X-Linked Hypophosphatemia in a Family Cohort: Clinical Variability, Genetic Confirmation and Modern Therapeutic Perspectives
Abstract
1. Introduction
2. Materials and Methods
3. Case Series
3.1. Case 1—S.G., 43-Year-Old Female (Index Patient)
3.1.1. Case Presentation
3.1.2. Laboratory Investigations
3.1.3. Imaging Assessment
3.1.4. Treatment and Management
3.2. Case Report 2—A.R., 20-Year-Old Male (Nephew of Index Patient)
3.2.1. Case Presentation
3.2.2. Laboratory Investigations
3.2.3. Imaging Assessment
3.2.4. Therapy
3.3. Case 3—A.D., Female, 55 Years Old
3.3.1. Case Presentation
3.3.2. Laboratory Investigations
3.3.3. Imaging Assessment
3.3.4. Therapy
4. Discussion
5. Conclusions
- X-linked hypophosphatemia (XLH) demonstrates marked clinical heterogeneity even within the same family, emphasizing the importance of early recognition and genetic confirmation.
- Delayed or missed diagnosis results in severe multisystem complications, including skeletal deformities, osteoarthritis, chronic pain, and complete edentulism.
- Conventional therapy with phosphate and active vitamin D provides partial benefit but fails to prevent long-term complications and carries risks such as secondary hyperparathyroidism and nephrocalcinosis.
- Burosumab directly addresses the underlying FGF23-driven pathophysiology, achieving sustained biochemical control, radiological improvement, and functional benefits in both children and adults.
- Cascade genetic screening and coordinated multidisciplinary management are essential for optimizing prognosis and guiding reproductive counseling in affected families.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haffner, D.; Emma, F.; Eastwood, D.M.; Duplan, M.B.; Bacchetta, J.; Schnabel, D.; Wicart, P.; Bockenhauer, D.; Santos, F.; Levtchenko, E.; et al. Clinical practice recommendations for the diagnosis and management of X-linked hypophosphataemia. Nat. Rev. Nephrol. 2019, 15, 435–455. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rowe, P.S. The molecular background to hypophosphataemic rickets. Arch. Dis. Child. 2000, 83, 192–194. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beck, L.; Karaplis, A.C.; Amizuka, N.; Hewson, A.S.; Ozawa, H.; Tenenhouse, H.S. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc. Natl. Acad. Sci. USA 1998, 95, 5372–5377. