The Potential of Focal Muscle Vibration Therapy in the Management of Parkinson’s Disease: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. The Search Strategy
2.2. The Study Selection
2.3. Data Extraction
2.4. The PICO Question
- -
- P (Population): People diagnosed with PD;
- -
- I (Intervention): Focal muscle vibration therapy;
- -
- C (Comparison): Compared to placebo;
- -
- O (Outcome): Improvement in symptoms of patients included in the study (effects of vibration therapy on motor/functional recovery and disability reduction).
2.5. Bias Assessment
2.6. Findings and Certainty of Evidence (GRADE Assessment)
3. Results
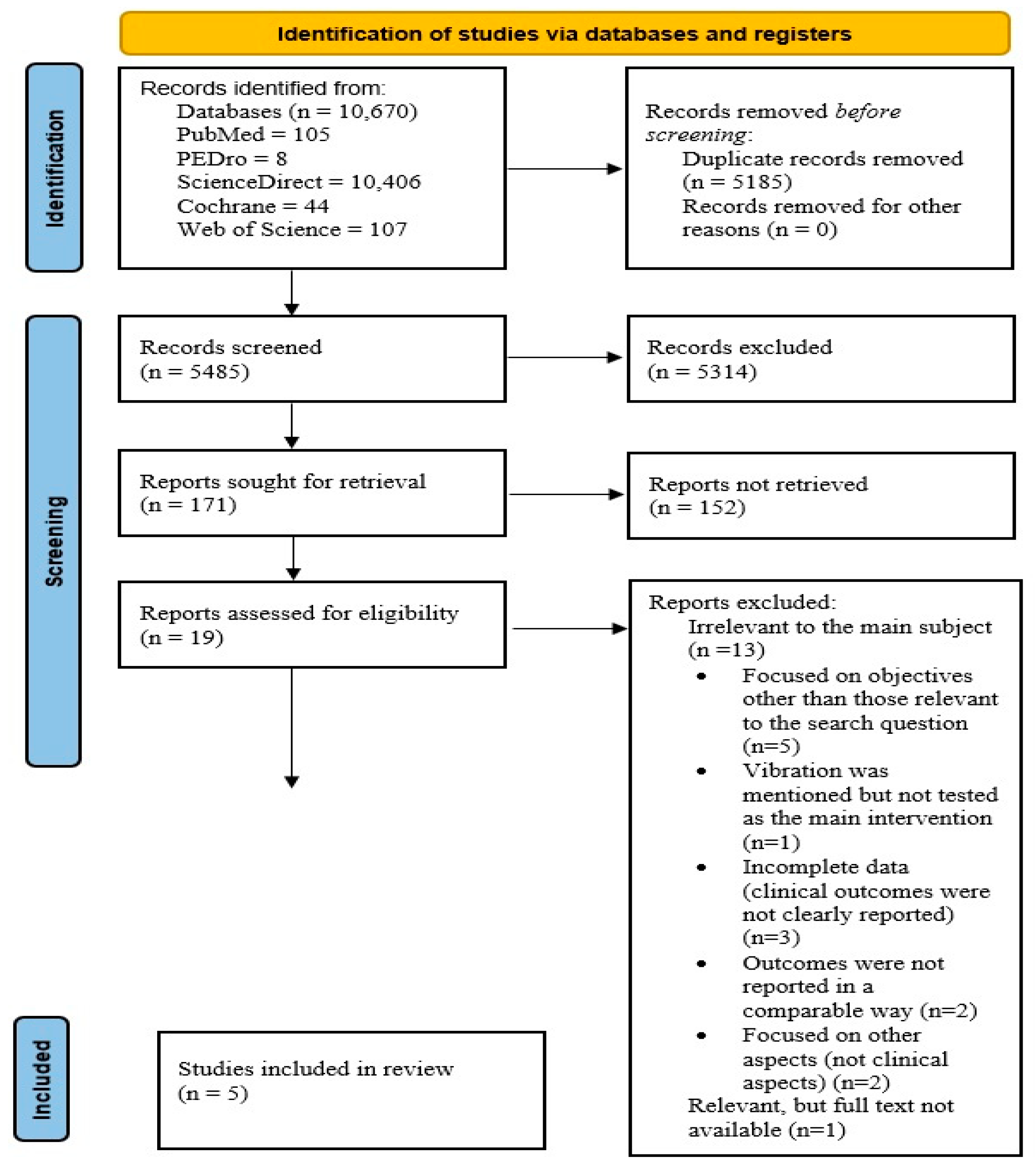
3.1. The Included Studies
3.2. Risk of Bias
3.3. GRADE Assessment
3.4. Detailed Description of the Studies
4. Discussion
4.1. Parkinson’s Disease in Light of Recent Evidence
4.2. Focal Vibration Therapy in PD—Evidence and Perspectives from the Literature
4.3. Evidence from the Studies Analyzed
4.4. The Strengths of the Study
4.5. Limitations of the Study
4.6. Future Research Directions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC-scale | Activities-specific Balance Confidence scale |
| AD | Alzheimer’s disease |
| AG | Active group |
| BBS | Berg Balance Scale |
| DBS | Deep brain stimulation |
| F | Female |
| FES | Falls Efficacy Scale |
| FMV | Focal muscle vibration |
| FOG | Freezing of gait |
| FVT | Focal vibration therapy |
| GA | Gait analysis |
| GRADE | Grading of Recommendations Assessment, Development and Evaluation |
| HFOs | High-frequency oscillations |
| LEDD | Levodopa equivalent daily dose |
| M | Male |
| MDUPDRS III | Movement disorder society-sponsored revision of the Unified Parkinson’s disease rating scale III |
| PD | Parkinson’s disease |
| PDQ-8 | Parkinson’s Disease Questionnaire |
| PDQ-39 | Parkinson’s Disease Questionnaire—39 |
| PEDro | Physiotherapy Evidence Database |
| PG | Placebo group |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| SCS | Spinal cord stimulation |
| SD | Standard deviation |
| sEMG | Surface electromyography |
| R-fMV | Repetitive sessions of focal muscle vibration |
| RoB 2 | Version 2 of the Cochrane risk-of-bias tool for randomized trials |
| TUG | Time Up and Go test |
| UPDRS-II | The Unified Parkinson’s Disease Rating Scale II |
| UPDRS-III | The Unified Parkinson’s Disease Rating Scale III |
| WBV | Whole-body vibration |
References
- Prajjwal, P.; Flores Sanga, H.S.; Acharya, K.; Tango, T.; John, J.; Rodriguez, R.S.C.; Dheyaa Marsool Marsool, M.; Sulaimanov, M.; Ahmed, A.; Hussin, O.A. Parkinson’s disease updates: Addressing the pathophysiology, risk factors, genetics, diagnosis, along with the medical and surgical treatment. Ann. Med. Surg. 2023, 85, 4887–4902. [Google Scholar] [CrossRef]
- Balestrino, R.; Schapira, A.H.V. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef]
- Zafar, S.; Yaddanapudi, S.S. Parkinson Disease. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Sveinbjornsdottir, S. The clinical symptoms of Parkinson’s disease. J. Neurochem. 2016, 139 (Suppl. 1), 318–324. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Murakami, H.; Shiraishi, T.; Umehara, T.; Omoto, S.; Iguchi, Y. Recent Advances in Drug Therapy for Parkinson’s Disease. Intern. Med. 2023, 62, 33–42. [Google Scholar] [CrossRef]
- Alotaibi, S.; Alfayez, L.; Alkhudhair, M. Parkinson’s Disease: Current Treatment Modalities and Emerging Therapies. Cureus 2024, 16, e75647. [Google Scholar] [CrossRef] [PubMed]
