Pulmonary Sequestration in Adults: Endovascular and Hybrid Treatment Strategies—A Systematic Review
Abstract
1. Introduction
| Characteristic | Intralobar Sequestration | Extralobar Sequestration |
|---|---|---|
| Pleural investment | Shares the visceral pleura with adjacent lung (no separate pleural covering) | Has its own, separate pleural covering |
| Venous drainage | Usually to pulmonary veins | Often to systemic veins (azygos, hemiazygos, inferior vena cava) |
| Typical location | Lower lobes; posterobasal segments; left predominance | Lower lobes; variable extrapulmonary attachments |
| Systemic arterial supply | Descending thoracic or abdominal aorta and branches | Similar origins; may involve diaphragmatic or abdominal branches |
| Associated congenital anomalies | Less frequent (~10–15%) | More frequent (~50–65%) |
| Clinical presentation | Recurrent infections, cough, chest/back pain, hemoptysis; may be incidental | Often neonatal/infantile symptoms; adult presentation less common |
| Age at diagnosis | Adolescence/adulthood common | Neonatal/infancy predominant; occasional later diagnosis |
| Side predominance | Left more common | Left somewhat more common |
| Risk of infection | Higher due to shared pleura and retained secretions | Lower; pleural separation limits contamination |
Research Question (PICOS)
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria and Outcomes
2.3. Information Sources and Search Strategy
2.4. Study Selection and Data Collection
2.5. Risk-of-Bias Assessment
2.6. Synthesis Methods
2.7. Reporting Bias Assessment
3. Results
3.1. Risk-of-Bias Summary
3.2. Study Selection
3.3. Patient Characteristics and Clinical Presentation
3.4. Anatomy, Lesion Characteristics, and Arterial Supply
3.5. Interventions and Outcomes
4. Discussion
4.1. Summary of Evidence
4.2. Clinical Implications
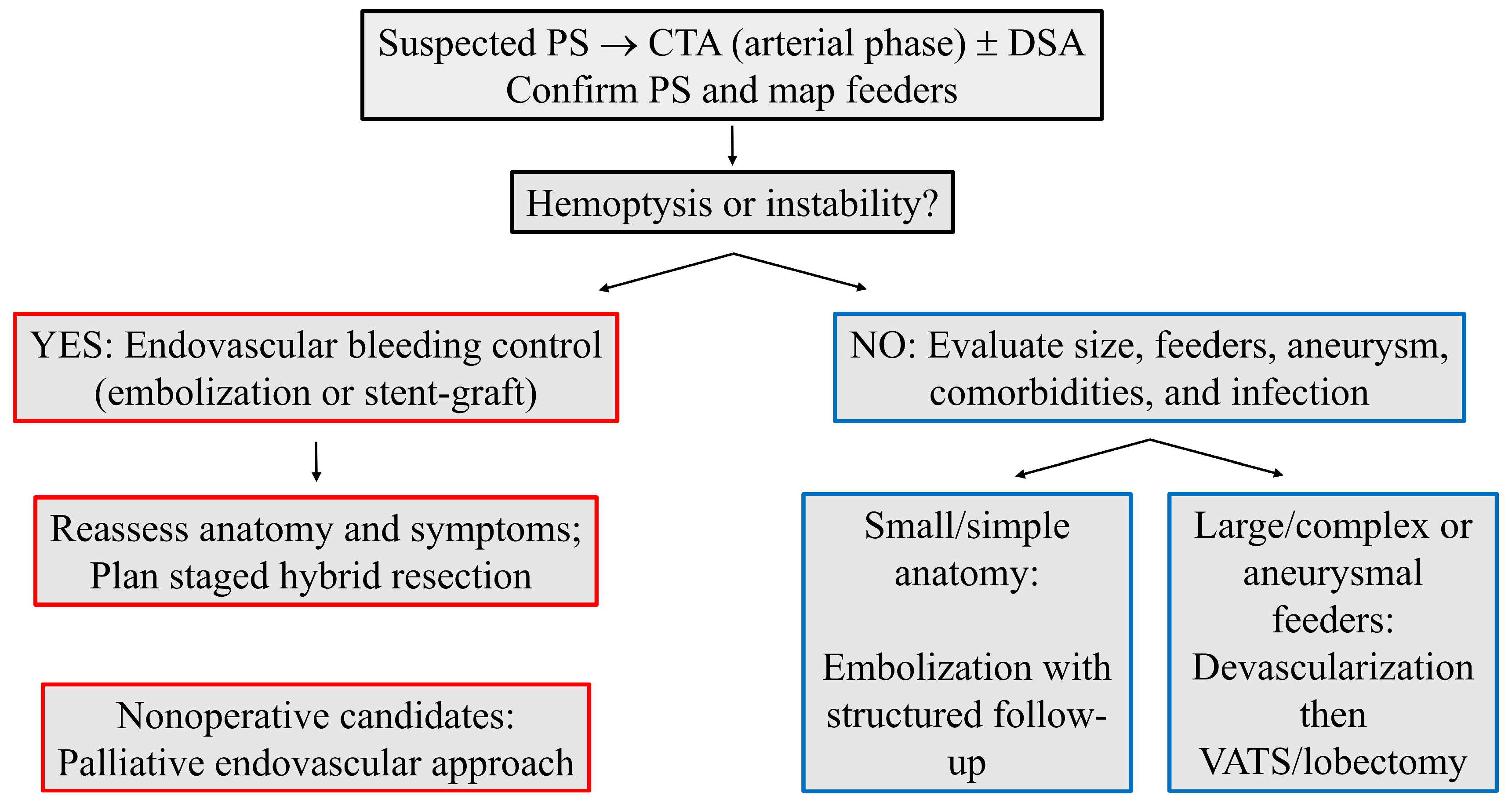
4.3. Technique Selection
4.4. Timing Within Hybrid Care
4.5. Complications and Re-Intervention
4.6. Practice Implications
4.7. Limitations
4.8. Future Work
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Durhan, G.; Ardali Duzgun, S.; Akpınar, M.G.; Demirkazık, F.; Arıyürek, O.M. Imaging of congenital lung diseases presenting in the adulthood: A pictorial review. Insights Imaging 2021, 12, 153. [Google Scholar] [CrossRef]
- Chakraborty, R.K.; Modi, P.; Sharma, S. Pulmonary Sequestration; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Abbey, P.; Das, C.J.; Pangtey, G.S.; Seith, A.; Dutta, R.; Kumar, A. Imaging in bronchopulmonary sequestration. J. Med. Imaging Radiat. Oncol. 2009, 53, 22–31. [Google Scholar] [CrossRef]
- Corbett, H.J.; Humphrey, G.M. Pulmonary sequestration. Paediatr. Respir. Rev. 2004, 5, 59–68. [Google Scholar] [CrossRef]
