Progression of Protruding Plaque in Acute Coronary Syndrome Diagnosed by Serial Optical Coherence Tomography
Abstract
1. Introduction
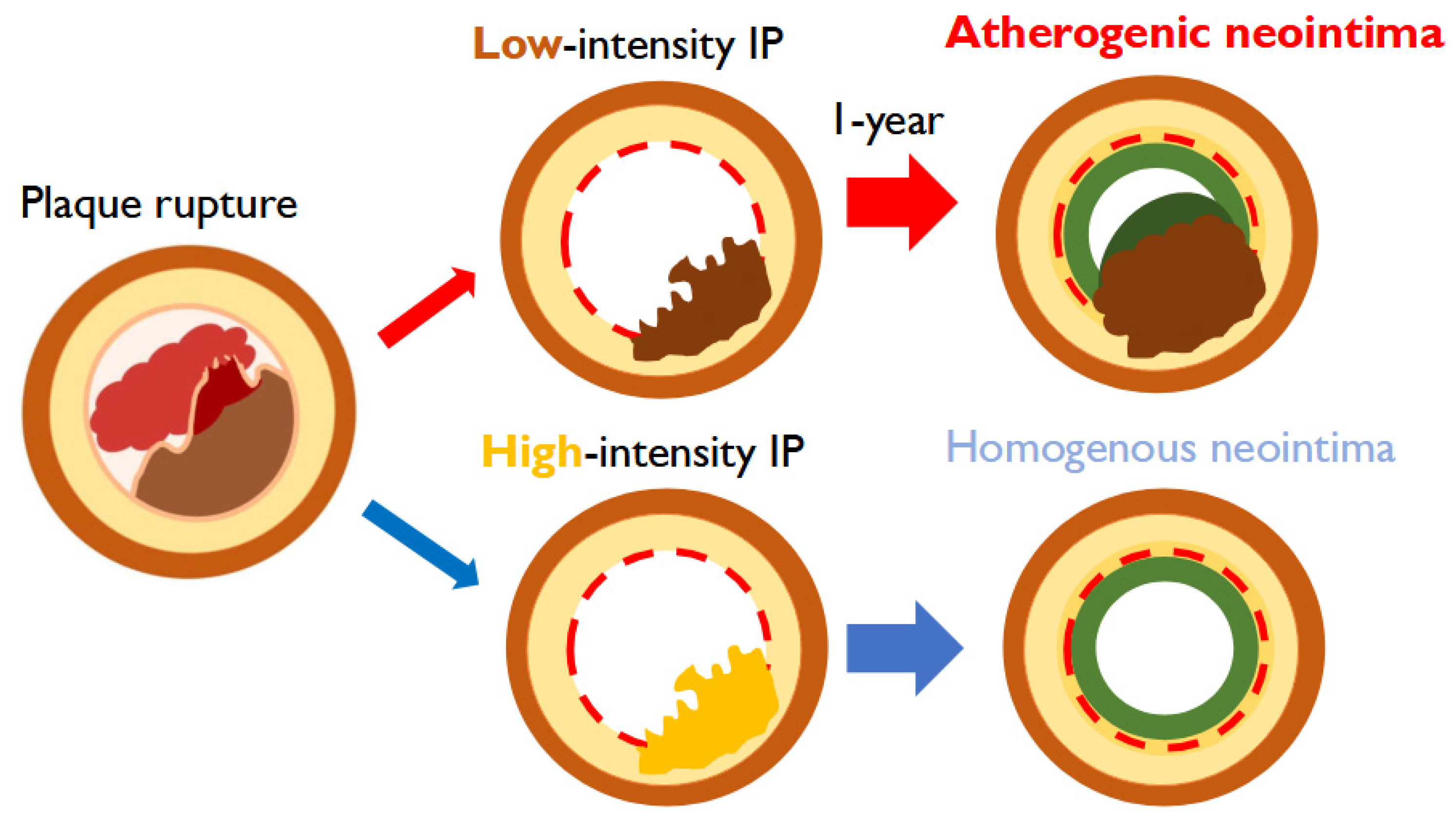
The Present Study Makes the Following Contributions
- First serial OCT evaluation of protruding plaque in ACS: We provide the first systematic observation of TP following stent implantation in ACS patients, using serial OCT from baseline to one year.
