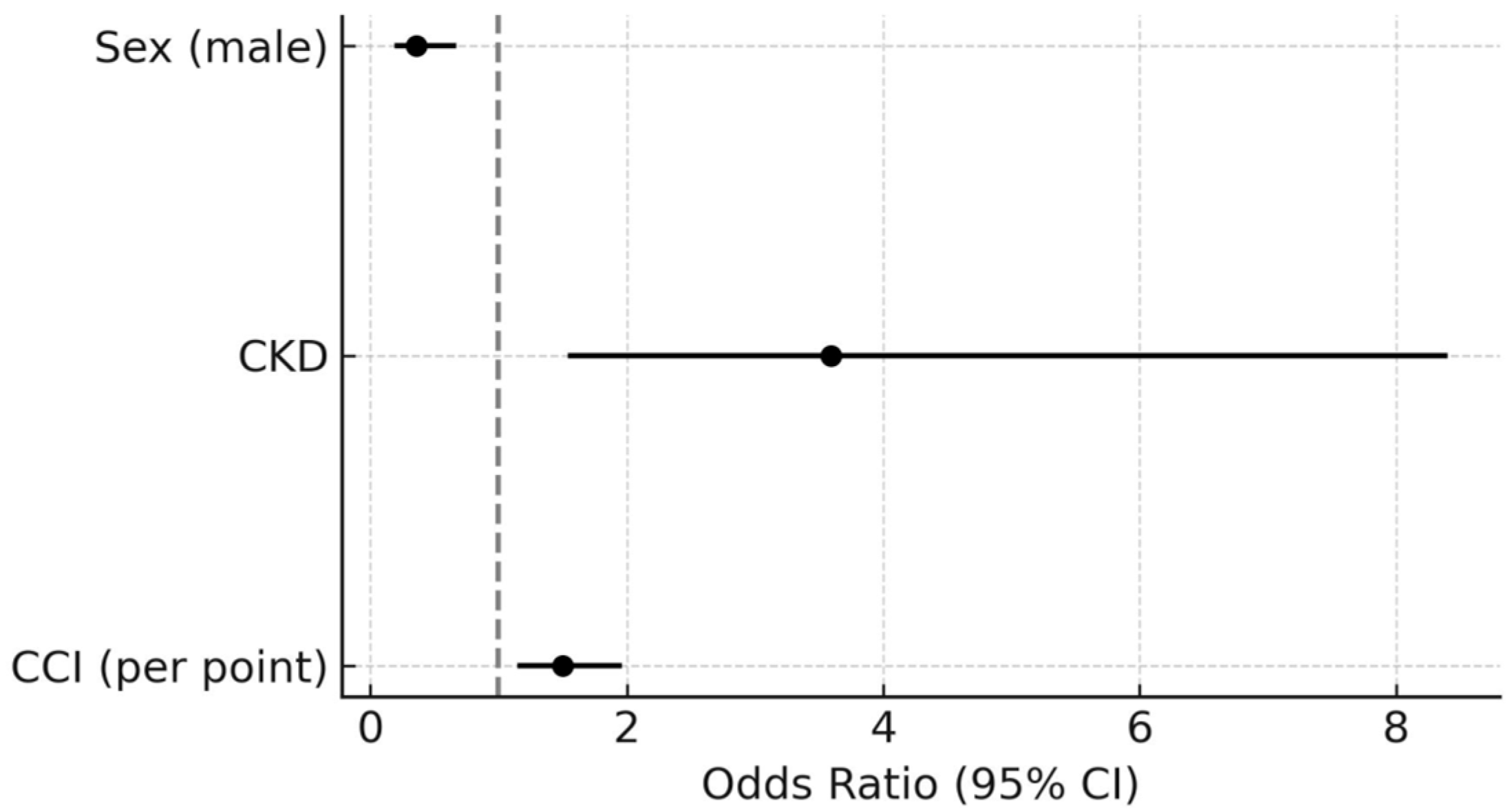
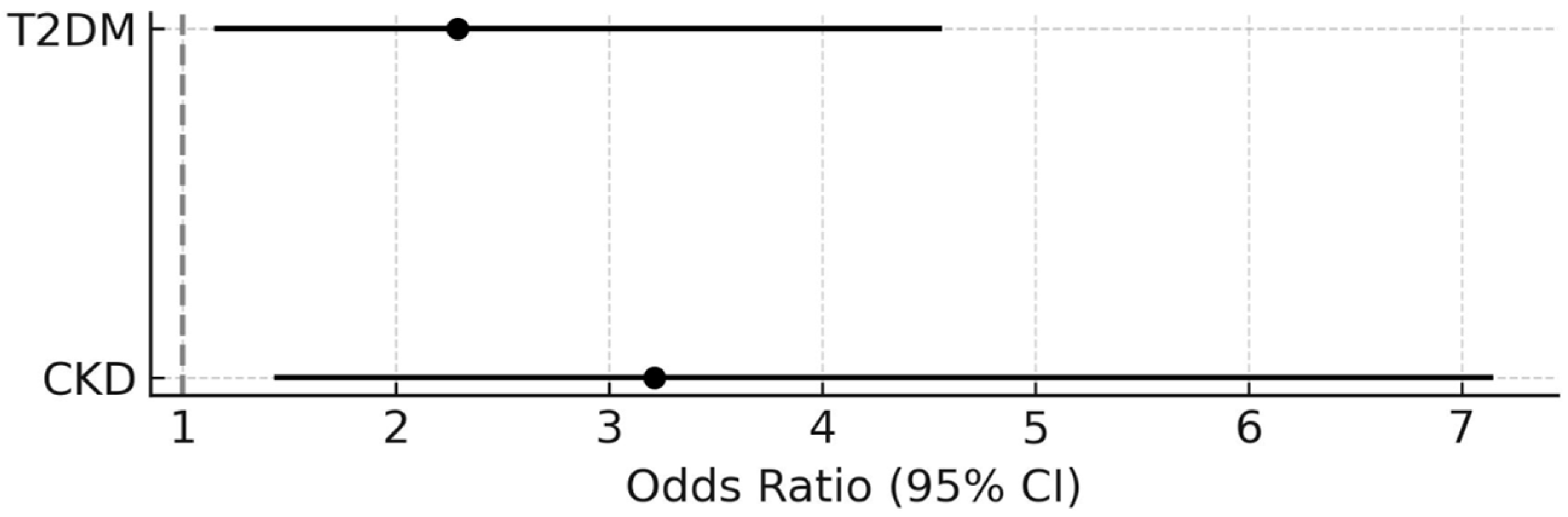
| VE/VCO2 Slope ≥36 | Peak VO2 <70% of Predicted Value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||||||
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Sex (male) | 0.50 | 0.30–0.84 | 0.079 | 0.36 | 0.19–0.67 | 0.001 | 1.91 | 1.13–3.22 | 0.015 | - | - | - |
| LVEF < 50% | 3.67 | 2.23–6.02 | <0.0001 | - | - | - | 2.62 | 1.71–4.03 | <0.0001 | - | - | - |
| HF | 5.32 | 3.10–9.11 | <0.0001 | - | - | - | 3.2 | 2.06–4.96 | <0.0001 | - | - | - |
| T2DM | 2.79 | 1.71–4.55 | <0.0001 | - | - | - | 2.11 | 1.36–3.28 | 0.0009 | 2.29 | 1.15–4.56 | 0.018 |
| PAD | 3.45 | 1.44–8.29 | 0.006 | - | - | - | 3.73 | 1.44–7.92 | 0.005 | - | - | - |
| CKD | 6.76 | 3.25–14.10 | <0.0001 | 3.59 | 1.54–8.40 | 0.003 | 5.43 | 2.61–11.31 | <0.0001 | 3.21 | 1.43–7.15 | 0.004 |
| Valvular disease | 3.71 | 2.15–6.38 | <0.0001 | - | - | - | 2.82 | 1.69–4.71 | 0.0001 | - | - | - |
| LVDD | 2.59 | 1.60–4.19 | 0.0001 | - | - | - | 1.97 | 1.28–3.03 | 0.002 | - | - | - |
| Anemia | 2.49 | 1.34–4.63 | 0.004 | - | - | - | 1.98 | 1.11–3.51 | 0.021 | - | - | - |