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abdullah, S.J.; Mahwi, T.O.; Mohamad Salih Saeed, A.; Abdulateef, D.S.; Rahman, H.S.; Ahmed, S.F.; Abdulqader, S.A. X-linked familial hypophosphatemia: A case report of a 27-year-old male and review of literature. Horm. Metab. Res. 2023, 55, 653–664. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Padidela, R.; Nilsson, O.; Mäkitie, O.; Beck-Nielsen, S.; Ariceta, G.; Schnabel, D.; Brandi, M.L.; Boot, A.; Levtchenko, E.; Smyth, M.; et al. The International X-Linked Hypophosphataemia (XLH) Registry (NCT03193476): Rationale for and Description of an International Observational Study. Orphanet J. Rare Dis. 2020, 15, 172. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baroncelli, G.I.; Mora, S. X-linked hypophosphatemic rickets: Multisystemic disorder in children requiring multidisciplinary management. Front. Endocrinol. 2021, 12, 688309. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ariceta, G.; Beck-Nielsen, S.S.; Boot, A.M.; Brandi, M.L.; Briot, K.; de Lucas Collantes, C.; Emma, F.; Giannini, S.; Haffner, D.; Keen, R.; et al. The International X-linked hypophosphatemia (XLH) registry: First interim analysis of baseline demographic, genetic and clinical data. Orphanet J. Rare Dis. 2023, 18, 304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sant’Ana, I.; Torrini, R.; Alves Coelho, M.C.; Cantoni, J.; Madeira, M.; Ribeiro, M. X-linked hypophosphatemic rickets: Description of seven new variants in patients followed up in reference hospitals in Rio de Janeiro. Mol. Genet. Genom. Med. 2022, 10, e1941. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carpenter, T.O.; Imel, E.A.; Holm, I.A.; Jan de Beur, S.M.; Insogna, K.L. A clinician’s guide to X-linked hypophosphatemia. J. Bone Miner. Res. 2011, 26, 1381–1388. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fratzl-Zelman, N.; Gamsjaeger, S.; Blouin, S.; Kocijan, R.; Plasenzotti, P.; Rokidi, S.; Nawrot-Wawrzyniak, K.; Roetzer, K.; Uyanik, G.; Haeusler, G.; et al. Alterations of bone material properties in adult patients with X-linked hypophosphatemia (XLH). J. Struct. Biol. 2020, 211, 107556. [Google Scholar] [CrossRef] [PubMed]