- Gabelloni, M.; Faggioni, L.; Accogli, S.; Aringhieri, G.; Neri, E. Pulmonary sequestration: What the radiologist should know. Clin. Imaging 2021, 73, 61–72. [Google Scholar] [CrossRef]
- Walker, C.M.; Wu, C.C.; Gilman, M.D.; Godwin, J.D., 2nd; Shepard, J.A.; Abbott, G.F. The imaging spectrum of bronchopulmonary sequestration. Curr. Probl. Diagn. Radiol. 2014, 43, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Pham, L.H.; Hamdaoui, Y.; Zeron, G.; El-Bershawi, A.; Alazzeh, A. Separating Out Pulmonary Sequestration. Cureus 2024, 16, e53190. [Google Scholar] [CrossRef]
- Wei, Y.; Li, F. Pulmonary sequestration: A retrospective analysis of 2625 cases in China. Eur. J. Cardiothorac. Surg. 2011, 40, e39–e42. [Google Scholar] [CrossRef] [PubMed]
- Sotto Mayor, J.; Rocha, D.; Esperança, S.; Oliveira e Silva, A. Intralobar pulmonary sequestration: Diagnostic expertise. BMJ Case Rep. 2015, 2015, bcr2015212384. [Google Scholar] [CrossRef] [PubMed]
- Alsumrain, M.; Ryu, J.H. Pulmonary sequestration in adults: A retrospective review of resected and unresected cases. BMC Pulm. Med. 2018, 18, 97. [Google Scholar] [CrossRef]
- Ren, S.; Yang, L.; Xiao, Y.; Tong, Z.; Wang, L.; Hu, Y. Pulmonary sequestration in adult patients: A single-center retrospective study. Respir. Res. 2023, 24, 13. [Google Scholar] [CrossRef]
- Liu, C.; Pu, Q.; Ma, L.; Mei, J.; Xiao, Z.; Liao, H.; Liu, L. Video-assisted thoracic surgery for pulmonary sequestration compared with posterolateral thoracotomy. J. Thorac. Cardiovasc. Surg. 2013, 146, 557–561. [Google Scholar] [CrossRef]
- Wang, L.M.; Cao, J.L.; Hu, J. Video-assisted thoracic surgery for pulmonary sequestration: A safe alternative procedure. J. Thorac. Dis. 2016, 8, 31–36. [Google Scholar]
- Zhang, Y.; Qiu, Y.; Li, Y. Debating the embolization of a large aberrant systemic artery for pulmonary sequestration using an Amplatzer duct occluder: A case report and literature review. Cardiol. Young 2022, 32, 331–336. [Google Scholar] [CrossRef]
- Berna, P.; Cazes, A.; Bagan, P.; Riquet, M. Intralobar sequestration in adult patients. Interact. Cardiovasc. Thorac. Surg. 2011, 12, 970–972. [Google Scholar] [CrossRef]
- Park, S.T.; Yoon, C.H.; Sung, K.B.; Yoon, H.K.; Goo, D.E.; Kim, K.S.; Pi, S.Y.; Auh, Y.H. Pulmonary sequestration in a newborn infant: Treatment with arterial embolization. J. Vasc. Interv. Radiol. 1998, 9, 648–650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.X.; Wang, H.D.; Yang, K.; Cheng, W.; Wu, W. Retrospective review of the diagnosis and treatment of pulmonary sequestration in 28 patients: Surgery or endovascular techniques? J. Thorac. Dis. 2017, 9, 5153–5160. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.S.; Kim, J.T.; Kim, E.A.; Kim, K.S.; Pi, S.Y.; Sung, K.B.; Yoon, C.H.; Goo, H.W. Neonatal pulmonary sequestration: Clinical experience with transumbilical arterial embolization. Pediatr. Pulmonol. 2008, 43, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Sung, K.B.; Yoon, H.K.; Ko, G.Y.; Yoon, C.H.; Goo, H.W.; Kim, E.A.; Kim, K.S.; Pi, S.Y. Transcatheter arterial embolization of pulmonary sequestration in neonates: Long-term follow-up results. J. Vasc. Interv. Radiol. 2003, 14, 363–367. [Google Scholar] [CrossRef]