- Fattorini, L.; Pettorossi, V.E.; Marchetti, E.; Rodio, A.; Filippi, G.M. A review about muscle focal vibration contribution on spasticity recovery. Front. Neurol. 2025, 16, 1579118. [Google Scholar] [CrossRef]
- Tahir, S.; Baig, M.O.; Rathore, F.; Aslam, H. The emerging role of focal muscle vibration in rehabilitation of neurological disorders. J. Pak. Med. Assoc. 2022, 72, 2126–2128. [Google Scholar] [CrossRef]
- Filippi, G.M.; Rodio, A.; Fattorini, L.; Faralli, M.; Ricci, G.; Pettorossi, V.E. Plastic changes induced by muscle focal vibration: A possible mechanism for long-term motor improvements. Front. Neurosci. 2023, 17, 1112232. [Google Scholar] [CrossRef]
- Viganò, A.; Celletti, C.; Giuliani, G.; Jannini, T.B.; Marenco, F.; Maestrini, I.; Zumpano, R.; Vicenzini, E.; Altieri, M.; Camerota, F.; et al. Focal Muscle Vibration (fMV) for Post-Stroke Motor Recovery: Multisite Neuroplasticity Induction, Timing of Intervention, Clinical Approaches, and Prospects from a Narrative Review. Vibration 2023, 6, 645–658. [Google Scholar] [CrossRef]
- Calderone, A.; Galasso, S.; De Nunzio, A.M.; Leo, A.; Balletta, T.; Quartarone, A.; Calabrò, R.S. Exploring the Impact of Muscle Vibration Therapy in Neurologic Rehabilitation: A Systematic Review. Arch. Rehabil. Res. Clin. Transl. 2025, 7, 100478. [Google Scholar] [CrossRef]
- Giorgi, F.; Donati, D.; Platano, D.; Tedeschi, R. Focal Vibration Therapy for Motor Deficits and Spasticity Management in Post-Stroke Rehabilitation. Brain Sci. 2024, 14, 1060. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Schiavo, J.H. PROSPERO: An International Register of Systematic Review Protocols. Med. Ref. Serv. Q. 2019, 38, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.K.; Al Dhubaib, B.E. Zotero: A bibliographic assistant to researcher. J. Pharmacol. Pharmacother. 2011, 2, 303–305. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Babić, A.; Barcot, O.; Visković, T.; Šarić, F.; Kirkovski, A.; Barun, I.; Križanac, Z.; Ananda, R.A.; Barreiro, Y.V.F.; Malih, N.; et al. Frequency of use and adequacy of Cochrane risk of bias tool 2 in non-Cochrane systematic reviews published in 2020: Meta-research study. Res. Synth. Methods 2024, 15, 430–440. [Google Scholar] [CrossRef]
- Cochrane Methods Bias. Available online: https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials (accessed on 5 August 2025).
- De Cassai, A.; Boscolo, A.; Zarantonello, F.; Pettenuzzo, T.; Sella, N.; Geraldini, F.; Munari, M.; Navalesi, P. Enhancing study quality assessment: An in-depth review of risk of bias tools for meta-analysis-a comprehensive guide for anesthesiologists. J. Anesth. Analg. Crit. Care 2023, 3, 44. [Google Scholar] [CrossRef] [PubMed]
- Cochrane. Available online: https://www.cochrane.org/authors/handbooks-and-manuals/handbook/current/chapter-08#section-8-2-3 (accessed on 5 August 2025).
- Brozek, J.L.; Canelo-Aybar, C.; Akl, E.A.; Bowen, J.M.; Bucher, J.; Chiu, W.A.; Cronin, M.; Djulbegovic, B.; Falavigna, M.; Guyatt, G.H.; et al. GRADE Guidelines 30: The GRADE approach to assessing the certainty of modeled evidence-An overview in the context of health decision-making. J. Clin. Epidemiol. 2021, 129, 138–150. [Google Scholar] [CrossRef]
- Bezerra, C.T.; Grande, A.J.; Galvão, V.K.; Santos, D.H.M.D.; Atallah, Á.N.; Silva, V. Assessment of the strength of recommendation and quality of evidence: GRADE checklist. A descriptive study. Sao Paulo Med. J. 2022, 140, 829–836. [Google Scholar] [CrossRef]