- Park, P.G.; Lim, S.H.; Lee, H.; Ahn, Y.H.; Cheong, H.I.; Kang, H.G. Genotype and phenotype analysis in X-linked hypophosphatemia. Front. Pediatr. 2021, 9, 699767. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Imel, E.A.; Glorieux, F.H.; Whyte, M.P.; Munns, C.F.; Ward, L.M.; Nilsson, O.; Simmons, J.H.; Padidela, R.; Namba, N.; Cheong, H.I.; et al. Burosumab versus conventional therapy in children with X-linked hypophosphataemia: A randomised, active-controlled, open-label, phase 3 trial. Lancet 2019, 393, 2416–2427. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brener, R.; Zeitlin, L.; Lebenthal, Y.; Brener, A. Dental health of pediatric patients with X-linked hypophosphatemia (XLH) after three years of burosumab therapy. Front. Endocrinol. 2022, 13, 947814. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gadion, M.; Hervé, A.; Herrou, J.; Rothenbuhler, A.; Smail-Faugeron, V.; Courson, F.; Linglart, A.; Chaussain, C.; Biosse Duplan, M. Burosumab and dental abscesses in children with X-linked hypophosphatemia. JBMR Plus 2022, 6, e10672. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamamoto, A.; Nakamura, T.; Ohata, Y.; Kubota, T.; Ozono, K. Phenotypes of a Family with XLH with a Novel PHEX Mutation. Hum. Genome Var. 2020, 7, 8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soto Barros, J.; Sanchez, S.I.; Cabral, K.; Beggs, A.H.; Agrawal, P.B.; Genetti, C.A.; Brownstein, C.A.; Carpenter, T.O. X-Linked Hypophosphatemia in Four Generations Due to an Exon 13–15 Duplication in PHEX, in the Absence of the c.*231A>G Variant. Bone 2023, 172, 116763. [Google Scholar] [CrossRef]
- Chesher, D.; Oddy, M.; Darbar, U.; Sayal, P.; Casey, A.; Ryan, A.; Sechi, A.; Simister, C.; Waters, A.; Wedatilake, Y.; et al. Outcome of Adult Patients with X-Linked Hypophosphatemia Caused by PHEX Gene Mutations. J. Inherit. Metab. Dis. 2018, 41, 865–876. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verge, C.F.; Lam, A.; Simpson, J.M.; Cowell, C.T.; Howard, N.J.; Silink, M. Effects of Therapy in X-Linked Hypophosphatemic Rickets. N. Engl. J. Med. 1991, 325, 1843–1848. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, T.O.; Whyte, M.P.; Imel, E.A.; Boot, A.M.; Högler, W.; Linglart, A.; Padidela, R.; Van’t Hoff, W.; Mao, M.; Chen, C.Y.; et al. Burosumab Therapy in Children with X-Linked Hypophosphatemia. N. Engl. J. Med. 2018, 378, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.J.; Imel, E.A.; Carpenter, T.O.; Peacock, M.; Portale, A.A.; Hetzer, J.; Merritt, J.L.; Insogna, K. Long-Term Burosumab Administration Is Safe and Effective in Adults with X-Linked Hypophosphatemia. J. Clin. Endocrinol. Metab. 