- Zener, R.; Bottoni, D.; Zaleski, A.; Fortin, D.; Malthaner, R.A.; Inculet, R.I.; Mujoomdar, A. Transarterial embolization of intralobar pulmonary sequestration in a young adult with hemoptysis. J. Thorac. Dis. 2017, 9, E188–E193. [Google Scholar] [CrossRef] [PubMed]
- Healy, J.; Healey, A.; Kitley, C. Embolization of symptomatic intralobar pulmonary sequestration—A minimally invasive treatment option. Radiol. Case Rep. 2019, 14, 759–762. [Google Scholar] [CrossRef]
- Borzelli, J.; Brahmbhatt, S.; Desmond, D.; Ching, B.; Hostler, J. Coil embolization of intralobar pulmonary sequestration—An alternative to surgery: A case report. J. Med. Case Rep. 2018, 12, 375. [Google Scholar]
- Chen, Y.; Liu, B.; Shao, J.; Liu, D.; Zheng, Y. Endovascular treatment of pulmonary sequestration with thoracic endograft: Two case reports. Medicine 2019, 98, e16666, Erratum in Medicine 2019, 98, e17603. [Google Scholar] [CrossRef]
- Wilder, F.G.; Minasyan, S.Z. Thoracic Stent Graft Accompanied by Coil Embolization for Pulmonary Sequestration. Innovations 2019, 14, 168–173. [Google Scholar] [CrossRef]
- Savic, B.; Birtel, F.J.; Tholen, W.; Funke, H.D.; Knoche, R. Lung sequestration: Report of seven cases and review of 540 published cases. Thorax 1979, 34, 96–101. [Google Scholar] [CrossRef]
- He, B.; Sun, M.S.; Niu, Y.; Zhang, J.B.; Nie, Q.Q.; Zheng, X.; Fan, X.Q.; Liu, P. Hybrid and Endovascular Treatment of Pulmonary Sequestration: Two Case Reports and Literature Review. Ann. Vasc. Surg. 2020, 69, 447.e1–447.e8. [Google Scholar] [CrossRef]
- Zhou, H.; Tang, S.; Fu, Q.; Yu, L.; Liu, L. Hybrid surgery in treatment of pulmonary sequestration with abdominal aorta feeding vessel: A case report. J. Cardiothorac. Surg. 2018, 13, 44. [Google Scholar] [CrossRef]
- Mohapatra, M.; Mishra, S.; Jena, P. Massive hemoptysis in a case of intralobar pulmonary sequestration associated with pulmonary hypoplasia and meandering right pulmonary vein: Diagnosis and management. Case Rep. Pulmonol. 2012, 2012, 960948. [Google Scholar] [CrossRef] [PubMed]
- Marine, L.M.; Valdes, F.E.; Mertens, R.M.; Bergoeing, M.R.; Kramer, A. Endovascular treatment of symptomatic pulmonary sequestration. Ann. Vasc. Surg. 2011, 25, 696.e11–696.e15. [Google Scholar] [CrossRef] [PubMed]
- Madhusudhan, K.S.; Das, C.J.; Dutta, R.; Kumar, A.; Bhalla, A.S. Endovascular embolization of pulmonary sequestration in an adult. J. Vasc. Interv. Radiol. 2009, 20, 1640–1642. [Google Scholar] [CrossRef]