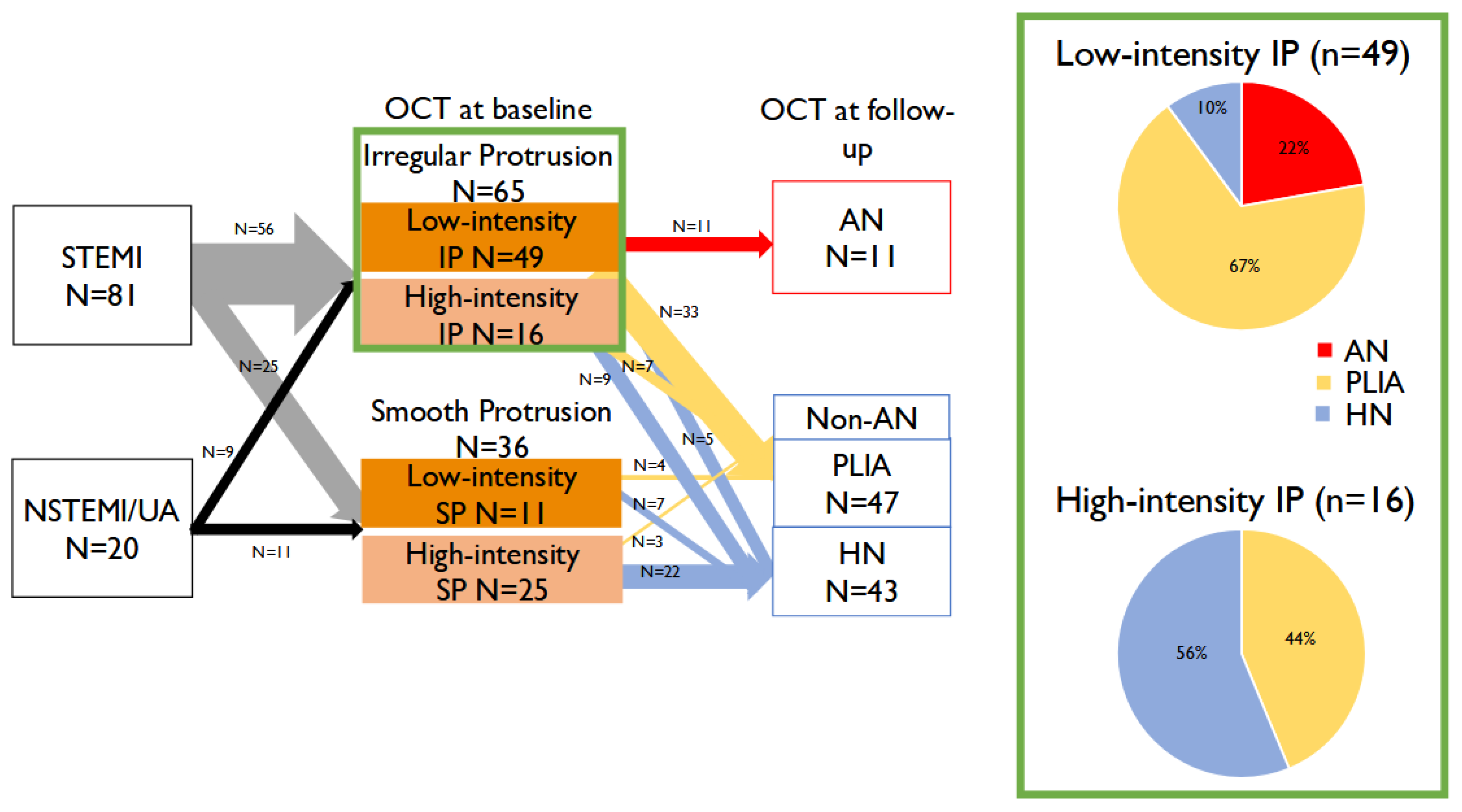
- Identification of pro-atherosclerosis progression: We demonstrate that low-intensity IP uniquely progresses to AN, introducing the novel concept of “pro-atherosclerosis” as a mechanistic pathway distinct from classical neoatherosclerosis.