- Volpe, D.; Giantin, M.G.; Fasano, A. A wearable proprioceptive stabilizer (Equistasi®) for rehabilitation of postural instability in Parkinson’s disease: A phase II randomized double-blind, double-dummy, controlled study. PLoS ONE 2014, 9, e112065. [Google Scholar] [CrossRef] [PubMed]
- Camerota, F.; Celletti, C.; Suppa, A.; Galli, M.; Cimolin, V.; Filippi, G.M.; La Torre, G.; Albertini, G.; Stocchi, F.; De Pandis, M.F. Focal Muscle Vibration Improves Gait in Parkinson’s Disease: A Pilot Randomized, Controlled Trial. Mov. Disord. Clin. Pract. 2016, 3, 559–566. [Google Scholar] [CrossRef]
- Peppe, A.; Paravati, S.; Baldassarre, M.G.; Bakdounes, L.; Spolaor, F.; Guiotto, A.; Pavan, D.; Sawacha, Z.; Bottino, S.; Clerici, D.; et al. Proprioceptive Focal Stimulation (Equistasi®) May Improve the Quality of Gait in Middle-Moderate Parkinson’s Disease Patients. Double-Blind, Double-Dummy, Randomized, Crossover, Italian Multicentric Study. Front. Neurol. 2019, 10, 998. [Google Scholar] [CrossRef]
- Romanato, M.; Guiotto, A.; Spolaor, F.; Bakdounes, L.; Baldassarre, G.; Cucca, A.; Peppe, A.; Volpe, D.; Sawacha, Z. Changes of biomechanics induced by Equistasi® in Parkinson’s disease: Coupling between balance and lower limb joints kinematics. Med. Biol. Eng. Comput. 2021, 59, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Spolaor, F.; Romanato, M.; Annamaria, G.; Peppe, A.; Bakdounes, L.; To, D.K.; Volpe, D.; Sawacha, Z. Relationship between Muscular Activity and Postural Control Changes after Proprioceptive Focal Stimulation (Equistasi®) in Middle-Moderate Parkinson’s Disease Patients: An Explorative Study. Sensors 2021, 21, 560. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shlomo, Y.; Darweesh, S.; Llibre-Guerra, J.; Marras, C.; San Luciano, M.; Tanner, C. The epidemiology of Parkinson’s disease. Lancet 2024, 403, 283–292. [Google Scholar] [CrossRef]
- Spolaor, F.; Guiotto, A.; Pavan, D.; Arab Yaghoubi, L.; Peppe, A.; Paone, P.; Sawacha, Z.; Volpe, D. The neurorehabilitation device Equistasi® impacts positively on the gait of Parkinson’s disease subjects. Gait Posture 2018, 66, S37–S38. [Google Scholar] [CrossRef]
- Han, J.; Jung, J.; Lee, J.; Kim, E.; Lee, M.; Lee, K. Effect of muscle vibration on postural balance of Parkinson’s diseases patients in bipedal quiet standing. J. Phys. Ther. Sci. 2013, 25, 1433–1435. [Google Scholar] [CrossRef]
- Nanhoe-Mahabier, W.; Allum, J.H.; Pasman, E.P.; Overeem, S.; Bloem, B.R. The effects of vibrotactile biofeedback training on trunk sway in Parkinson’s disease patients. Parkinsonism Relat. Disord. 2012, 18, 1017–1021. [Google Scholar] [CrossRef]
- Kim, S.H.; Yun, S.J.; Dang, Q.K.; Chee, Y.; Chung, S.G.; Oh, B.M.; Kim, K.; Seo, H.G. Measurement and Correction of Stooped Posture during Gait Using Wearable Sensors in Patients with Parkinsonism: A Preliminary Study. Sensors 2021, 21, 2379. [Google Scholar] [CrossRef]
- Abbruzzese, G.; Trompetto, C.; Mori, L.; Pelosin, E. Proprioceptive rehabilitation of upper limb dysfunction in movement disorders: A clinical perspective. Front. Hum. Neurosci. 2014, 8, 961. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, J.; Morigaki, R.; Yamamoto, N.; Oda, T.; Nakanishi, H.; Izumi, Y.; Takagi, Y. Therapeutic Devices for Motor Symptoms in Parkinson’s Disease: Current Progress and a Systematic Review of Recent Randomized Controlled Trials. Front. Aging Neurosci. 2022, 14, 807909. [Google Scholar] [CrossRef]
- Abbruzzese, G.; Marchese, R.; Avanzino, L.; Pelosin, E. Rehabilitation for Parkinson’s disease: Current outlook and future challenges. Parkinsonism Relat. Disord. 2016, 22 (Suppl. 1), S60–S64. [Google Scholar] [CrossRef]