- Goto, T.; Toya, K.; Wakaki, M.; Kato, R. Resection of intralobar pulmonary sequestration after coil embolization of aberrant arteries: Report of a case. Surg. Today 2013, 43, 923–925. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Balasubramanian, S.; Jackson, R.; Agrawal, D. Combined endovascular and surgical approaches to treat intralobar pulmonary sequestration: A case report. Ann. R. Coll. Surg. Engl. 2021, 103, e35–e37. [Google Scholar] [CrossRef] [PubMed]
- Chataut, D.; Katwal, S.; Suwal, S.; Thapa, A.; Bhusal, A. Endovascular embolization for massive hemoptysis in intralobar pulmonary sequestration with celiac artery supply: A comprehensive case report. Radiol. Case Rep. 2024, 19, 2239–2244. [Google Scholar] [CrossRef]
- Marine, L.; Torrealba, J.I.; Valdes, F.; Mertens, R.; Vargas, F.; Bergoeing, M.; Vallejos, D. Endovascular treatment of a right pulmonary sequestration supplied by an aneurysmal aberrant artery originating from the abdominal aorta. J. Vasc. Bras. 2022, 21, e20190160. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Fang, X.; Wu, B. Coil embolization to treat pulmonary sequestration in the right upper lobe. Interact. Cardiovasc. Thorac. Surg. 2022, 35, ivac178. [Google Scholar] [CrossRef]
- Borzelli, A.; Paladini, A.; Giurazza, F.; Tecame, S.; Giordano, F.; Cavaglià, E.; Amodio, F.; Corvino, F.; Beomonte Zobel, D.; Frauenfelder, G.; et al. Successful endovascular embolization of an intralobar pulmonary sequestration. Radiol. Case Rep. 2017, 13, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, D.; Cascione, F.; Modesti, M.; Gianardi, D.; Caputo, R.; Galatioto, C.; Chiarugi, M. Hemoptysis caused by pulmonary sequestration in perforated appendicitis: A rare case report. Ulus. Travma Acil Cerrahi Derg. 2016, 22, 569–571. [Google Scholar]
- Ojha, V.; Samui, P.P.; Dakshit, D. Role of endovascular embolization in improving the quality of life in a patient suffering from complicated intralobar pulmonary sequestration—A case report. Respir. Med. Case Rep. 2015, 16, 24–28. [Google Scholar] [CrossRef]
- Kim, T.E.; Kwon, J.H.; Kim, J.S. Transcatheter embolization for massive hemoptysis from an intralobar pulmonary sequestration: A case report. Clin. Imaging 2014, 38, 326–329. [Google Scholar] [CrossRef]
- Greben, C.R.; Goldstein, G.E.; Gandras, E.J.; Setton, A. Pulmonary sequestration aneurysm embolization. J. Vasc. Interv. Radiol. 2012, 23, 477. [Google Scholar] [CrossRef]
- Leoncini, G.; Rossi, U.G.; Ferro, C.; Chessa, L. Endovascular treatment of pulmonary sequestration in adults using Amplatzer® vascular plugs. Interact. Cardiovasc. Thorac. Surg. 2011, 12, 98–100. [Google Scholar] [CrossRef]
- Nemoto, M.; Koyama, K.; Tadokoro, Y.; Watanabe, T.; Suzuki, H.; Kiyoshima, M.; Yoshimi, F. Treatment of an Aberrant Arterial Aneurysm with Intralobar Pulmonary Sequestration: A Case Report. Ann. Vasc. Surg. 2020, 69, 453.e11–453.e14. [Google Scholar] [CrossRef]
