Pancreatic Ultrasound Features at Diagnosis of Type 1 Diabetes: Age-Related Differences in Children
Abstract
1. Introduction
2. Subjects, Materials and Methods
2.1. Laboratory Tests
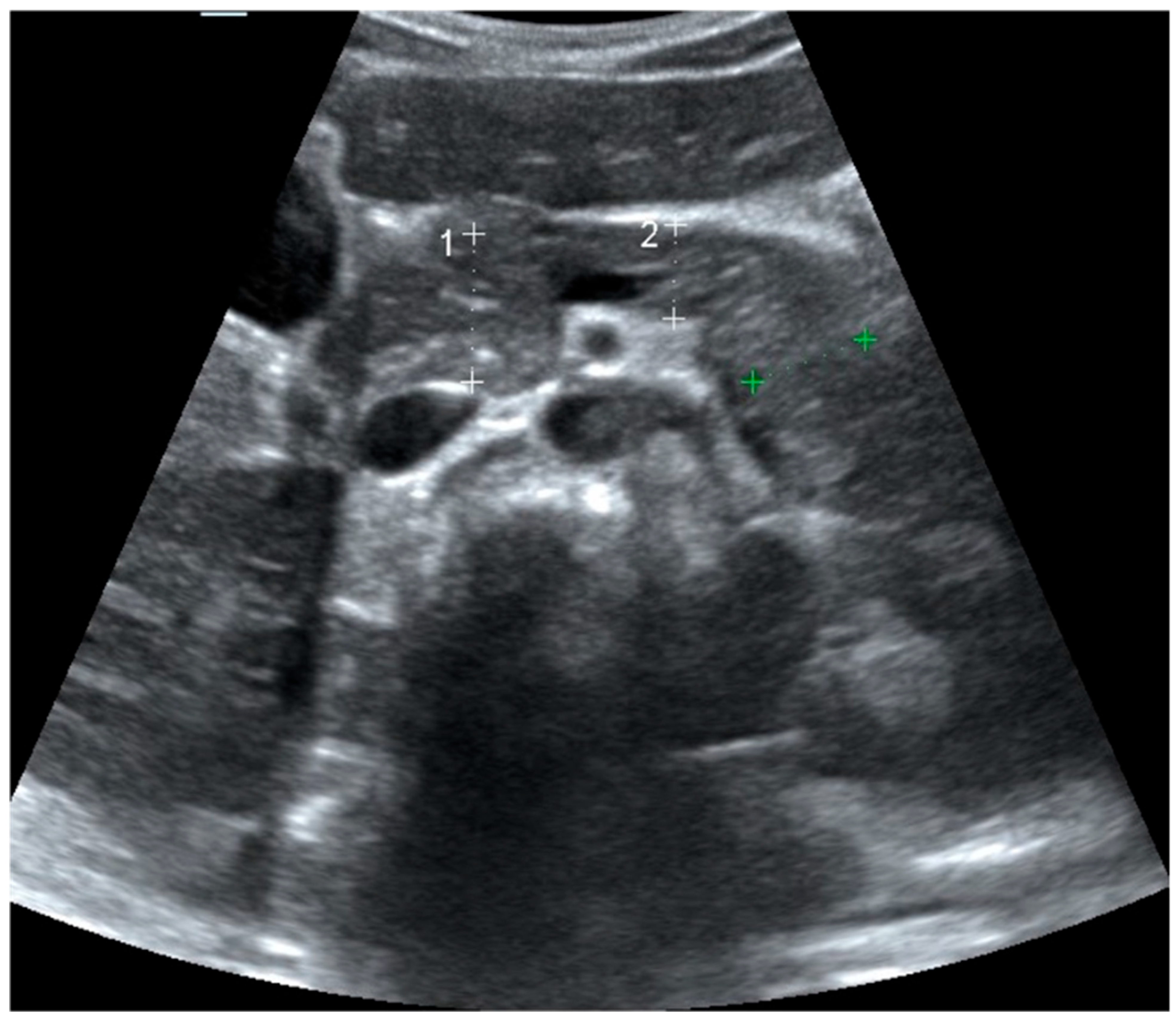
2.2. Pancreas Ultrasonography: Pancreatic Size and Echogenicity
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Anti-GAD | Anti-Glutamic Acid Decarboxylase (antibody) |
| BMI SDS | Body Mass Index Standard Deviation Score |
| CT | Computed Tomography |
| DKA | Diabetic Ketoacidosis |
| HbA1c | Hemoglobin A1c |
| HCO3 | Bicarbonate |
| IFNγ | Interferon Gamma |
| IL-10 | Interleukin-10 |
| MRI | Magnetic Resonance Imaging |
| SD | Standard Deviation |
| SPSS | Statistical Package for the Social Sciences |
| T1DE1 | Type 1 Diabetes Endotype 1 (early childhood onset, <7 years) |
| T1DE2 | Type 1 Diabetes Endotype 2 (adolescent onset, ≥13 years) |
| T1DM | Type 1 Diabetes Mellitus |
| USG | Ultrasonography |
References
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45, S17–S38. [Google Scholar] [CrossRef] [PubMed]
- Libman, I.; Haynes, A.; Lyons, S.; Pradeep, P.; Rwagasor, E.; Tung, J.Y.; Jefferies, C.A.; Oram, R.A.; Dabelea, D.; Craig, M.E. ISPAD Clinical Practice Consensus Guidelines 2022: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr. Diabetes 2022, 23, 1160–1174. [Google Scholar] [CrossRef]
- Neu, A.; Bürger-Büsing, J.; Danne, T.; Dost, A.; Holder, M.; Holl, R.W.; Holterhus, P.M.; Kapellen, T.; Karges, B.; Kordonouri, O.; et al. Diagnosis, Therapy and Follow-Up of Diabetes Mellitus in Children and Adolescents. Exp. Clin. Endocrinol. Diabetes 2019, 127, S39–S72. [Google Scholar] [CrossRef]
- Ilonen, J.; Lempainen, J.; Veijola, R. The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 635–650. [Google Scholar] [CrossRef]
- Herold, K.C.; Delong, T.; Perdigoto, A.L.; Biru, N.; Brusko, T.M.; Walker, L.S. The immunology of type 1 diabetes. Nat. Rev. Immunol. 2024, 24, 435–451. [Google Scholar] [CrossRef]
- Atkinson, M.A.; Campbell-Thompson, M.; Kusmartseva, I.; Kaestner, K.H. Organisation of the human pancreas in health and in diabetes. Diabetologia 2020, 63, 1966–1973. [Google Scholar] [CrossRef]
- Zajec, A.; Trebušak Podkrajšek, K.; Tesovnik, T.; Šket, R.; Čugalj Kern, B.; Jenko Bizjan, B.; Šmigoc Schweiger, D.; Battelino, T.; Kovač, J. Pathogenesis of Type 1 Diabetes: Established Facts and New Insights. Genes 2022, 13, 706. [Google Scholar] [CrossRef]