- Goldman, J.G.; Volpe, D.; Ellis, T.D.; Hirsch, M.A.; Johnson, J.; Wood, J.; Aragon, A.; Biundo, R.; Di Rocco, A.; Kasman, G.S.; et al. Delivering Multidisciplinary Rehabilitation Care in Parkinson’s Disease: An International Consensus Statement. J. Parkinsons Dis. 2024, 14, 135–166. [Google Scholar] [CrossRef]
- Fattorini, L.; Rodio, A.; Filippi, G.M.; Pettorossi, V.E. Effectiveness of Focal Muscle Vibration in the Recovery of Neuromotor Hypofunction: A Systematic Review. J. Funct. Morphol. Kinesiol. 2023, 8, 103. [Google Scholar] [CrossRef]
- Murillo, N.; Valls-Sole, J.; Vidal, J.; Opisso, E.; Medina, J.; Kumru, H. Focal vibration in neurorehabilitation. Eur. J. Phys. Rehabil. Med. 2014, 50, 231–242. [Google Scholar]
- Punin, C.; Barzallo, B.; Clotet, R.; Bermeo, A.; Bravo, M.; Bermeo, J.P.; Llumiguano, C. A Non-Invasive Medical Device for Parkinson’s Patients with Episodes of Freezing of Gait. Sensors 2019, 19, 737. [Google Scholar] [CrossRef]
- Cruciani, A.; Lanzone, J.; Musumeci, G.; Di Lazzaro, V.; Marano, M. Focal vibrations enhance somatosensory facilitation in healthy subjects: A pilot study on Equistasi® and high-frequency oscillations. Front. Neurol. 2022, 13, 1052989. [Google Scholar] [CrossRef] [PubMed]
- Buonanno, P.; Iacovazzo, C.; Marra, A.; de Siena, A.U.; Josu, T.; Zampi, M.; Sedda, D.; Servillo, G.; Vargas, M. Potential Role of Focal Microvibration (Equistasi®) in the Management of Chronic Pain: A Pilot Study. Pain. Ther. 2024, 13, 185–198. [Google Scholar] [CrossRef]
- De Nunzio, A.M.; Grasso, M.; Nardone, A.; Godi, M.; Schieppati, M. Alternate rhythmic vibratory stimulation of trunk muscles affects walking cadence and velocity in Parkinson’s disease. Clin. Neurophysiol. 2010, 121, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Moggio, L.; de Sire, A.; Marotta, N.; Demeco, A.; Ammendolia, A. Vibration therapy role in neurological diseases rehabilitation: An umbrella review of systematic reviews. Disabil. Rehabil. 2022, 44, 5741–5749. [Google Scholar] [CrossRef]
- Alashram, A.R.; Annino, G.; Romagnoli, C.; Raju, M.; Padua, E. Proprioceptive Focal Stimulation (Equistasi®) for gait and postural balance rehabilitation in patients with Parkinson’s disease: A systematic review. Proc. Inst. Mech. Eng. H 2023, 237, 179–189. [Google Scholar] [CrossRef]
- Serio, F.; Minosa, C.; De Luca, M.; Conte, P.; Albani, G.; Peppe, A. Focal Vibration Training (Equistasi®) to Improve Posture Stability. A Retrospective Study in Parkinson’s Disease. Sensors 2019, 19, 2101. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.J.; Park, H.S. Analysis of the tonic vibration reflex: Influence of vibration variables on motor unit synchronization and fatigue. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 75, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Azzollini, V.; Fragapane, N.; Baster, Z.; Carozzo, S.; Dalise, S.; Chisari, C. Focal muscle vibration and action observation: A combined approach for muscle strengthening. Eur. J. Transl. Myol. 2024, 34, 12366. [Google Scholar] [CrossRef] [PubMed]
- Alashram, A.R.; Padua, E.; Romagnoli, C.; Raju, M.; Annino, G. Clinical effectiveness of focal muscle vibration on gait and postural stability in individuals with neurological disorders: A systematic review. Physiother. Res. Int. 2022, 27, e1945. [Google Scholar] [CrossRef]