- Clinical implications for lipid management: We show that inadequate LDL control (>70 mg/dL) is strongly associated with this progression, highlighting the importance of intensive lipid-lowering strategies in ACS patients after PCI.
2. Methods
2.1. Study Design and Population
2.2. PCI Procedure
2.3. OCT Imaging
2.4. Evaluation of Intensity of Protrusion by ImageJ
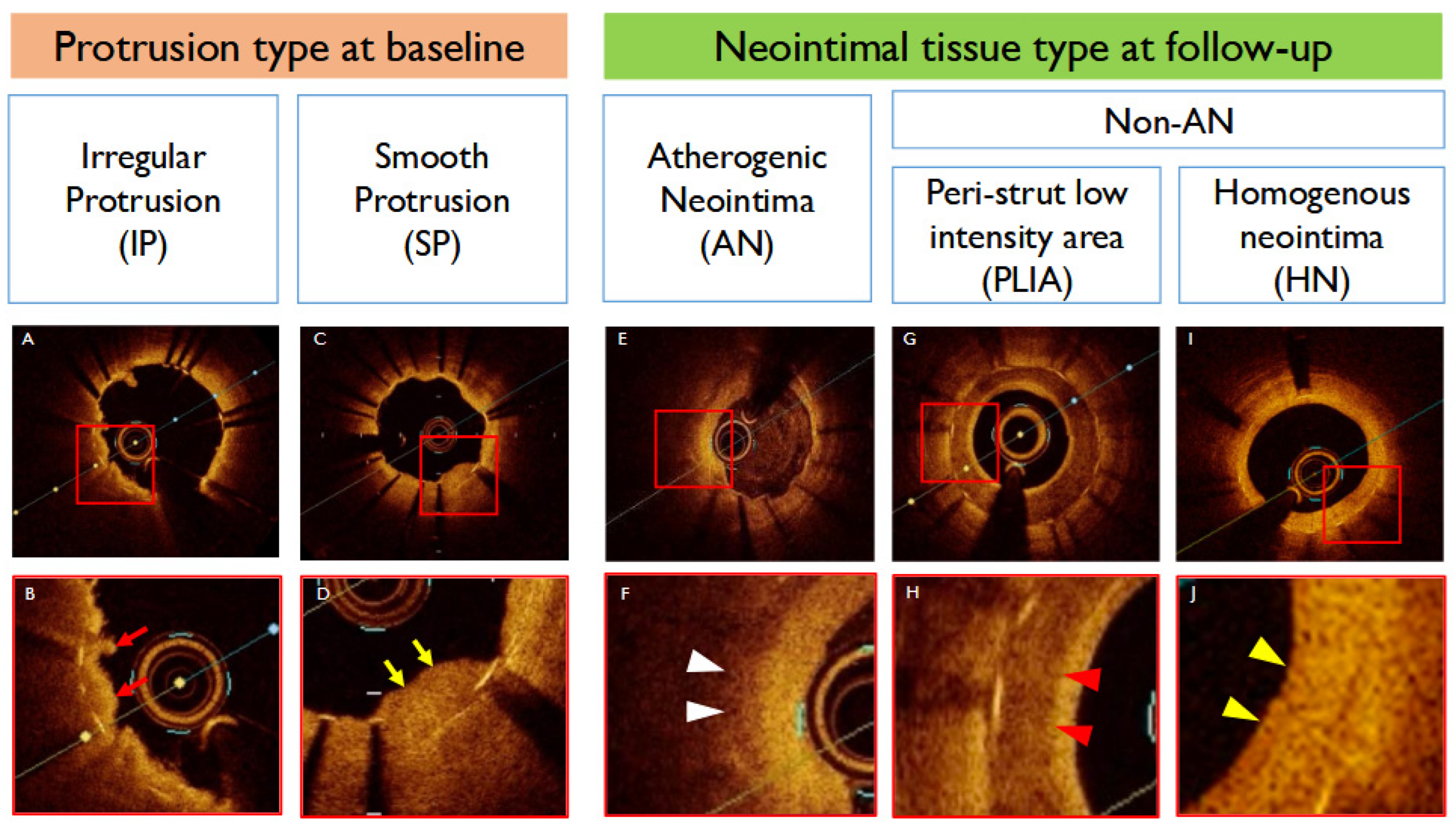
2.5. Protrusion Classification
2.6. Quantitative Coronary Angiography (QCA)
2.7. Statistical Analysis
2.8. Ethical Approval
3. Results
3.1. Baseline and Procedural Characteristics
3.2. OCT Findings at Baseline and 1-Year Follow-Up
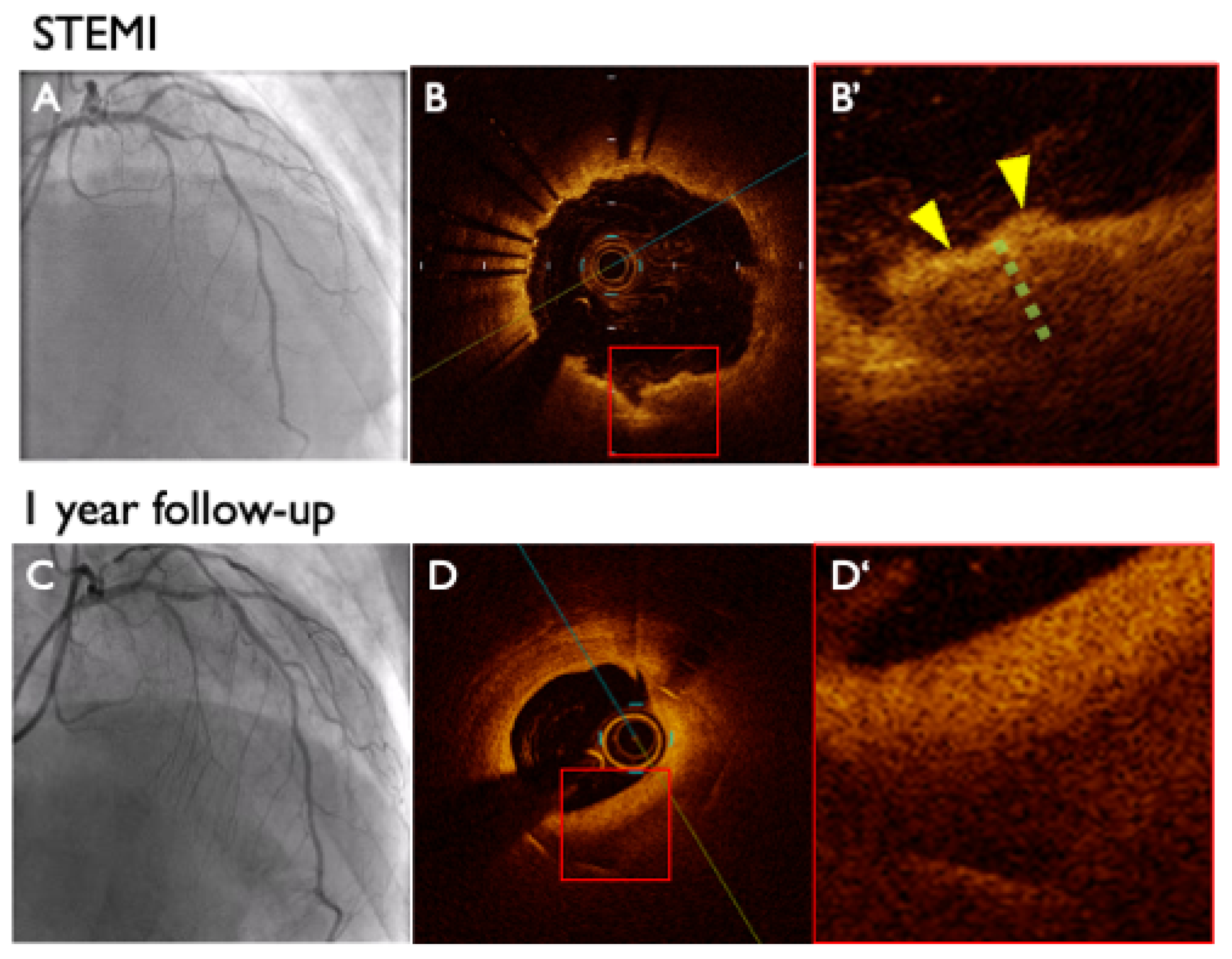
3.2.1. Baseline OCT Findings
3.2.2. Follow-Up OCT Findings
3.2.3. Quantitative Coronary Angiography (QCA) Findings
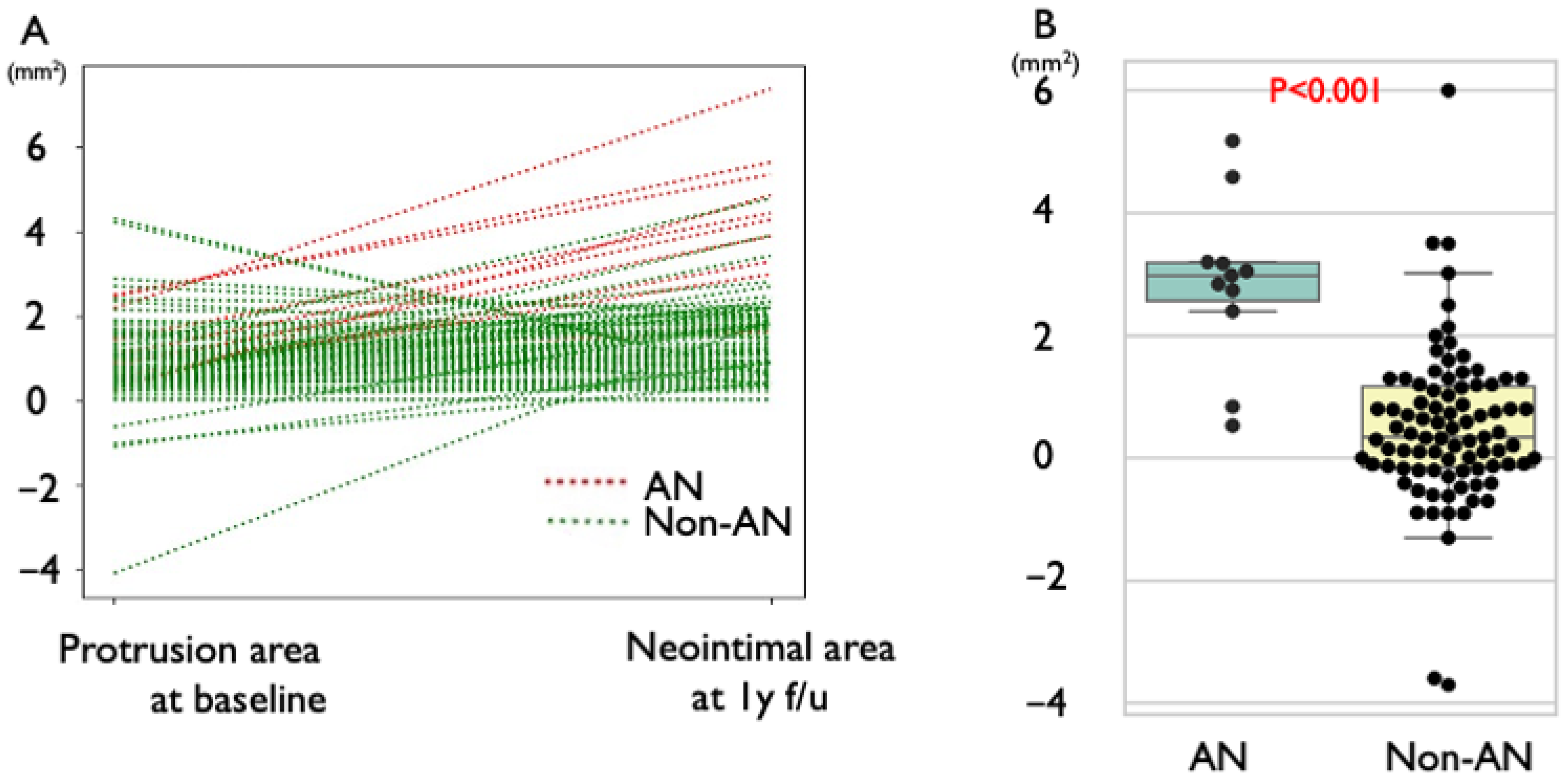
3.3. Gray Value Analysis of Protruding Plaque
3.4. Clinical Outcomes
3.5. Lipid Profile and Neointimal Progression
4. Discussion
5. Limitation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACS | acute coronary syndrome |
| AN | atherogenic neointima |
| HDL-C | high-density lipoprotein cholesterol |
| IP | irregular protrusion |
| LDL-C | low-density lipoprotein cholesterol |
| OCT | optical coherence tomography |
| PCI | percutaneous coronary intervention |
| TP | tissue protrusion |
References
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; Bhatt, D.L.; Birtcher, K.K.; Blankstein, R.; Boyd, J.; Bullock-Palmer, R.P.; Conejo, T.; et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 144, e368–e454. [Google Scholar] [CrossRef]