2022, 108, 155–165. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bubbear, J.; Lachmann, R.; Murphy, E.; Krishna, G.; Clunie, G.P.R.; Walsh, J.; Schini, M.; Salam, S.; Roy, M.; Finezilber, Y.; et al. Real-World Effectiveness of Burosumab in Adults with X-Linked Hypophosphataemia (XLH) in the UK. Calcif. Tissue Int. 2025, 116, 122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abdullah, A.; Wuersching, S.N.; Kollmuss, M.; Poxleitner, P.; Dewenter, I.; Brandenburg, L.S.; Steybe, D.; Fegg, F.N.; Smolka, W.; Otto, S.; et al. X-Linked Hypophosphatemia: Does Targeted Therapy Modify Dental Impairment? J. Clin. Med. 2023, 12, 7546. [Google Scholar] [CrossRef] [PubMed Central]
- Arhar, A.; Pavlič, A.; Hočevar, L. Characteristics of Oral Health of Patients with X-Linked Hypophosphatemia: Case Reports and Literature Review. BDJ Open 2024, 10, 42. [Google Scholar] [CrossRef]
- Lambert, A.S.; Zhukouskaya, V.; Rothenbuhler, A.; Linglart, A. X-Linked Hypophosphatemia: Management and Treatment Prospects. Jt. Bone Spine 2019, 86, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Seefried, L.; Smyth, M.; Keen, R.; Harvengt, P. Burden of disease associated with X-linked hypophosphataemia in adults: A systematic literature review. Osteoporos. Int. 2021, 32, 7–22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alsagheir, A.I.; Bin-Abbas, B.; Alghamdi, N.M.; Alhuthil, R.T.; Murad, S.A.; Husein, T.H.; Mughal, M.Z.; Alsabban, Z.E.; Almarzoug, L.M.; Ramzan, K. Quality of life of 26 family members from four generations with X-linked hypophosphatemia: A cross-sectional study. Front. Endocrinol. 2025, 16, 1544016. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akta, C.; Wenzel-Schwarz, F.; Stauffer, A.; Kranzl, A.; Raimann, A.; Kocijan, R.; Ganger, R.; Mindler, G.T. The ankle in XLH: Reduced motion, power and quality of life. Front. Endocrinol. 2023, 14, 1111104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


| Test | Initial Evaluation | First Year After Treatment | Second Year After Treatment | Reference Range |
|---|---|---|---|---|
| Ph | 2.3 ↓ | 2.0 ↓ | 1.7 ↓ | 2.5–5.5 mg/dL |
| Total Ca | 8.1 ↓ | 8.2 ↓ | 8.1 ↓ | 8.3–10.6 mg/dL |
| ALP | 121 | 90 | - | 38–126 U/L |