- Ciortea, V.M.; Motoasca, I.; Borda, I.M.; Ungur, R.A.; Bondor, C.I.; Iliescu, M.G.; Ciubean, A.D.; Lazar, I.; Bendea, E.; Irsay, L. Effects of High-Intensity Electromagnetic Stimulation on Reducing Upper Limb Spasticity in Post-Stroke Patients. Appl. Sci. 2022, 12, 2125. [Google Scholar] [CrossRef]
- Irsay, L.; Ungur, R.A.; Borda, I.M.; Tica, I.; Iliescu, M.G.; Ciubean, A.D.; Popa, T.; Cinteza, D.; Popa, F.L.; Bondor, C.I.; et al. Safety of Electrotherapy Treatment in Patients with Knee Osteoarthritis and Cardiac Diseases. Life 2022, 12, 1690. [Google Scholar] [CrossRef]
- Fattorini, L.; Rodio, A.; Pettorossi, V.E.; Filippi, G.M. Is the Focal Muscle Vibration an Effective Motor Conditioning Intervention? A Systematic Review. J. Funct. Morphol. Kinesiol. 2021, 6, 39. [Google Scholar] [CrossRef]
- Avvantaggiato, C.; Casale, R.; Cinone, N.; Facciorusso, S.; Turitto, A.; Stuppiello, L.; Picelli, A.; Ranieri, M.; Intiso, D.; Fiore, P.; et al. Localized muscle vibration in the treatment of motor impairment and spasticity in post-stroke patients: A systematic review. Eur. J. Phys. Rehabil. Med. 2021, 57, 44–60. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, S.; Canet-Vintró, M.; López-de-Celis, C.; Shen-Chen, Z.; Caballero-Martínez, I.; García-Ribell, E.; Rodríguez-Sanz, J. Immediate Effects of Focal Muscle Vibration on Squat Power and Velocity in Amateur Athletes: A Randomized Controlled Trial. J. Funct. Morphol. Kinesiol. 2025, 10, 60. [Google Scholar] [CrossRef] [PubMed]


| PubMed | PEDro | Science Direct | Cochrane Library | Web of Science | Total | |
|---|---|---|---|---|---|---|
| Focal vibration AND Parkinson’s | 27 | 2 | 2203 | 9 | 31 | 2272 |
| Focal vibration AND Parkinson’s disease | 24 | 2 | 2000 | 9 | 26 | 2061 |
| Focal muscle vibration AND Parkinson’s | 12 | 2 | 1655 | 7 | 20 | 1696 |
| Focal muscle vibration AND Parkinson’s disease | 9 | 2 | 1612 | 7 | 17 | 1647 |
| Focal vibration therapy AND Parkinson’s | 16 | 0 | 1436 | 6 | 6 | 1464 |
| Focal vibration therapy AND Parkinson’s disease | 15 | 0 | 1416 | 6 | 5 | 1442 |
| FVT AND Parkinson | 1 | 0 | 47 | 0 | 1 | 49 |
| FVT AND Parkinson’s disease | 1 | 0 | 37 | 0 | 1 | 39 |
| Total | 105 | 8 | 10,406 | 44 | 107 | 10,670 |
| PICOS | The Inclusion Criteria | The Exclusion Criteria |
|---|---|---|
| Population (P) | PD diagnosis | Patients with another medical condition, besides PD; animal studies or preclinical studies without clinical validation |
| Intervention (I) | Studies that used focal muscle vibration therapy | Other therapies used, apart from focal muscle vibration therapy |
| Comparison (C) | Control group studies or crossover studies | Studies that do not have a control group |
| Outcomes (O) | Improvement of symptoms: motor function, functional capacity, quality of life, reduction in disability | Results not relevant for focal muscle vibration therapy or not presented |
| Study design (S) | Original studies (randomized controlled trials, case–control studies, cohort studies, observational studies, trials) | Systematic reviews, meta-analyses, books, book chapters, editorials, conference abstracts, and notes |
| Author’s Name | Year of Publication | Study Design | Sample Size |
|---|---|---|---|
| Volpe et al. [24] | 2014 | A double-blind, double-dummy, parallel group, randomized controlled trial, placebo | 40 |
| Camerota et al. [25] | 2016 | A single-blind, parallel-group study design, Pilot Randomized, Controlled Trial, placebo | 20 |
| Peppe et al. [26] | 2019 | A multicentric randomized, double-blind crossover study, placebo | 40 |
| Romanato et al. [27] | 2021 | Crossover double-dummy, double-blind, randomized, controlled study, placebo | 24 |