- Yamamoto, F.; Natsuaki, M.; Kuramitsu, S.; Ohya, M.; Otake, H.; Horie, K.; Yamanaka, F.; Shiomi, H.; Nakazawa, G.; Ando, K.; et al. Outcomes of Drug-Eluting Stent Thrombosis After Treatment for Acute Versus Chronic Coronary Syndrome. JACC Cardiovasc. Interv. 2021, 14, 1082–1090. [Google Scholar] [CrossRef]
- Soeda, T.; Uemura, S.; Park, S.J.; Jang, Y.; Lee, S.; Cho, J.M.; Kim, S.J.; Vergallo, R.; Minami, Y.; Ong, D.S.; et al. Incidence and Clinical Significance of Poststent Optical Coherence Tomography Findings: One-Year Follow-Up Study from a Multicenter Registry. Circulation 2015, 132, 1020–1029. [Google Scholar] [CrossRef]
- Sanuki, Y.; Sonoda, S.; Muraoka, Y.; Shimizu, A.; Kitagawa, M.; Takami, H.; Anai, R.; Miyamoto, T.; Oginosawa, Y.; Tsuda, Y.; et al. Contribution of Poststent Irregular Protrusion to Subsequent In-Stent Neoatherosclerosis After the Second-Generation Drug-Eluting Stent Implantation. Int. Heart J. 2018, 59, 307–314. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; The Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Mintz, G.S.; Witzenbichler, B.; Metzger, D.C.; Rinaldi, M.J.; Duffy, P.L.; Weisz, G.; Stuckey, T.D.; Brodie, B.R.; Parvataneni, R.; et al. Prevalence and Clinical Impact of Tissue Protrusion After Stent Implantation: An ADAPT-DES Intravascular Ultrasound Substudy. JACC Cardiovasc. Interv. 2016, 9, 1499–1507. [Google Scholar] [CrossRef]
