The Contemporary Role of Cardiovascular Magnetic Resonance in Ischemic Cardiomyopathy
Abstract
1. Introduction
2. CMR Overview
3. CMR Role for Functional Assessment
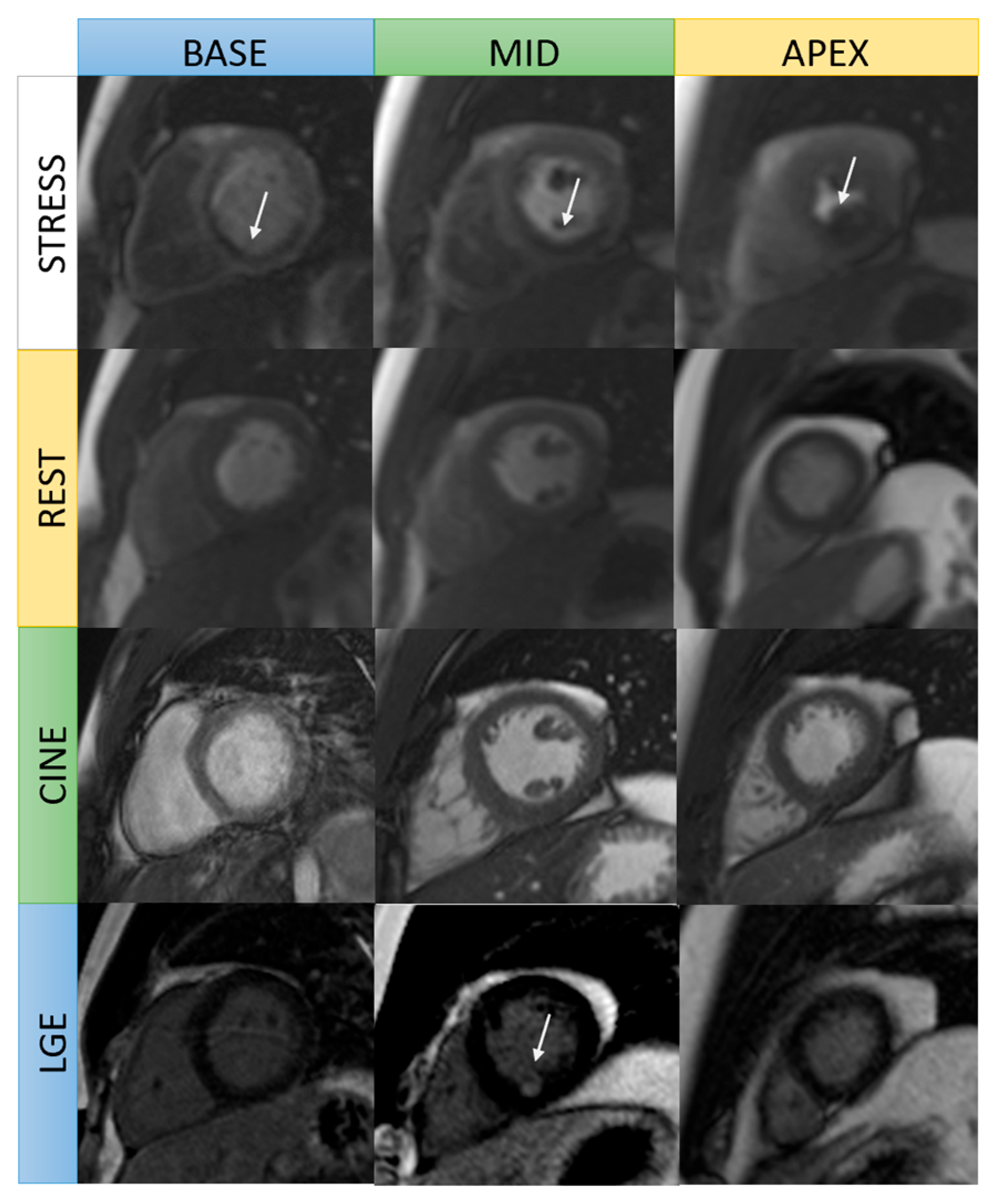
4. Stress Perfusion CMR
4.1. Vasodilator Stress Perfusion
4.2. Inotropic Stress (Dobutamine) for Wall Motion and Contractile Reserve
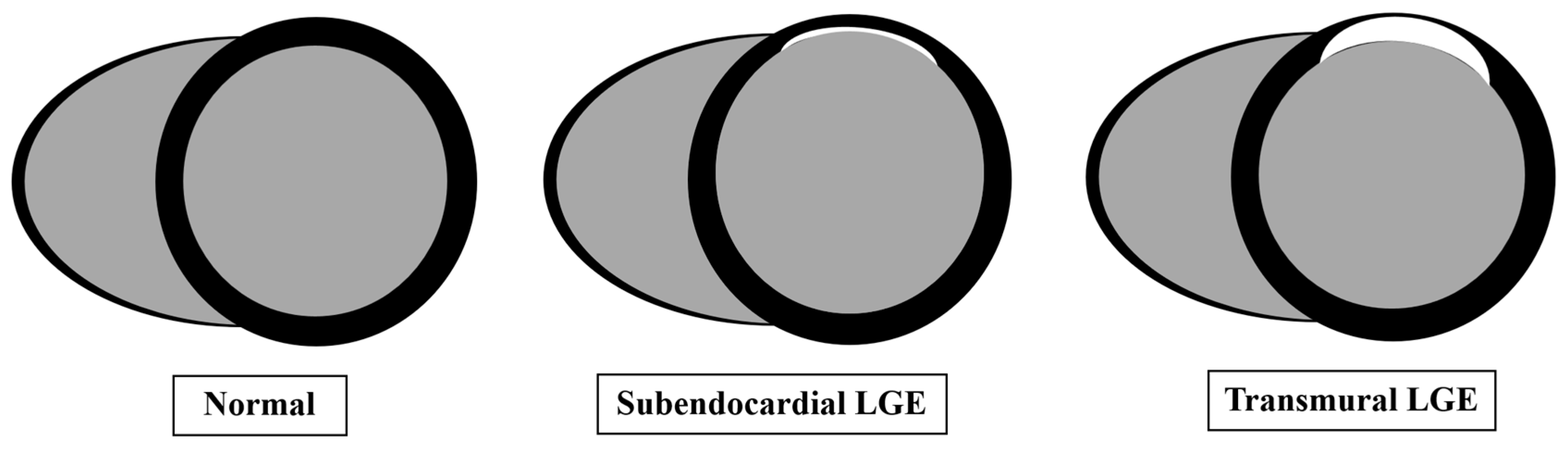
5. CMR Role in Viability
6. CMR Role in Prognostication
7. Recent Trials and Clinical CMR Application in Guiding Revascularization
8. Future Directions
9. Conclusions and Future Outlook
10. Take-Home Messages
- Contemporary CMR has evolved from a diagnostic adjunct to a central tool in the the phenotyping and prognostication of ischemic cardiomyopathy.
- Quantitative mapping and automated perfusion analyses now bridge the gap between structural and functional assessment, offering reproducible metrics that could reshape clinical decision-making.
- While the prognostic value of LGE is established, its integration with artificial intelligence and stress perfusion imaging offers opportunities for personalized risk stratification.
- The divergence between STICH and REVIVED trials highlights the need to redefine the role of CMR-derived viability beyond revascularization candidacy, toward comprehensive myocardial health assessment. This requires further research.