- Türk, İ.; Özdemir, M.; Çetin, M.; Bıçakçıoğlu, P. Coil embolization for pulmonary sequestration may serve as an alternative to surgical treatment: Under which circumstances? Indian J. Thorac. Cardiovasc. Surg. 2025, 41, 1464–1468. [Google Scholar] [CrossRef]
- Hordijk, M.; Buimer, M.G. Rupture of an Aneurysmal Pulmonary Sequestration Artery. Vasc. Endovasc. Surg. 2025, 59, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Cho, Y.K.; Lee, J.H.; Woo, J.J.; Choi, Y.S. Endovascular Treatment of Intralobar Pulmonary Sequestration with Vascular Plug: A Case Report. J. Korean Soc. Radiol. 2012, 67, 105–108. [Google Scholar] [CrossRef]
- Grossi, W.; Londero, F.; Vit, A.; De Franceschi, E.; Masullo, G.; Sponza, M.; Morelli, A. Hybrid minimally invasive treatment of intralobar pulmonary sequestration: A single-centre experience. Interact. Cardiovasc. Thorac. Surg. 2022, 34, 255–257. [Google Scholar] [CrossRef]
- Hakiri, S.; Fukui, T.; Chen-Yoshikawa, T.F. Combined surgical therapy for pulmonary sequestration and aberrant artery from the abdominal aorta. Gen. Thorac. Cardiovasc. Surg. 2021, 69, 1031–1034. [Google Scholar] [CrossRef] [PubMed]
- Sakai, M.; Ozawa, Y.; Konishi, T.; Watanabe, A.; Shiigai, M. Endostapling the aberrant artery filled with embolized coils for intralobar pulmonary sequestration: A report of two cases. J. Thorac. Dis. 2018, 10, E304–E308. [Google Scholar] [CrossRef]
- Petty, L.; Joseph, A.; Sanchez, J. Case report: Pulmonary sequestration in an adult. Radiol. Case Rep. 2017, 13, 21–23. [Google Scholar] [CrossRef]
- Fabbri, N.; Tamburini, N.; Galeotti, R.; Quarantotto, F.; Maniscalco, P.; Rinaldi, R.; Salviato, E.; Cavallesco, G. A rare case of intralobar pulmonary sequestration: Combined endovascular and video-assisted thoracoscopic approach. AME Case Rep. 2018, 2, 19. [Google Scholar] [CrossRef]
- Ferland, N.; Couture, C.; Provencher, S. Near-fatal haemoptysis as presentation of a giant intralobar pulmonary sequestration. Eur. Respir. Rev. 2015, 24, 155–156. [Google Scholar] [CrossRef]
- Ragusa, M.; Vannucci, J.; Lenti, M.; Cieri, E.; Cao, P.; Puma, F. Pulmonary sequestration supplied by giant aneurysmal aortic branch. Ann. Thorac. Surg. 2010, 89, e7–e8. [Google Scholar] [CrossRef]
- Nahal, C.C.; Lackland, T.; Lowe, H.; Platz, J. Presentation and Management of Pulmonary Sequestration with an Aneurysmal Aberrant Pulmonary Artery. Cureus 2024, 16, e60225. [Google Scholar] [CrossRef]