- Hong, Y.J.; Jeong, M.H.; Choi, Y.H.; Song, J.A.; Kim, D.H.; Lee, K.H.; Yamanaka, F.; Lee, M.G.; Park, K.H.; Sim, D.S.; et al. Impact of tissue prolapse after stent implantation on short- and long-term clinical outcomes in patients with acute myocardial infarction: An intravascular ultrasound analysis. Int. J. Cardiol. 2013, 166, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.J.; Jeong, M.H.; Ahn, Y.; Sim, D.S.; Chung, J.W.; Cho, J.S.; Yoon, N.S.; Yoon, H.J.; Moon, J.Y.; Kim, K.H.; et al. Plaque prolapse after stent implantation in patients with acute myocardial infarction: An intravascular ultrasound analysis. JACC Cardiovasc. Imaging 2008, 1, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Kimura, S.; Akiyama, D.; Hishikari, K.; Kawaguchi, N.; Kamiishi, T.; Hikita, H.; Takahashi, A.; Isobe, M. Quantitative assessment of tissue prolapse on optical coherence tomography and its relation to underlying plaque morphologies and clinical outcome in patients with elective stent implantation. Int. J. Cardiol. 2014, 176, 182–190. [Google Scholar] [CrossRef]
- Kawamori, H.; Shite, J.; Shinke, T.; Otake, H.; Matsumoto, D.; Nakagawa, M.; Nagoshi, R.; Kozuki, A.; Hariki, H.; Inoue, T.; et al. Natural consequence of post-intervention stent malapposition, thrombus, tissue prolapse, and dissection assessed by optical coherence tomography at mid-term follow-up. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; ESC Scientific Document Group. Fourth universal definition of myocardial infarction (2018). Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e18–e114. [Google Scholar] [CrossRef] [PubMed]