- Future work should focus on standardizing quantitative techniques and integrating CMR with multimodal imaging and molecular markers to refine management of ischemic LV dysfunction.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Airhart, S.; Murali, S. Chapter Ischemic Cardiomyopathy. In Encyclopedia of Cardiovascular Research and Medicine; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Pastena, P.; Frye, J.T.; Ho, C.; Goldschmidt, M.E.; Kalogeropoulos, A.P. Ischemic Cardiomyopathy: Epidemiology, Pathophysiology, Outcomes, and Therapeutic Options. Heart Fail. Rev. 2024, 29, 287–299. [Google Scholar] [CrossRef]
- Buono, M.G.D.; Moroni, F.; Montone, R.A.; Azzalani, L. Ischemic Cardiomyopathy and Heart Failure After Acute Myocardial Infarction. Curr. Cardiol. Rep. 2022, 24, 1505–1515. [Google Scholar] [CrossRef]
- Briceno, N.; Schuster, A.; Lumley, M.; Perera, D. Ischaemic Cardiomyopathy: Pathophysiology, Assessment and The Role of Revascularisation. Heart 2016, 102, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Panza, J.A.; Chrzanowski, L.; Bonow, R.O. Myocardial Viability Assessment Before Surgical Revascularization in Ischemic Cardiomyopathy. JACC 2021, 78, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Scatteia, A.; Dellegrottaglie, S. Cardiac Magnetic Resonance in Ischemic Cardiomyopathy: Present Role and Future Directions. Eur. Heart J. Suppl. 2023, 25 (Suppl. C), C58–C62. [Google Scholar] [CrossRef]
- Emrich, T.; Halfmann, M.; Schoepf, U.J.; Kreitner, K.F. CMR for Myocardial Characterization in Ischemic Heart Disease: State-Of-The-Art and Future Developments. Eur. Radiol. Exp. 2021, 5, 14. [Google Scholar] [CrossRef]
- Doesch, C.; Papavassiliu, T. Diagnosis and Management of Ischemic Cardiomyopathy: Role of Cardiovascular Magnetic Resonance Imaging. World J. Cardiol. 2014, 6, 1166–1174. [Google Scholar] [CrossRef]
- Garcia, M.J.; Kwong, R.Y.; Scherrer-Crosbie, M.; Taub, C.C.; Blankstein, R.; Lima, J.; Bonow, R.O.; Eshtehardi, P.; Bois, J.P. State of the Art: Imaging for Myocardial Viability: A Scientific Statement From the American Heart Association. Circ. Cardiovasc. Imaging 2020, 13, e000053. [Google Scholar] [CrossRef]
- Schiau, C.; Dudea, S.M.; Manole, S. Cardiovascular Magnetic Resonance: Contribution to The Exploration of Cardiomyopathies. Med. Pharm. Rep. 2019, 92, 326–336. [Google Scholar] [CrossRef]
- Winchester, D.; Maron, D.; Blankstein, R.; Chang, I.C.; Kirtane, A.J.; Kwong, R.Y.; Pellikka, P.A.; Prutkin, J.M.; Russell, R.; Sandhu, A.T. ACC/AHA/ASE/ASNC/ASPC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2023 Multimodality Appropriate Use Criteria for the Detection and Risk Assessment of Chronic Coronary Disease. JACC 2023, 81, 2445–2467. [Google Scholar] [CrossRef] [PubMed]
- Arlene, S.; Mojdeh, M.; Seth, J.K.; Daniel, W.G.; Allen, P.B.; Faraz, K.; Charles, S.W.; Andrew, E.A. Ischemic Heart Disease: Noninvasive Imaging Techniques and Findings. RadioGraphics 2021, 41, 990–1021. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Guglielmo, M.; Serra, A.; Gatti, M.; Volpato, V.; Schoepf, U.J.; Saba, L.; Cau, R.; Faletti, R.; McGill, L.J.; et al. Multimodality Imaging in Ischemic Chronic Cardiomyopathy. J. Imaging 2022, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Gaine, S.P.; Sharma, G.; Tower-Rader, A.; Botros, M.; Kovell, L.; Parakh, A.; Wood, M.J.; Harrington, C.M. Multimodality Imaging in the Detection of Ischemic Heart Disease in Women. J. Cardiovasc. Dev. Dis 2022, 9, 350. [Google Scholar] [CrossRef] [PubMed]