| Spolaor et al. [28] | 2021 | A multicenter, randomized, double-blinded crossover study, placebo | 20 |
| Outcomes | Anticipated Absolute Effects * (95% CI) | Relative Effect (95% CI) | Number of Participants (Studies) | Certainty of the Evidence (GRADE) | |
|---|---|---|---|---|---|
| Risk with [Comparison] | Risk with [Intervention] | ||||
| Effectiveness of the balance training program | Static posturography parameters have not changed. No significant effect was found for the fall rate. | Improving clinical variables assessing self-confidence, balance, and disability. Having an overall positive impact on health-related quality of life. A significant effect was found for the fall rate. | - | 40 (1 study) | Low a,b,c ⊕⊕⊝⊝ |
| Effects on gait | No significant differences in all the gait variables. | Gait improvement as a result of increased walking velocity and stride length. | - | 60 (2 studies) | Low d,e,f ⊕⊕⊝⊝ |
| Coupling between balance and lower limb joints kinematics | Initial kinematic maintenance. | Improvements in trunk flexion, extension, and ankle dorsi-plantar flexion. Balance assessment—improvements at the frequencies corresponding to the vestibular system. | - | 24 (1 study) | Low g,h,i ⊕⊕⊝⊝ |
| Relationship between muscular activity and postural control changes | Increased surface electromyography activity was observed in both gastrocnemius lateralis and biceps femoris. | A reduction in the postural efforts and the peripheral vestibular disorders. Surface electromyography revealed a change in the motor control after the treatment. Increase in stride length. | - | 20 (1 study) | Low j,k,l ⊕⊕⊝⊝ |
| Author’s Name/Country | The Site of the Therapy Application | Intervention | The Main Objective | Follow-Up Period |
|---|---|---|---|---|
| Volpe et al. [24] Italy | Each patient wore 3 Equistasi devices, applied over the 7th cervical vertebra and on each soleus muscle tendons. | A 60-min physiotherapy session, five days a week, for two months. During the first three weeks of rehabilitation, both groups wore the devices six days per week for: 60 min per day in the first week, 120 min in the second, and 180 min in the third. From the fourth week, the devices were worn five days a week for four hours each day. | Effectiveness of a wearable proprioceptive stabilizer (Equistasi) for the rehabilitation of postural instability. | Baseline measurements (T0) were collected within one week before enrollment. The second evaluation (T1) was conducted within one week after completing the two-month therapy period The final assessment (T2) took place two months after T1. |
| Camerota et al. [25] Italy | The transducer was placed bilaterally on the quadriceps tendon near the insertion of the rectus femoris, approximately 2 cm from the medial border of the patella, as well as on the lumbar paraspinal muscles. | For each muscle group, r-FMV was administered in three 10-min sessions, separated by 1-min intervals, resulting in a total application time of 60 min. This protocol was repeated over three consecutive days to induce cumulative after-effects. | Beneficial effects of repetitive sessions of r-fMV on gait (using the Cro system). | A GA evaluation was done before r-fMV (T0) and 24 h (T1), 1 week (T2), and 3 weeks (T3) after the last session of r-fMV. |