- Monfregola, A.; De Angelis, L.; Comune, R.; Arienzo, F.; Barbato, G.; Di Stasio, M.; Pourmolkara, D.; Rosano, N.; Picchi, S.G.; Galluzzo, M.; et al. Interlobar pulmonary sequestration with celiac aberrant artery in an elderly patient treated with combined endovascular and video-assisted thoracoscopic approach. Radiol. Case Rep. 2024, 19, 3418–3424. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Okada, H.; Nakashima, J.; Anayama, T. Thoracic endovascular aortic repair of an aberrant arterial aneurysm with pulmonary sequestration. Interact. Cardiovasc. Thorac. Surg. 2020, 30, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, P.; Hardouin, S.; Cheng, T.; Farber, A.; Suzuki, K.; Jones, D.W. Endovascular exclusion and open resection of aberrant pulmonary artery aneurysm associated with intralobar pulmonary sequestration. J. Vasc. Surg. 2019, 70, 1328–1329. [Google Scholar] [CrossRef]
- Hewett, L.; Kwon, J.; Adams, J.D.; Denlinger, C.E.; Klapper, J.A. Intralobar Pulmonary Sequestration With Aneurysmal Feeding Vessel: Use of Hybrid Surgical Management. Ann. Thorac. Surg. 2016, 102, e533–e535. [Google Scholar] [CrossRef]
- Yamasaki, M.; Suzuki, M.; Misumi, H.; Abe, K.; Ito, J.; Kawazoe, K. Hybrid surgery for intralobar pulmonary sequestration with aortic aneurysm. Ann. Thorac. Surg. 2014, 98, e11–e13. [Google Scholar] [CrossRef]
- Nakagiri, T.; Sawabata, N.; Kuratani, T.; Okumura, M. Endovascular stent-graft implantation for a cecum of an aberrant artery from a systemic arterial supply to the basal segment of the left pulmonary lobe. Gen. Thorac. Cardiovasc. Surg. 2010, 58, 640–643. [Google Scholar] [CrossRef]
- Porez, F.; Singhal, S.; Mercier, O.; Fabre, D. One-stage hybrid minimally invasive treatment by thoracic endovascular repair and video-assisted thoracoscopic surgery for symptomatic pulmonary sequestration. JTCVS Tech. 2025, 30, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, K.; Funakoshi, Y. Successful Concomitant Thoracic Endovascular Aortic Repair and Lobectomy for Pulmonary Sequestration. Ann. Thorac. Surg. Short Rep. 2023, 1, 483–485. [Google Scholar] [CrossRef]
- Tatli, S.; Yucel, E.K.; Couper, G.S.; Henderson, J.M.; Colson, Y.L. Aneurysm of an aberrant systemic artery to the lung. AJR Am. J. Roentgenol. 2005, 184, 1241–1244. [Google Scholar] [CrossRef] [PubMed]
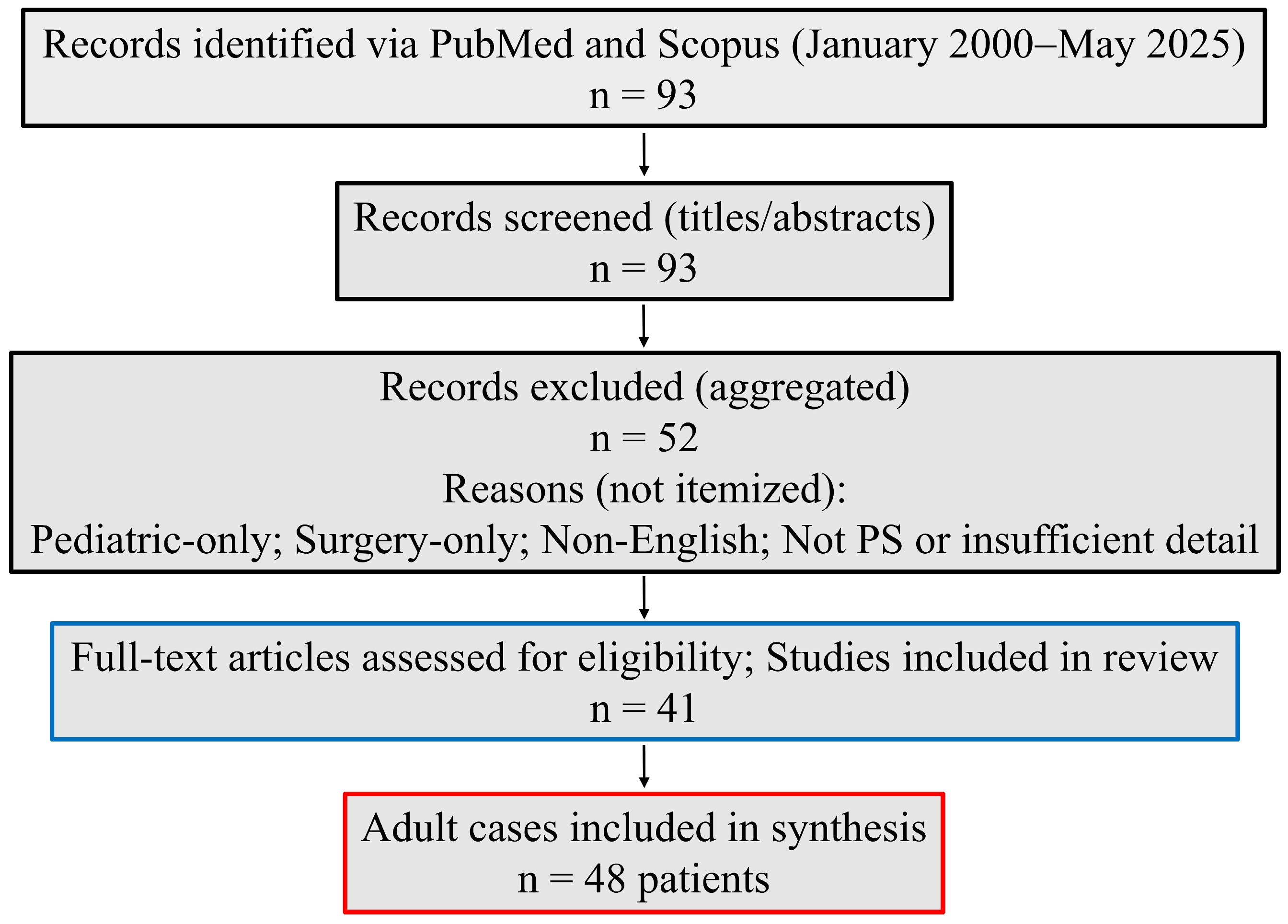
| Domain | Definition |
|---|---|
| Population | Adults (≥18 years) with intralobar or extralobar PS confirmed by imaging and, where available, intraoperative findings. |
| Interventions | Endovascular embolization (coils, vascular plugs, particles, or liquid agents); aortic stent-graft exclusion of aberrant arterial origins; or hybrid strategies combining endovascular therapy with surgical resection. |
| Comparators | Not required; single-arm reports included due to rarity and design constraints. |
| Outcomes | Technical success, clinical success, complications, recurrence, re-intervention, imaging-based lesion evolution, and follow-up duration. |
| Study designs | English-language human case reports, case series, and cohorts including an endovascular or hybrid arm. |