| CCI score (per point) | 1.67 | 1.46–1.97 | <0.0001 | 1.5 | 1.15–1.96 | 0.003 | 1.26 | 1.12–1.41 | 0.0001 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurpaska, M.; Krzesiński, P.; Banak, M.; Piotrowicz, K. Predictors of Impaired Exercise Performance in Patients Qualified for Cardiac Rehabilitation: The Impact of Sex and Comorbidities. J. Clin. Med. 2025, 14, 7512. https://doi.org/10.3390/jcm14217512
Kurpaska M, Krzesiński P, Banak M, Piotrowicz K. Predictors of Impaired Exercise Performance in Patients Qualified for Cardiac Rehabilitation: The Impact of Sex and Comorbidities. Journal of Clinical Medicine. 2025; 14(21):7512. https://doi.org/10.3390/jcm14217512
Chicago/Turabian StyleKurpaska, Małgorzata, Paweł Krzesiński, Małgorzata Banak, and Katarzyna Piotrowicz. 2025. "Predictors of Impaired Exercise Performance in Patients Qualified for Cardiac Rehabilitation: The Impact of Sex and Comorbidities" Journal of Clinical Medicine 14, no. 21: 7512. https://doi.org/10.3390/jcm14217512
APA StyleKurpaska, M., Krzesiński, P., Banak, M., & Piotrowicz, K. (2025). Predictors of Impaired Exercise Performance in Patients Qualified for Cardiac Rehabilitation: The Impact of Sex and Comorbidities. Journal of Clinical Medicine, 14(21), 7512. https://doi.org/10.3390/jcm14217512