- Tearney, G.J.; Regar, E.; Akasaka, T.; Adriaenssens, T.; Barlis, P.; Bezerra, H.G.; Bouma, B.; Bruining, N.; Cho, J.M.; Chowdhary, S.; et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: A report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J. Am. Coll. Cardiol. 2012, 59, 1058–1072. [Google Scholar] [CrossRef]
- Fujii, K.; Kubo, T.; Otake, H.; Nakazawa, G.; Sonoda, S.; Hibi, K.; Shinke, T.; Kobayashi, Y.; Ikari, Y.; Akasaka, T. Expert consensus statement for quantitative measurement and morphological assessment of optical coherence tomography: Update 2022. Cardiovasc. Interv. Ther. 2022, 37, 248–254. [Google Scholar] [CrossRef]
- Jinnouchi, H.; Otsuka, F.; Sato, Y.; Bhoite, R.R.; Sakamoto, A.; Torii, S.; Yahagi, K.; Cornelissen, A.; Mori, M.; Kawakami, R.; et al. Healthy Strut Coverage After Coronary Stent Implantation: An Ex Vivo Human Autopsy Study. Circ. Cardiovasc. Interv. 2020, 13, e008869. [Google Scholar] [CrossRef] [PubMed]
- Jinnouchi, H.; Sato, Y.; Torii, S.; Sakamoto, A.; Cornelissen, A.; Bhoite, R.R.; Kuntz, S.; Guo, L.; Paek, K.H.; Fernandez, R.; et al. Detection of cholesterol crystals by optical coherence tomography. EuroIntervention 2020, 16, 395–403. [Google Scholar] [CrossRef]
- Torii, S.; Nakazawa, G.; Ijichi, T.; Yoshikawa, A.; Murakami, T.; Natsumeda, M.; Fujii, T.; Shinozaki, N.; Yoshimachi, F.; Morino, Y.; et al. Simultaneous Intravascular Ultrasound Usage Overcomes Misinterpretation When Evaluating Lipid-Rich Plaques with Optical Frequency Domain Imaging—Ex Vivo Study. Circ. J. 2015, 79, 2641–2647. [Google Scholar] [CrossRef]