| Exclusions | Pediatric-only reports; surgery-only reports without an endovascular or hybrid component; non-PS lesions; non-English publications; insufficient detail to classify intervention or outcomes; duplicate reports merged where identified. |
| Domain | Yes, n (%) | No, n (%) | NR, n (%) |
|---|---|---|---|
| Case definition | 38 (92.7) | 3 (7.3) | 0 (0) |
| Diagnostic ascertainment | 40 (97.6) | 1 (2.4) | 0 (0) |
| Intervention description | 39 (95.1) | 2 (4.9) | 0 (0) |
| Outcome ascertainment | 36 (87.8) | 5 (12.2) | 0 (0) |
| Follow-up adequacy | 23 (56.1) | 7 (17.1) | 11 (26.8) |
| Characteristic | All (n = 48) | Endovascular Treatment (n = 25) | Hybrid Treatment (n = 23) | |||
|---|---|---|---|---|---|---|
| Embolization (n = 21) | Stent-Graft (n = 2) | Embolization + Stent-Graft (n = 2) | Surgery + Embolization (n = 16) | Surgery + Stent-Graft (n = 7) | ||
| Sex (male/female/not reported) | 29/18/1 | 11/9/1 | 2/0/0 | 2/0/0 | 7/9/0 | 7/0/0 |
| Age (years), mean ± SD | 44.9 ± 14.9 | 40 ± 14.6 | 36.5 ± 0.7 | 39 ± 5.7 | 47.3 ± 14.8 | 58.1 ± 11 |
| Symptomatic, n (%) | 44 (91.7) | 19 (90.5) | 2 (100) | 1 (50) | 15 (93.8) | 7 (100) |
| Hemoptysis, n (%) | 22 (45.8) | 15 (71.4) | 0 (0) | 1 (50) | 5 (31.3) | 1 (14.3) |
| Chest, back, or abdominal pain, n (%) | 18 (37.5) | 7 (33.3) | 1 (50) | 1 (50) | 6 (37.5) | 3 (42.9) |
| Recurrent infections, n (%) | 15 (31.3) | 7 (33.3) | 1 (50) | 0 (0) | 3 (18.8) | 4 (57.1) |
| Cough, expectoration, n (%) | 9 (18.8) | 4 (19) | 0 (0) | 0 (0) | 5 (31.3) | 0 (0) |
| Fever, n (%) | 7 (14.6) | 2 (9.5) | 0 (0) | 0 (0) | 5 (31.3) | 0 (0) |
| Characteristic | All (n = 48) | Endovascular Treatment (n = 25) | Hybrid Treatment (n = 23) | |||
|---|---|---|---|---|---|---|
| Embolization (n = 21) | Stent-Graft (n = 2) | Embolization + Stent-Graft (n = 2) | Surgery + Embolization (n = 16) | Surgery + Stent-Graft (n = 7) | ||
| Type | ||||||
| ILS, n (%) | 36 (75) | 13 (61.9) | 0 (0) | 2 (100) | 15 (93.8) | 6 (85.7) |
| ELS, n (%) | 2 (4.2) | 2 (9.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| NR, n (%) | 10 (20.8) | 6 (28.6) | 2 (100) | 0 (0) | 1 (6.3) | 1 (14.3) |
| Location | ||||||
| Left lower lobe, n (%) | 24 (50) | 9 (42.9) | 2 (100) | 2 (100) | 7 (43.8) | 4 (57.1) |
| Right lower lobe, n (%) | 19 (39.6) | 9 (42.9) | 0 (0) | 0 (0) | 8 (50) | 2 (28.6) |
| Other, n (%) | 5 (10.4) | 3 (14.3) | 0 (0) | 0 (0) | 1 (6.3) | 1 (14.3) |
| PS maximum diameter (mm), mean ± SD | 67.1 ± 31.5 (n = 12) | 51.5 ± 36.1 (n = 2) | NR | NR | 74.5 ± 33 (n = 8) | 53 ± 25.5 (n = 2) |
| Feeding artery origin | ||||||
| Thoracic aorta, n (%) | 28 (58.3) | 14 (66.7) | 2 (100) | 2 (100) | 4 (25) | 6 (85.7) |
| Celiac trunk, n (%) | 8 (16.7) | 2 (9.5) | 0 (0) | 0 (0) | 6 (37.5) | 0 (0) |