- Zamboni, G.A.; Ambrosetti, M.C.; D’Onofrio, M.; Pozzi Mucelli, R. Ultrasonography of the pancreas. Radiol. Clin. N. Am. 2012, 50, 395–406. [Google Scholar] [CrossRef]
- Raut, D.S.; Raje, D.V.; Dandge, V.P.; Singh, D. Percentile reference curves for normal pancreatic dimensions in Indian children. Indian J. Radiol. Imaging 2018, 28, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.W.; Kim, G.H.; Kang, D.H.; Kim, H.W.; Kim, D.U.; Heo, J.; Song, G.A.; Park, D.Y.; Kim, S. Associated factors for a hyperechogenic pancreas on endoscopic ultrasound. World J. Gastroenterol. 2010, 16, 4329–4334. [Google Scholar] [CrossRef] [PubMed]
- Ćwik, G.; Gierbliński, I.W. Errors and mistakes in the ultrasound diagnosis of the pancreas. J. Ultrason. 2013, 13, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Okaniwa, S. How Does Ultrasound Manage Pancreatic Diseases? Ultrasound Findings and Scanning Maneuvers. Gut Liver 2020, 14, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Scharfmann, R.; Staels, W.; Albagli, O. The supply chain of human pancreatic β cell lines. J. Clin. Investig. 2019, 129, 3511–3520. [Google Scholar] [CrossRef]
- Campbell-Thompson, M.; Rodriguez-Calvo, T.; Battaglia, M. Abnormalities of the Exocrine Pancreas in Type 1 Diabetes. Curr. Diabetes Rep. 2015, 15, 79. [Google Scholar] [CrossRef]
- Campbell-Thompson, M.L.; Filipp, S.L.; Grajo, J.R.; Nambam, B.; Beegle, R.; Middlebrooks, E.H.; Gurka, M.J.; Atkinson, M.A.; Schatz, D.A.; Haller, M.J. Relative Pancreas Volume Is Reduced in First-Degree Relatives of Patients with Type 1 Diabetes. Diabetes Care 2019, 42, 281–287. [Google Scholar] [CrossRef]
- Wright, J.J.; Dulaney, A.; Williams, J.M.; Hilmes, M.A.; Du, L.; Kang, H.; Powers, A.C.; Moore, D.J.; Virostko, J. Longitudinal MRI Shows Progressive Decline in Pancreas Size and Altered Pancreas Shape in Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2023, 108, 2699–2707. [Google Scholar] [CrossRef]
- Evans-Molina, C.; Sims, E.K.; DiMeglio, L.A.; Ismail, H.M.; Steck, A.K.; Palmer, J.P.; Krischer, J.P.; Geyer, S.; Xu, P.; Sosenko, J.M. β Cell dysfunction exists more than 5 years before type 1 diabetes diagnosis. JCI Insight 2018, 3, e120877. [Google Scholar] [CrossRef]
- Virostko, J.; Williams, J.; Hilmes, M.; Bowman, C.; Wright, J.J.; Du, L.; Kang, H.; Russell, W.E.; Powers, A.C.; Moore, D.J. Pancreas Volume Declines During the First Year After Diagnosis of Type 1 Diabetes and Exhibits Altered Diffusion at Disease Onset. Diabetes Care 2019, 42, 248–257. [Google Scholar] [CrossRef]