- Sugiyama, T.; Kimura, S.; Ohtani, H.; Hishikari, K.; Kojima, K.; Sagawa, Y.; Hayasaka, K.; Mizusawa, M.; Misawa, T.; Yamakami, Y.; et al. Relationship between quantities of tissue prolapse after percutaneous coronary intervention and neointimal hyperplasia at follow-up on serial optical coherence tomography examination. Int. J. Cardiol. 2017, 241, 470–477. [Google Scholar] [CrossRef]
- Miura, T.; Sonoda, S.; Sanuki, Y.; Naka, Y.; Okabe, H.; Setoyama, K.; Inoue, K.; Shimizu, A.; Anai, R.; Tsuda, Y.; et al. Comparison of post-stent irregular protrusion and subsequent neointimal characteristics between second- and third-generation drug-eluting stent implantation. J. Cardiol. 2020, 76, 464–471. [Google Scholar] [CrossRef]
- Torii, S.; Jinnouchi, H.; Sakamoto, A.; Kutyna, M.; Cornelissen, A.; Kuntz, S.; Guo, L.; Mori, H.; Harari, E.; Paek, K.H.; et al. Drug-eluting coronary stents: Insights from preclinical and pathology studies. Nat. Rev. Cardiol. 2020, 17, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Sakai, K.; Torii, S.; Aoki, Y.; Turcotte-Gosselin, F.; Fujinuma, K.; Ohwaki, A.; Aihara, K.; Noda, S.; Miyamoto, J.; et al. Lipid profile and risk factors for neoatherosclerosis after drug-eluting stent implantation in acute coronary syndrome. J. Clin. Lipidol. 2024, 18, e977–e985. [Google Scholar] [CrossRef] [PubMed]
| Overall | AN (n = 11) | Non-AN (n = 90) | p | |
|---|---|---|---|---|
| Age, mean ± SD, y | 63.6 ± 11.5 | 67.8 ± 3.42 | 63.1 ± 1.21 | 0.14 |
| Male sex, n (%) | 83.0 (82.2) | 8.0 (72.7) | 75.0 (83.3) | 0.41 |
| BMI, mean ± SD, kg/m2 | 24.6 ± 3.01 | 25.5 ± 0.89 | 24.4 ± 0.32 | 0.17 |