- Kitai, T.; Kohsaka, S.; Kato, T.; Kato, E.; Sato, K.; Teramoto, K.; Yaku, H.; Akiyama, E.; Ando, M.; Izumi, C.; et al. JCS/JHFS 2025 Guideline on Diagnosis and Treatment of Heart Failure. Circ. J. 2025, 89, 1579–1581. [Google Scholar] [CrossRef] [PubMed]
- Ralota, K.K.; Layland, J.; Han Win, K.T.; Htun, N.M. Myocardial Viability: Evolving Insights and Challenges in Revascularization and Functional Recovery. J. Cardiovasc. Dev. Dis 2025, 12, 106. [Google Scholar] [CrossRef]
- Baer, F.M.; Theissen, P.; Schneider, C.A.; Voth, E.; Sechtem, U.; Schicha, H.; Erdmann, E. Dobutamine Magnetic Reso-nance Imaging Predicts Contractile Recovery of Chronically Dysfunctional Myocardium After Successful Revasculari-zation. J. Am. Coll. Cardiol. 1998, 31, 1040–1048. [Google Scholar] [CrossRef]
- Shah, D.J.; Kim, H.W.; James, O.; Parker, M.; Wu, E.; Bonow, R.O.; Judd, R.M.; Kim, R.J. Prevalence of regional myocardial thinning and relationship with myocardial scarring in patients with coronary artery disease. JAMA 2013, 309, 909–918. [Google Scholar] [CrossRef]
- Siddiqui, T.A.; Chamarti, K.S.; Tou, L.C.; Demirjian, G.A.; Noorani, S.; Zink, S.; Umair, M. The Merits, Limitations, and Future Directions of Cost-Effectiveness Analysis in Cardiac MRI with a Focus on Coronary Artery Disease: A Literature Review. J. Cardiovasc. Dev. Dis. 2022, 9, 357. [Google Scholar] [CrossRef]
- Baritussio, A.; Scatteia, A.; Bucciarelli-Ducci, C. Role of Cardiovascular Magnetic Resonance in Acute and Chronic Ischemic Heart Disease. Int. J. Cardiovasc. Imaging 2018, 4, 67–80. [Google Scholar] [CrossRef]
- Vrints, C.; Andreotti, V.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for The Management of Chronic Coronary Syndromes. Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef]
- Catania, R.; Quinn, S.; Rahsepar, A.A.; Trabzonlu, T.A.; Bisen, J.B.; Chow, K.; Lee, D.C.; Avery, R.; Kellman, P.; Allen, B.D. Quantitative Stress First-Pass Perfusion Cardiac MRI: State of the Art. RadioGraphics 2025, 45, e240115. [Google Scholar] [CrossRef]
- Motwani, M.; Kidambi, A.; Greenwood, J.P.; Plein, S. Advances in Cardiovascular Magnetic Resonance in Ischaemic Heart Disease and Non-Ischaemic Cardiomyopathies. Heart 2014, 100, 1722–1733. [Google Scholar] [CrossRef]
- Dhorl-Pathil, A.; Aneja, A. Role of Cardiovascular Magnetic Resonance in Ischemic Cardiomyopathy. Heart Fail. Clin. 2021, 17, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Sin, J.; Yan, A.T.; Wang, H.; Lu, J.; Li, Y.; Kim, P.; Patel, A.R.; Ng, M.Y. Qualitative and Quantitative Stress Perfusion Cardiac Magnetic Resonance in Clinical Practice: A Comprehensive Review. Diagnostics 2023, 13, 524. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, J.; Karamitsos, T.D.; Myerson, S.G.; Francis, J.M.; Neubauer, S. Stress Perfusion Imaging Using Cardiovascular Magnetic Resonance: A Review. Heart Lung Circ. 2010, 19, 697–705. [Google Scholar] [CrossRef]