- Redondo, M.J.; Morgan, N.G. Heterogeneity and endotypes in type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2023, 19, 542–554. [Google Scholar] [CrossRef]
- Morgan, N.G. Insulitis in human type 1 diabetes: Lessons from an enigmatic lesion. Eur. J. Endocrinol. 2024, 190, R1–R9. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, P.; Hippich, M.; Zapardiel-Gonzalo, J.; Karges, B.; Holl, R.W.; Petrera, A.; Bonifacio, E.; Ziegler, A.G. A classification and regression tree analysis identifies subgroups of childhood type 1 diabetes. eBioMedicine 2022, 82, 104118. [Google Scholar] [CrossRef] [PubMed]
- Leete, P.; Oram, R.A.; McDonald, T.J.; Shields, B.M.; Ziller, C.; TIGI Study Team; Hammersley, A.T.; Richardson, S.J.; Morgan, N.G. Studies of insulin and proinsulin in pancreas and serum support the existence of aetiopathological endotypes of type 1 diabetes associated with age at diagnosis. Diabetologia 2020, 63, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Tridgell, D.M.; Spiekerman, C.; Wang, R.S.; Greenbaum, C.J. Interaction of onset and duration of diabetes on the percent of GAD and IA-2 antibody–positive subjects in the Type 1 Diabetes Genetics Consortium Database. Diabetes Care 2011, 34, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Bruggeman, B.S.; Schatz, D.A. Type 1 diabetes: A disorder of the exocrine and endocrine pancreas. J. Cell. Immunol. 2023, 5, 120. [Google Scholar] [CrossRef]
- Felton, J.L.; Redondo, M.J.; Oram, R.A.; Speake, C.; Long, S.A.; Onengut-Gumuscu, S.; Rich, S.S.; Monaco, G.S.; Harris-Kawano, A.; Perez, D. Islet autoantibodies as precision diagnostic tools to characterize heterogeneity in type 1 diabetes: A systematic review. Commun. Med. 2024, 4, 66. [Google Scholar] [CrossRef]
- Abdulrahman, S.; Ibrahim, A.A.; Mohamed, M.A.; Gameraddin, M.; Alelyani, M. Sonographic Evaluation of the Pancreas in Type 1 Diabetes Mellitus: A Case-control Study. J. Med. Ultrasound 2021, 29, 167–170. [Google Scholar] [CrossRef]
- Rogers, M.A.M.; Kim, C.; Banerjee, T.; Lee, J.M. Fluctuations in the incidence of type 1 diabetes in the United States from 2001 to 2015: A longitudinal study. BMC Med. 2017, 15, 199. [Google Scholar] [CrossRef]