| Peppe et al. [26] Italy | The three plaques were placed on the skin as follows: one over the seventh cervical vertebra and one on the tendon of each soleus muscle. | The device was worn for 6 days/week, starting with 1 h/day in the first week and increasing by 1 h weekly until reaching 4 h/day by week 4. This duration was then maintained for another 4 weeks, worn 5 days/week. At the end of the first 8-week period (T1), patients were evaluated for primary and secondary endpoints, followed by a 4-week wash-out phase. After re-evaluation (T2), patients began the crossover phase with the alternate kit. | This study aims to evaluate the clinical effects of proprioceptive system modulation on gait performance in individuals with PD using the Equistasi device. | The baseline assessment (T0) was conducted before enrollment. The second evaluation (T1) took place at the end of the first 8-week treatment period. The third assessment (T2) was performed at the start of the second 8-week treatment phase, following a 4-week wash-out period. The final evaluation (T3) occurred at the end of the second 8-week treatment period. |
| Romanato et al. [27] Italy | The device was applied to the skin in the following locations: one over the seventh cervical vertebra and one on each soleus muscle. | Participants underwent two 8-week treatment phases (active/placebo), separated by a 4-week wash-out. The device was worn 6 days/week in the first week for 1 h/day, increasing to 4 h/day by week 4, then maintained at 4 h/day, 5 days/week, for the remaining 4 weeks. | The study aimed to investigate the effects of the Equitasi device on gait and balance. | T0: Baseline assessment conducted before enrollment; T1: Second evaluation at the end of the first 8-week treatment period; T2: Third evaluation following the 4-week wash-out period, marking the start of the second treatment phase; T3: Final assessment at the end of the second 8-week treatment period. |
| Spolaor et al. [28] Italy | The device was applied on the skin as follows: One over the 7th cervical vertebra and two over each soleus muscle. | Participants received treatment for 8 weeks, without any additional rehabilitation procedures. After a 4-week wash-out period, they crossed over to the other treatment for another 8 weeks. During the first week, the device was worn for 1 h/day, 6 days/week. Over the next 3 weeks, the wearing time increased by 1 h per week, reaching 4 h/day by week 4. In the final 4 weeks, the device was worn for 4 h/day, 5 days/week. | This study aimed to examine how the Equistasi wearable device influences the relationship between muscle activity and changes in postural control. | Patients were evaluated at four time points: (T0) baseline before enrollment; (T1) after the first 8-week treatment period; (T2) following the 4-week wash-out period, which marked the start of the second treatment phase; and (T3) after the second 8-week treatment period. |
| Author’s Name | Age | Sex | Disease Stage | Disease Duration | Medication –ON State | Adverse Events | Treatment Adherence | Intensity of Therapies | Tools Used | Therapists’ Experience |
|---|---|---|---|---|---|---|---|---|---|---|
| Volpe et al. [24] | AG = 66.5 (64.0; 78.0) PG = 69.5 (65.0; 73.8) p value = 0.947 | AG = 7 M/13 F PG = 9 M/11 F p value = 0.747 | Hoehn and Yahr stage: AG = 3.0 (3.0; 3.0) PG = 3.0 (2.0; 3.0) p value = 0.429 | Active group: 6.0 (4.0; 10.8); Placebo group: 6.5 (4.0; 9.0) | Levodopa L-dopa LEDD: AG = 487.5 (315.0; 690.0) PG = 450.0 (293.8; 600.0) p value = 0.892 | No | Successfully with high adherence in both groups | Equistasi- mechanical vibratory energy < 0.8 N, 9000 Hz | Clinical scales: UPDRS-II, UPDRS-III, BBS, ABC Scale, FES, TUG, PDQ-39; -Other measures: number of falls during the observation period. | Blinded hospital physiotherapists delivered the standardized rehabilitation and applied indistinguishable active/placebo devices |