- Korosoglou, G.; Lehrke, S.; Wochele, A.; Hoerig, B.; Lossnitzer, D.; Steen, H.; Giannitsis, E.; Osman, N.F.; Katus, H.A. Strain-Encoded CMR For The Detection of Inducible Ischemia During Intermediate Stress. JACC Cardiovasc. Imaging 2010, 3, 361–371. [Google Scholar] [CrossRef]
- Velazquez, E.J.; Lee, K.L.; Deja, M.A.; Jain, A.; Sopko, G.; Marchenko, A.; Ali, I.S.; Pohost, G.; Gradinac, S.; Abraham, W.T.; et al. Coronary-Artery Bypass Surgery in Patients with Left Ventricular Dysfunction. N. Engl. J. Med. 2011, 364, 1607–1616. [Google Scholar] [CrossRef]
- Kim, R.J.; Wu, E.; Rafael, A.; Chen, E.L.; Parker, M.A.; Simonetti, O.; Klocke, F.J.; Bonow, R.O.; Judd, R.M. The Use of Contrast-Enhanced Magnetic Resonance Imaging to Identify Reversible Myocardial Dysfunction. N. Engl. J. Med. 2000, 343, 1445–1453. [Google Scholar] [CrossRef]
- Meier, C.; Eisenblätter, M.; Gielen, S. Myocardial Late Gadolinium Enhancement (LGE) in Cardiac Magnetic Resonance Imaging (CMR)—An Important Risk Marker for Cardiac Disease. J. Cardiovasc. Dev. Dis. 2024, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Aquaro, G.D.; Gori, C.D.; Faggioni, L.; Parisella, M.L.; Cioni, D.; Lencioni, R.; Neri, A. Diagnostic and Prognostic Role of Late Gadolinium Enhancement in Cardiomyopathies. Eur. Heart J. Suppl. 2023, 25 (Suppl. C), C130–C136. [Google Scholar] [CrossRef]
- Becker, M.A.J.; Van der Lingen, A.L.C.J.; Cornel, J.H.; van de Ven, P.; van Rossum, A.C.; Allaart, C.P.; Germans, T. Septal Midwall Late Gadolinium Enhancement in Ischemic Cardiomyopathy and Nonischemic Dilated Cardiomyopathy—Characteristics and Prognosis. Am. J. Cardiol. 2023, 201, 294–301. [Google Scholar] [CrossRef]
- Unger, A.; Toupin, S.; Hovasse, T.; Garot, P.; Champagne, S.; Unterseeh, T.; Duhamel, S.; Hamzi, L.; Goncalves, T.; Dillinger, J.G.; et al. Prognostic Value of Additional Midwall Late Gadolinium Enhancement in Patients with Ischemic Cardiomyopathy. Eur. Heart J. 2024, 45, ehae666.230. [Google Scholar] [CrossRef]
- Romero, J.; Xue, X.; Gonzalez, W.; Garcia, M.J. CMR Imaging Assessing Viability in Patients with Chronic Ventricular Dysfunction Due to Coronary Artery Disease: A Meta-Analysis Of Prospective Trials. JACC Cardiovasc. Imaging 2012, 5, 494–508. [Google Scholar] [CrossRef]
- Kiberu, Y.; Jathanna, N.; Narayanan, N.; Vanezis, A.P.; Erhayiem, B.; Graham, A.; Copley, S.J. Myocardial Scar Imaging: Viability Beyond REVIVED. Curr. Cardiovasc. Imaging Rep. 2024, 17, 107–114. [Google Scholar] [CrossRef]
- Ge, Y.; Antiochos, P.; Steel, K.; Bingham, S.; Abdullah, S.; Chen, Y.Y.; Mikolich, J.R.; Arai, A.E.; Bandettini, W.P.; Shanbhag, S.M.; et al. Prognostic Value of Stress CMR Perfusion Imaging in Patients with Reduced Left Ventricular Function. JACC Cardiovasc. Imaging 2020, 13, 2132–2145. [Google Scholar] [CrossRef]
- Betemariam, T.A.; Morgan, H.; Perera, D. REVIVED BCIS-2: Update and key learnings. Curr. Opin. Cardiol. 2024, 39, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Ezad, A.M.; McEntegart, M.; Dodd, M.; Didagelos, M.; Sidik, N.; Wa, M.L.K.; Morgan, H.P. Impact of Anatomical and Viability-Guided Completeness of Revascularization on Clinical Outcomes in Ischemic Cardiomyopathy. JACC 2024, 84, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Liga, R.; Colli, A.; Taggart, D.P.; Boden, W.E.; Caterina, R.D. Myocardial Revascularization in Patients with Ischemic Cardiomyopathy: For Whom and How. J. Am. Heart Assoc. 2023, 12, e026943. [Google Scholar] [CrossRef]