| Patient (n = 69) | Control (n = 78) | p | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Sex | Female | 19 | 27.5 | 24 | 30.8 | 0.667 * |
| Male | 50 | 72.5 | 54 | 69.2 | ||
| Age (years) | 9.9 ± 4.7 (8.8–11.0) | 10.0 ± 4.6 (9.0–11.0) | 0.894 ** | |||
| Pancreatic head (mm) | 11.6 ± 2.4 (11.0–12.2) | 14.4 ± 2.5 (13.8–15.0) | <0.001 ** | |||
| Pancreatic body (mm) | 9.1 ± 1.7 (8.7–9.5) | 12.0 ± 2.1 (11.5–12.5) | <0.001 ** | |||
| Pancreatic tail (mm) | 9.9 ± 1.9 (9.4–10.4) | 12.4 ± 2.0 (11.9–12.9) | <0.001 ** | |||
| Pancreatic echogenicity (compared to liver) | Isoechoic | 42 | 60.9 | 60 | 76.9 | 0.035 * |
| Hypo/Hyperechoic | 27 | 39.1 | 18 | 23.1 | ||
| Isoechoic | Non-Isoechogenic | p * | ||||
|---|---|---|---|---|---|---|
| Patient Group (n:69) | n | % | n | % | ||
| Sex | Female | 11 | 57.9 | 8 | 42.1 | 0.755 |
| Male | 31 | 62.0 | 19 | 38.0 | ||
| Age, years, Mean ± SD | 8.6 ± 4.5 (7.2–10.0) | 11.8 ± 4.5 (10.1–13.5) | 0.005 ** | |||
| Diagnosis Glucose, mg/dL, Mean ± SD | 463.9 ± 149.9 (417.2–510.6) | 473.4 ± 149.8 (414.1–532.7) | 0.798 ** | |||
| Diagnosis Insulin, Mean ± SD | 2.9 ± 4.5 (1.5–4.3) | 2.9 ± 2.0 (2.1–3.7) | 0.996 ** | |||
| Diagnosis C-peptide, µg/L, Mean ± SD | 0.5 ± 0.3 (0.4–0.6) | 0.6 ± 0.4 (0.4–0.8) | 0.080 ** | |||
| Diagnosis HgbA1C, Mean ± SD | 11.8 ± 2.3% (11.1–12.5) | 12.2 ± 1.7% (11.5–12.9) | 0.368 ** | |||
| Diagnosis pH, Mean ± SD | 7.3 ± 0.2 (7.2–7.4) | 7.3 ± 0.1 (7.3–7.3) | 0.568 ** | |||
| Diagnosis HCO3, mmol/L, Mean ± SD | 15.0 ± 7.2 (12.8–17.2) | 15.5 ± 7.7 (12.5–18.5) | 0.810 ** | |||
| Diagnosis Ketone, mmol/L, Mean ± SD | 4.0 ± 2.1 (3.3–4.7) | 3.3 ± 2.0 (2.5–4.1) | 0.202 ** | |||
| Anti GAD | Positive | 24 | 60.0 | 16 | 40.0 | 0.953 |
| Negative | 17 | 60.7 | 11 | 39.3 | ||
| Anti-GAD Level IU/mL, Mean ± SD | 76.7 ± 95.6 (46.9–106.5) | 48.1 ± 61.8 (23.7–72.5) | 0.139 ** | |||
| Islet Cell Antibody | Positive | 22 | 64.7 | 12 | 35.3 | 0.457 |
| Negative | 19 | 55.9 | 15 | 44.1 | ||
| Islet Cell Antibody Level U/mL, Mean ± SD | 61.2 ± 76.0 (37.5–84.9) | 46.5 ± 57.7 (23.7–69.3) | 0.396 ** | |||
| Insulin Antibody | Positive | 6 | 85.7 | 1 | 14.3 | 0.223 |
| Negative | 31 | 54.4 | 26 | 45.6 | ||
| Insulin Antibody Level, Mean ± SD | 6.1 ± 5.0% (4.5–7.7) | 5.3 ± 4.2% (3.6–7.0) | 0.465 ** | |||
| Number of Antibody Positivity | None | 8 | 44.4 | 10 | 55.6 | 0.506 |
| 1 | 13 | 68.4 | 6 | 31.6 | ||
| 2 | 15 | 60.0 | 10 | 40.0 | ||
| 3 | 1 | 50.0 | 1 | 50.0 | ||