| Abdominal aorta, n (%) | 7 (14.6) | 3 (14.3) | 0 (0) | 0 (0) | 4 (25) | 0 (0) |
| Other or no data, n (%) | 5 (10.4) | 2 (9.5) | 0 (0) | 0 (0) | 2 (12.5) | 1 (14.3) |
| Aneurysmal feeding artery, n (%) | 12 (25) | 4 (19) | 0 (0) | 1 (50) | 3 (18.8) | 4 (57.1) |
| Feeding artery maximum diameter (mm), mean ± SD | 30.6 ± 38.5 (n = 21) | 26.2 ± 41.8 (n = 9) | NR | 27 (n = 1) | 37 ± 52.2 (n = 5) | 32.3 ± 29.5 (n = 6) |
| Parameter | All (n = 48) | Endovascular Treatment (n = 25) | Hybrid Treatment (n = 23) | |||
|---|---|---|---|---|---|---|
| Embolization (n = 21) | Stent-Graft (n = 2) | Embolization + Stent-Graft (n = 2) | Surgery + Embolization (n = 16) | Surgery + Stent-Graft (n = 7) | ||
| Embolic agents used | ||||||
| Coils, n (%) | 29 (60.4) | 15 (71.4) | - | 2 (100) | 12 (75) | - |
| Vascular plug, n (%) | 12 (25) | 6 (28.6) | - | 1 (50) | 5 (31.3) | - |
| PVA, n (%) | 8 (16.7) | 8 (38.1) | - | 0 (0) | 0 (0) | - |
| Other agents, n (%) | 4 (8.3) | 3 (14.3) | - | 0 (0) | 1 (6.3) | - |
| Hospitalization (days), mean ± SD | 4.1 ± 2.7 (n = 36) | 2.9 ± 3 (n = 15) | 3 (n = 1) | 4 ± 4.2 (n = 2) | 5.4 ± 2.1 (n = 13) | 4.8 ± 1.9 (n = 5) |
| Complications, n (%) | 10 (20.8) | 8 (38.1) | 0 (0) | 1 (50) | 1 (6.3) | 0 (0) |
| Recurrence, n (%) | 2 (4.2) | 2 (9.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Re-intervention, n (%) | 3 (6.3) | 3 (14.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Follow-up (months), mean ± SD | 15.3 ± 15.4 (n = 32) | 20.8 ± 17.5 (n = 18) | 7.5 ± 6.4 (n = 2) | 0.5 ± 0.7 (n = 2) | 8 ± 5.3 (n = 7) | 13.7 ± 15 (n = 3) |
| Imaging-based size reduction, involution, n | 9 (of 48) | 7 (of 21) | 1 (of 2) | 1 (of 2) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szablics, F.É.; Bérczi, Á.; Nyárády, B.B.; Philippovich, M.; Szőnyi, Á.; Dósa, E. Pulmonary Sequestration in Adults: Endovascular and Hybrid Treatment Strategies—A Systematic Review. J. Clin. Med. 2025, 14, 7493. https://doi.org/10.3390/jcm14217493
Szablics FÉ, Bérczi Á, Nyárády BB, Philippovich M, Szőnyi Á, Dósa E. Pulmonary Sequestration in Adults: Endovascular and Hybrid Treatment Strategies—A Systematic Review. Journal of Clinical Medicine. 2025; 14(21):7493. https://doi.org/10.3390/jcm14217493
Chicago/Turabian StyleSzablics, Fanni Éva, Ákos Bérczi, Balázs Bence Nyárády, Márton Philippovich, Ádám Szőnyi, and Edit Dósa. 2025. "Pulmonary Sequestration in Adults: Endovascular and Hybrid Treatment Strategies—A Systematic Review" Journal of Clinical Medicine 14, no. 21: 7493. https://doi.org/10.3390/jcm14217493
APA StyleSzablics, F. É., Bérczi, Á., Nyárády, B. B., Philippovich, M., Szőnyi, Á., & Dósa, E. (2025). Pulmonary Sequestration in Adults: Endovascular and Hybrid Treatment Strategies—A Systematic Review. Journal of Clinical Medicine, 14(21), 7493. https://doi.org/10.3390/jcm14217493