| BALP | 18.9 ↑ | 12.3 | - | 4.5–16.9 mcg/L |
| PTH | 35.9 | 42.9 | 42.2 | 18.5–88 pg/mL |
| 25-OH Vit D | 29.75 ↓ | - | - | 30–50 ng/mL |
| 1,25-OH2D | 42.8 | 40.2 | - | 19.9–79.3 pg/mL |
| TmP/GFR | 2.03 | 1.76 | 1.32 | 2.5–4.2 mg/dL |
| eGFR | 108.1 | 108.1 | 98 | ≥90 mL/min/1.73 m2 |
| Comments | Baseline persistent hypo- phosphatemia, Vit D deficiency, ALP and marginally increased BALP | Phosphate remains low, ALP and BALP improved | Persistent hypo-phosphatemia |
| Category | Finding | Description |
|---|---|---|
| General | Age/Sex | 43 years, female |
| Family relation | Index patient | |
| Genetic result | PHEX exon 16 deletion (heterozygous) | |
| Clinical presentation | Onset | Early childhood—short stature, delayed walking, poor motor endurance |
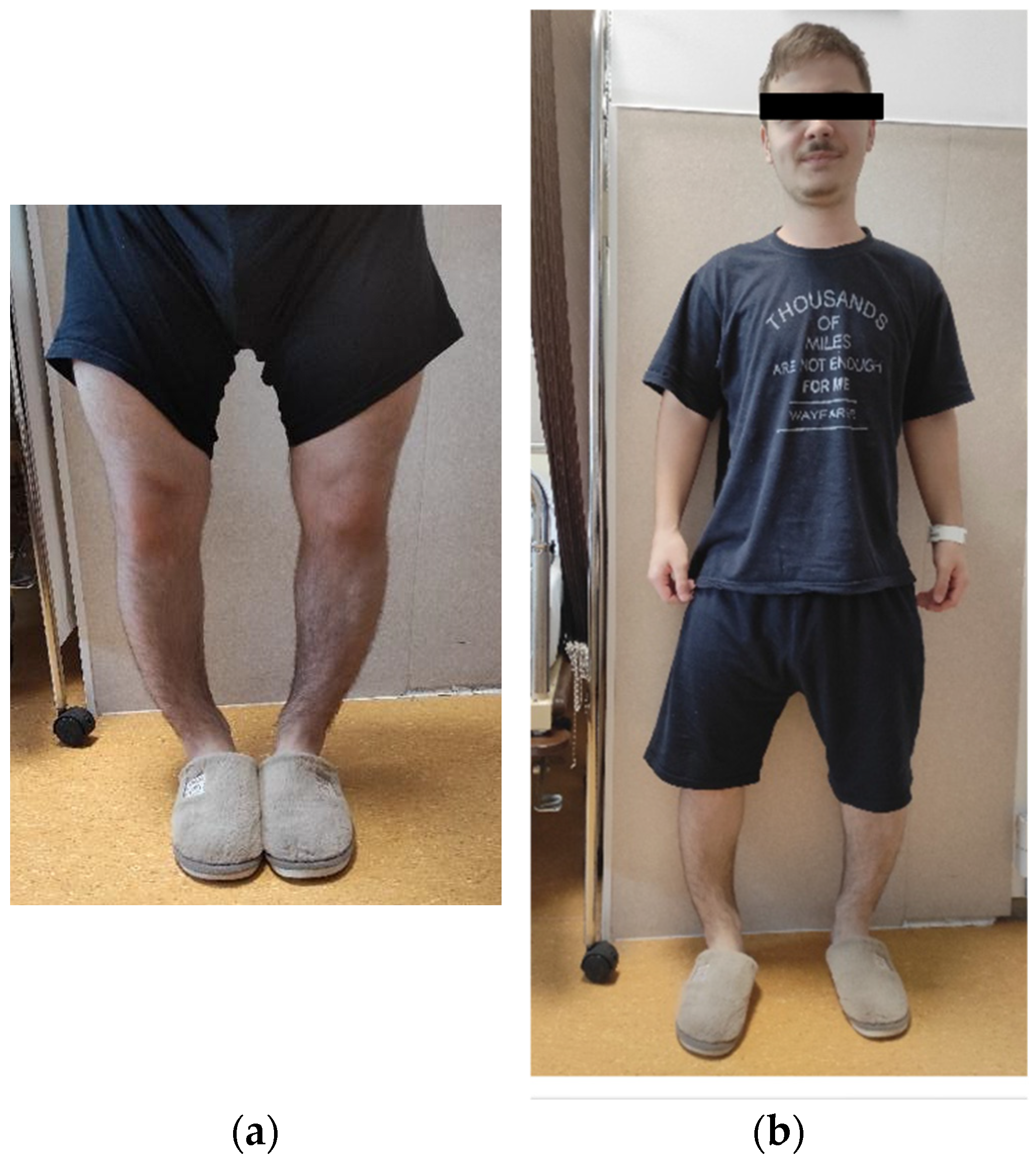
| Main features at clinical examination | Frontal bossing, genu varum, waddling gait, recurrent dental abscesses, chronic musculoskeletal pain; height of 139 cm (−4.35 SD), weight 75 kg, and a BMI of 37.7 kg/m2 | |
| Clinical course | Misdiagnosed as pituitary dwarfism and later as achondroplasia; progressive skeletal deformities and pain in adulthood | |
| Comorbidities | Multinodular goiter, obesity | |
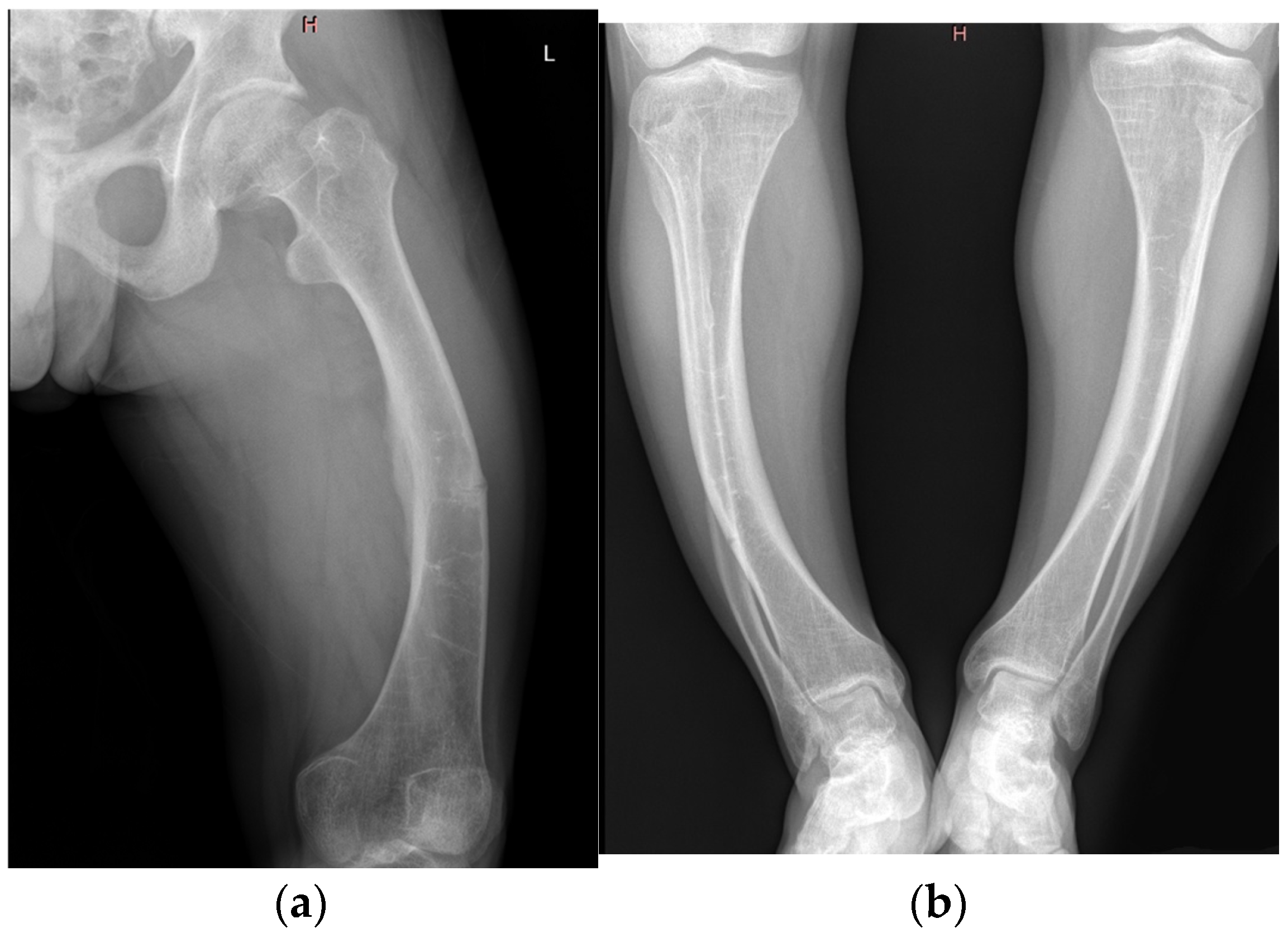
| Imaging findings | Radiography | Bilateral coxarthrosis and gonarthrosis, tibial osteosclerosis, no pseudofractures |
| DXA | Preserved bone mineral density with an osteomalacia pattern | |
| Functional status | Mobility, dental status | Waddling gait, limited mobility, requires assistance for longer distances, complete edentulism secondary to recurrent abscesses |
| Treatment history | Prior therapy | None until 2022; analgesics and physiotherapy |
| Conventional therapy | Alfacalcidol and oral phosphate (discontinued due to intolerance) | |
| Current management | Continues alfacalcidol, under multidisciplinary follow-up; planned burosumab therapy pending thyroid surgery |
| Test | Initial Evaluation | Conventional Treatment | First Year Burosumab | Second Year Burosumab | Reference Range |
|---|---|---|---|---|---|
| Ph | 1.8 ↓ | 2.2 ↓ | 3.2 | 2.5 | 2.5–5.5 mg/dL |
| Total Ca | 9.3 | 9.3 | 9.1 | 9 | 8.3–10.6 mg/dL |
| ALP | 131 ↑ | 113 | 107 | 114 | 38–126 U/L |
| BALP | 30.3 ↑ | 29.5 ↑ | - | 11.4 | 10–28.8 mcg/L |
| PTH | 100↑ | 109.5 ↑ | 42.2 | 34.8 | 18.5–88 pg/mL |