| 0–6 Year Olds | 7–12 Year Olds | ≥13 Year Olds | p | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Sex | Female | 3 | 15.0 | 12 | 44.4 | 4 | 18.2 | 0.041 * |
| Male | 17 | 85.0 | 15 | 55.6 | 18 | 81.8 | ||
| Pancreatic echogenicity | Isoechoic | 16 | 80.0 a | 17 | 63.0 a,b | 9 | 40.9 b | 0.033 * |
| Hypo/Hyperechoic | 4 | 20.0 | 10 | 37.0 | 13 | 59.1 | ||
| Diagnosis Glucose, mg/dL, Mean ± SD | 473.3 ± 92.9 (428–518) | 472.9 ± 177.8 (406–543) | 456.0 ± 156.7 (386–525) | 0.909 ** | ||||
| Diagnosis Insulin, Mean ± SD | 3.1 ± 6.4 (0.1–6.1) | 2.8 ± 1.8 (2.1–3.5) | 3.0 ± 1.6 (2.3–3.7) | 0.981 ** | ||||
| Diagnosis C-peptide, µg/L, Mean ± SD | 0.4 ± 0.2 a (0.3–0.5) | 0.6 ± 0.3 a,b (0.5–0.7) | 0.7 ± 0.3 b (0.6–0.8) | 0.002 ** | ||||
| Diagnosis HgbA1C, Mean ± SD | 11.1 ± 1.9 a (10.2–12.0) | 11.9 ± 2.0 a,b (11.1–12.7) | 12.7 ± 2.1 b (11.8–13.6) | 0.048 ** | ||||
| Diagnosis pH, Mean ± SD | 7.3 ± 0.1 (7.3–7.3) | 7.3 ± 0.2 (7.2–7.4) | 7.3 ± 0.1 (7.3–7.3) | 0.519 ** | ||||
| Diagnosis HCO3, mmol/L, Mean ± SD | 12.4 ± 7.0 (9.1–15.7) | 16.8 ± 6.9 (14.1–19.5) | 15.9 ± 7.8 (12.4–19.4) | 0.117 ** | ||||
| Diagnosis Ketone, mmol/L, Mean ± SD | 4.7 ± 1.8 a (3.9–5.5) | 3.0 ± 2.0 b (2.2–3.8) | 3.7 ± 2.1 a,b (2.8–4.6) | 0.035 ** | ||||
| Anti GAD | Positive | 7 | 36.8 a | 16 | 59.3 a,b | 17 | 77.3 b | 0.032 * |
| Negative | 12 | 63.2 | 11 | 40.7 | 5 | 22.7 | ||
| Islet Cell Antibody | Positive | 6 | 31.6 | 17 | 63.0 | 11 | 50.0 | 0.111 * |
| Negative | 13 | 68.4 | 10 | 37.0 | 11 | 50.0 | ||
| Insulin Antibody | Positive | 6 | 31.6 a | 0 | 0.0 b | 1 | 4.8 a,b | 0.002 * |
| Negative | 13 | 68.4 | 24 | 100.0 | 20 | 95.2 | ||
| Anti-GAD Level IU/mL, Mean ± SD | 44.0 ± 65.0 (13.6–74.4) | 75.3 ± 97.4 (36.6–113.8) | 71.6 ± 82.6 (35.0–108.2) | 0.432 ** | ||||
| Islet Cell Antibody Level U/mL, Mean ± SD | 28.9 ± 41.8 (9.3–48.5) | 67.9 ± 77.6 (37.2–98.6) | 62.9 ± 73.6 (30.3–95.5) | 0.140 ** | ||||
| Insulin Antibody Level, Mean ± SD | 7.9 ± 6.4 a (4.9–10.9) | 4.6 ± 1.6 b (4.0–5.2) | 5.2 ± 4.8 a,b (3.1–7.3) | 0.045 ** | ||||
| Number of Antibody Positivity | 0 | 9 | 47.4 | 5 | 20.8 | 4 | 19.0 | 0.160 * |
| 1 | 2 | 10.5 | 9 | 37.5 | 8 | 38.1 | ||
| 2 | 7 | 36.8 | 10 | 41.7 | 8 | 38.1 | ||
| 3 | 1 | 5.3 | 0 | 0.0 | 1 | 4.8 | ||
| Symptom Duration (Days), Mean ± SD | 24.5 ± 23.2 (18.9–30.1) | ||
|---|---|---|---|
| BMI SDS, Mean ± SD | 0.01 ± 1.12 (−0.3–0.3) | ||
| Dehydration Severity | Mild | 4 | 5.8 |