| Camerota et al. [25] | AG = 67 ± 7.96 (53–74) PG = 65.5 ± 9.85 (48–79) p value = 0.74 Mean age: 64.85 ± 8.74 years | 8 males and 12 females | Hoehn and Yahr stage: AG = 3 ± 0.45 (2–3) PG = 2.5 ± 0.39 (2–3) p value = 0.11 | Study group: Median 8.0 5.57, Range 3.0–12.0; Control group: Median 7.5 3.70, Range 4.0–12.0 | Levodopa L-DOPA LEDD: AG = 740 ± 53.75 (580–750) PG = 690 ± 116.14 (580–800) | None of the patients showed any side effects | Not reported | Cro System- low amplitude 200–500 μm and high frequency (100 Hz) | UPDRS-III, 3D gait analysis. | Standardized instructions; blinded to real/sham stimulation |
| Peppe et al. [26] | Age not reported; age at onset 60.3 ± 9.9 years | 26 males and 14 females | Hoehn and Yahr stage: mean ± SD: 2.45 ± 0.50 | Mean: 8.347, ±SD: 3.6 | Levodopa L-DOPA LEDD: Therapy dose mean ± SD: 743.3 ± 293 | No major adverse events during the study period were reported. | Not reported | Equistasi-frequency not reported | MDUPDRS III scores and 3D gait analysis (BTS system, stereophotogrammetry) | Therapists were blinded to treatment allocation (double-blind, double-dummy design) |
| Romanato et al. [27] | 67.46 ± 10.27 years | 15 males and 9 females | Hoehn and Yahr stage: 2.46 ± 0.51 | Mean: 11.88, ±SD: 4.89 | Levodopa L-DOPA LEDD: 757.14 ± 290.88 | No adverse events or falls were recorded during the trial | Not reported (intervention described as tolerable) | Equistasi vibration intensity not reported | UPDRS-III, Posturography (incl. instrumented Romberg test), 3D gait analysis, ABC Scale, PDQ-39, and fall rate (last month) | Therapists were blinded to treatment allocation (double-blind, double-dummy design) |
| Spolaor et al. [28] | 67.46 ± 10.27 years | 13 males and 7 females | Hoehn and Yahr stage: 2.46 ± 0.51 | Mean: 11.88, ±SD: 3.23 | Levodopa L-DOPA LEDD: 757.14 ± 290.88 | Not reported | Not reported | Equistasi- ~9000 Hz nanovibrations | UPDRS III, ABC scale, PDQ-39, Fall Rate (last month), sEMG; posturography (instrumented Romberg) | Therapists were blinded in a double-blind crossover design |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafti, D.; Uzun, A.-B.; Bodeanu, L.; Stanciu, L.-E.; Popescu, M.-N.; Iliescu, M.-G. The Potential of Focal Muscle Vibration Therapy in the Management of Parkinson’s Disease: A Systematic Review. J. Clin. Med. 2025, 14, 7472. https://doi.org/10.3390/jcm14217472
Rafti D, Uzun A-B, Bodeanu L, Stanciu L-E, Popescu M-N, Iliescu M-G. The Potential of Focal Muscle Vibration Therapy in the Management of Parkinson’s Disease: A Systematic Review. Journal of Clinical Medicine. 2025; 14(21):7472. https://doi.org/10.3390/jcm14217472
Chicago/Turabian StyleRafti, Daniel, Andreea-Bianca Uzun, Lavinia Bodeanu, Liliana-Elena Stanciu, Marius-Nicolae Popescu, and Madalina-Gabriela Iliescu. 2025. "The Potential of Focal Muscle Vibration Therapy in the Management of Parkinson’s Disease: A Systematic Review" Journal of Clinical Medicine 14, no. 21: 7472. https://doi.org/10.3390/jcm14217472
APA StyleRafti, D., Uzun, A.-B., Bodeanu, L., Stanciu, L.-E., Popescu, M.-N., & Iliescu, M.-G. (2025). The Potential of Focal Muscle Vibration Therapy in the Management of Parkinson’s Disease: A Systematic Review. Journal of Clinical Medicine, 14(21), 7472. https://doi.org/10.3390/jcm14217472