- Perera, D.; Ryan, M.; Morgan, H.P.; Greenwood, J.P.; Petrie, M.C.; Dodd, M.; Weerackody, R.; O’kane, P.D.; Masci, P.G.; Nazir, M.S.; et al. Viability and Outcomes With Revascularization or Medical Therapy in Ischemic Ventricular Dysfunction: A Prespecified Secondary Analysis of the REVIVED-BCIS2 Trial. JAMA Cardiol. 2023, 8, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Amedeo, C.; Andrew, E.A.; Edward, D.; Li-Yueh, H.; Masaki, I.; Michael, J.; Sebastian, K.; Xenios, M.; Reza, N.; Sven, P.; et al. SCMR Expert Consensus Statement on Quantitative Myocardial Perfusion Cardiovascular Magnetic Resonance Imaging. JCMR, 2025, in press. [CrossRef]

| Modality | Advantages | Limitations |
|---|---|---|
| Echocardiography |
|
|
| Stress echocardiography |
|
|
| CCTA |
|
|
| Fractional flow reserve (FFRct) |
|
|
| Stress CT perfusion |
|
|
| Stress CMR |
|
|
| Single-photon emission computed tomography (SPECT) |
|
|
| Myocardial Positron Emission Tomography (PET) imaging |
|
|
| Step | Sequences | Acquisition Planes | Primary Goals |
|---|---|---|---|
| Anatomic survey | Bright blood and dark blood single-shot imaging | Axial, sagittal, coronal stack | Assessment of cardiovascular anatomy, planning of planes, and evaluation of extracardiac findings |
| Cine imaging | Balanced steady-state free precession imaging | Long axis-single slice: 2 chamber, 3 chamber, 4 chamber view | Assessment of cardiac anatomy and function |
| Stress perfusion (with adenosine infusion) | Real-time cine | 3 slices short axis: basal, mid, apical LV | Detection of inducible ischemia |
| Cine imaging | Balanced steady-state free precession imaging | Short-axis stack | Assessment of cardiac anatomy and 3D volume, and function |
| Rest perfusion | Real-time cine | 3 slices short axis: basal, mid, apical LV | Comparison to stress perfusion imaging |
| Late gadolinium enhancement (LGE) | LGE imaging: magnitude and phase sensitive inversion recovery | Short-axis stack Long axis-single slice: 2 chamber, 3 chamber, 4 chamber view | Detection of infarction or fibrosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Widyawati, D.G.; Teo, L.L.S.; Ong, C.C.; Houdmont, M.; Chai, P.; Sia, C.-H. The Contemporary Role of Cardiovascular Magnetic Resonance in Ischemic Cardiomyopathy. J. Clin. Med. 2025, 14, 7479. https://doi.org/10.3390/jcm14217479
Widyawati DG, Teo LLS, Ong CC, Houdmont M, Chai P, Sia C-H. The Contemporary Role of Cardiovascular Magnetic Resonance in Ischemic Cardiomyopathy. Journal of Clinical Medicine. 2025; 14(21):7479. https://doi.org/10.3390/jcm14217479
Chicago/Turabian StyleWidyawati, Desak Gede, Lynette L. S. Teo, Ching Ching Ong, Marie Houdmont, Ping Chai, and Ching-Hui Sia. 2025. "The Contemporary Role of Cardiovascular Magnetic Resonance in Ischemic Cardiomyopathy" Journal of Clinical Medicine 14, no. 21: 7479. https://doi.org/10.3390/jcm14217479
APA StyleWidyawati, D. G., Teo, L. L. S., Ong, C. C., Houdmont, M., Chai, P., & Sia, C.-H. (2025). The Contemporary Role of Cardiovascular Magnetic Resonance in Ischemic Cardiomyopathy. Journal of Clinical Medicine, 14(21), 7479. https://doi.org/10.3390/jcm14217479