| 25-OH Vit D | 14.5 ↓ | 13.3 ↓ | 31.7 | 39,4 | 30–50 ng/mL |
| 1,25-OH2D | 25.3 | 51.5 | 72.5 ↑ | 59.5↑ | 19.9–79.3 pg/mL |
| TmP/GFR | 1.16 ↓ | 1.25 ↓ | 2.42 ↓ | 1.70 ↓ | 2.5–4.2 mg/dL |
| eGFR | 130 | 135 | 129.9 | 131 | ≥90 mL/min/1.73 m2 |
| Comments | Severe hypophosphatemia, vitamin D deficiency, high ALP/BALP, preserved renal function | Partial biochemical improvement, persistent phosphate wasting and elevated PTH | Improved phosphate and vitamin D, but TmP/GFR still low | Persistently low TmP/GFR despite stable calcium, PTH and phosphate |
| Category | Finding | Description |
|---|---|---|
| General | Age/Sex | 20 years, male |
| Family relation | Nephew of S.G. | |
| Genetic result | PHEX exon 16 deletion (heterozygous) | |
| Clinical presentation | Onset | Age 2—delayed walking and progressive bowing of the lower limbs |
| Main features at clinical examination | Short stature, genu varum, intercondylar gap of 10 cm, dental abscesses, height was 154–156 cm (−2.75 SD), weight 59 kg, BMI 24 kg/m2, and head circumference 60.5 cm | |
| Clinical course | Childhood diagnosis of familial vitamin D–resistant rickets; intermittent conventional therapy with poor adherence | |
| Imaging findings | Radiography | Bowing of femurs and tibiae, pseudofracture in mid-femur |
| DXA | Borderline osteopenia | |
| Functional status | Mobility, dental status | Waddling gait; improved mobility after burosumab initiation, multiple dental abscesses requiring repeated interventions |
| Treatment history | Prior therapy | Oral phosphate and alfacalcidol (ages 2–7), later discontinued |
| Conventional therapy | Oral phosphate and alfacalcidol (ages 2–7), later discontinued | |
| Current management | Burosumab since 2022 (60–70 mg monthly, adjusted to body weight) |
| Test | Initial Evaluation | Treatment with Cholecalciferol | Follow-Up—Treatment with Alfacalcidol | Reference Range |
|---|---|---|---|---|
| Ph | 2.8 ↓ | 2.0 ↓ | 1.8 ↓ | 2.5–5.5 mg/dL |
| Total Ca | 8.6 | 8.7 | 8.7 | 8.3–10.6 mg/dL |
| ALP | 137 ↑ | 134 ↑ | 139 ↑ | 38–126 U/L |
| PTH | 97.1 ↑ | 109 ↑ | 81.8 | 18.5–88 pg/mL |
| 25-OH Vit D | 4.98 ↓ | 13.27 ↓ | 18.13 ↓ | 30–50 ng/mL |
| TmP/GFR | 2.41 ↓ | 1.34 ↓ | 1.18 ↓ | 2.5–4.2 mg/dL |
| eGFR | 104 | 105 | 95 | ≥90 mL/min/1.73 m2 |