| Moderate | 51 | 73.9 | |
| Severe | 6 | 8.7 | |
| None | 8 | 11.6 | |
| Initial Diagnosis | Hyperglycemia | 26 | 37.7 |
| Ketosis | 16 | 23.2 | |
| Ketoacidosis | 27 | 39.1 | |
| DKA Severity | Mild | 8 | 29.6 |
| Moderate | 7 | 25.9 | |
| Severe | 12 | 44.4 | |
| Diagnosis Glucose, mg/dL, Mean ± SD | 467.6 ± 148.8 (431.9–503.3) | ||
| Diagnosis Ketone, mmol/L, Mean ± SD | 3.8 ± 2.1 (3.3–4.3) | ||
| Diagnosis Insulin, Mean ± SD | 2.9 ± 3.7 (2.0–3.8) | ||
| Diagnosis C-peptide, µg/L, Mean ± SD | 0.5 ± 0.3 (0.4–0.6) | ||
| Diagnosis HgbA1C, Mean ± SD | 11.9 ± 2.1% (11.4–12.4) | ||
| Diagnosis pH, Mean ± SD | 7.3 ± 0.1 (7.3–7.3) | ||
| Diagnosis HCO3, mmol/L, Mean ± SD | 15.2 ± 7.3 (13.4–17.0) | ||
| Anti GAD | Positive | 40 | 58.8 |
| Negative | 28 | 41.2 | |
| Anti-GAD Level (IU/mL), Mean ± SD | 65.4 ± 84.5 (45.1–85.7) | ||
| Islet Cell Antibody | Positive | 34 | 50.0 |
| Negative | 34 | 50.0 | |
| Islet Cell Antibody Level U/mL, Mean ± SD | 55.4 ± 69.2 (38.8–72.0) | ||
| Insulin Antibody | Positive | 7 | 10.9 |
| Negative | 57 | 89.1 | |
| Insulin Antibody Level, Mean ± SD | 5.8 ± 4.7% (4.7–6.9) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özer, E.; Tığrak, S.; Seçil Ekşioğlu, A.; Kocaay, P.; Bitkay, A.; Toksoy Adıgüzel, K.; Boyraz, M.; Gürbüz, F. Pancreatic Ultrasound Features at Diagnosis of Type 1 Diabetes: Age-Related Differences in Children. J. Clin. Med. 2025, 14, 7490. https://doi.org/10.3390/jcm14217490
Özer E, Tığrak S, Seçil Ekşioğlu A, Kocaay P, Bitkay A, Toksoy Adıgüzel K, Boyraz M, Gürbüz F. Pancreatic Ultrasound Features at Diagnosis of Type 1 Diabetes: Age-Related Differences in Children. Journal of Clinical Medicine. 2025; 14(21):7490. https://doi.org/10.3390/jcm14217490
Chicago/Turabian StyleÖzer, Emre, Sefa Tığrak, Ayşe Seçil Ekşioğlu, Pınar Kocaay, Abdurrahman Bitkay, Keziban Toksoy Adıgüzel, Mehmet Boyraz, and Fatih Gürbüz. 2025. "Pancreatic Ultrasound Features at Diagnosis of Type 1 Diabetes: Age-Related Differences in Children" Journal of Clinical Medicine 14, no. 21: 7490. https://doi.org/10.3390/jcm14217490
APA StyleÖzer, E., Tığrak, S., Seçil Ekşioğlu, A., Kocaay, P., Bitkay, A., Toksoy Adıgüzel, K., Boyraz, M., & Gürbüz, F. (2025). Pancreatic Ultrasound Features at Diagnosis of Type 1 Diabetes: Age-Related Differences in Children. Journal of Clinical Medicine, 14(21), 7490. https://doi.org/10.3390/jcm14217490