| Comments | Hypophosphatemia with vitamin D deficiency, elevated ALP, high PTH | Partial biochemical response, persistent phosphate wasting, secondary hyperparathyroidism | Persistent hypophosphatemia ALP still elevated |
| Category | Finding | Description |
|---|---|---|
| General | Age/Sex | 55 years, female |
| Family relation | Mother of A.R., sister of S.G. | |
| Genetic result | PHEX exon 16 deletion (heterozygous) | |
| Clinical presentation | Onset | Early childhood with disproportionate short stature and gait abnormality |
| Main features at clinical examination | Genu varum, thoracic kyphosis, recurrent dental infections, degenerative joint disease | |
| Clinical course | Progressive mobility limitation and pain; surgical correction of limb deformity in adulthood | |
| Imaging findings | Radiography | Bone demineralization, bilateral coxarthrosis and gonarthrosis, genu varum |
| Functional status | Mobility, dental status | Reduced range of motion, moderate pain, Partial edentulism following recurrent abscesses |
| Treatment history | Prior therapy | None |
| Conventional therapy | Cholecalciferol followed by alfacalcidol; phosphate unavailable | |
| Current management | Burosumab planned following vitamin D correction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, O.; Balaș, M.; Golu, I.; Amzăr, D.; Dorogi, C.; Vlad, M. X-Linked Hypophosphatemia in a Family Cohort: Clinical Variability, Genetic Confirmation and Modern Therapeutic Perspectives. J. Clin. Med. 2025, 14, 7496. https://doi.org/10.3390/jcm14217496
Popa O, Balaș M, Golu I, Amzăr D, Dorogi C, Vlad M. X-Linked Hypophosphatemia in a Family Cohort: Clinical Variability, Genetic Confirmation and Modern Therapeutic Perspectives. Journal of Clinical Medicine. 2025; 14(21):7496. https://doi.org/10.3390/jcm14217496
Chicago/Turabian StylePopa, Oana, Melania Balaș, Ioana Golu, Daniela Amzăr, Carmen Dorogi, and Mihaela Vlad. 2025. "X-Linked Hypophosphatemia in a Family Cohort: Clinical Variability, Genetic Confirmation and Modern Therapeutic Perspectives" Journal of Clinical Medicine 14, no. 21: 7496. https://doi.org/10.3390/jcm14217496
APA StylePopa, O., Balaș, M., Golu, I., Amzăr, D., Dorogi, C., & Vlad, M. (2025). X-Linked Hypophosphatemia in a Family Cohort: Clinical Variability, Genetic Confirmation and Modern Therapeutic Perspectives. Journal of Clinical Medicine, 14(21), 7496. https://doi.org/10.3390/jcm14217496