| Clinical presentation, n (%) | ||||
| STEMI, n (%) | 81.0 (80.2) | 8.0 (72.7) | 73.0 (81.1) | 0.51 |
| NSTE-ACS, n (%) | 20.0 (19.8) | 3.0 (27.3) | 17.0 (18.9) | 0.45 |
| Previous PCI, n (%) | 1.0 (0.99) | 0.0 (0.0) | 1.0 (1.1) | 1.0 |
| Previous CABG, n (%) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | NA |
| Hypertension, n (%) | 65.0 (64.4) | 8.0 (72.7) | 57.0 (63.3) | 0.60 |
| Dyslipidemia, n (%) | 75.0 (74.3) | 11.0 (100.0) | 64.0 (71.1) | 0.01 |
| Diabetes mellitus, n (%) | 21.0 (20.8) | 6.0 (54.5) | 15.0 (16.6) | 0.01 |
| Chronic kidney disease, n (%) | 25.0 (24.8) | 3.0 (27.3) | 22.0 (24.4) | 0.87 |
| Dialysis, n (%) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | NA |
| Smoking history, n (%) | 63.0 (62.4) | 5.0 (45.5) | 58.0 (64.4) | 0.18 |
| LVEF, mean ± SD, % | 63.3 ± 10.1 | 62.8 ± 10.0 | 65.2 ± 9.79 | 0.27 |
| Laboratory data at admission | ||||
| 14.5 ± 1.82 | 14.6 ± 0.50 | 14.4 ± 0.24 | 0.93 |
| 0.92 ± 0.25 | 0.82 ± 0.11 | 0.87± 0.02 | 0.44 |
| 203.1 ± 34.4 | 193.0 ± 10.3 | 205.5 ± 4.60 | 0.28 |
| 51.1 ± 12.1 | 50.6 ± 3.72 | 51.1 ± 1.30 | 0.84 |
| 153.1 ± 35.0 | 142.4 ± 10.5 | 155.6 ± 4.72 | 0.18 |
| 127.0 ± 32.4 | 117.6 ± 9.81 | 128.3 ± 3.50 | 0.22 |
| 151.8 ± 15.5 | 136.7 ± 48.1 | 154.7 ± 16.4 | 0.43 |
| 6.3 ± 1.42 | 6.6 ± 1.63 | 6.8 ± 0.55 | 0.14 |
| Medications at 1-year follow-up | ||||
| 69.0 (69.7) | 7.0 (63.6) | 62.0 (68.8) | 0.65 |
| 82.0 (82.8) | 10.0 (90.9) | 72.0 (80.0) | 0.45 |
| 6.0 (1.7) | 0.0 (0.0) | 6.0 (6.66) | 0.37 |
| 96.0 (95.0) | 11.0 (100.0) | 85.0 (94.4) | 0.53 |
| 91.0 (90.1) | 11.0 (100.0) | 80.0 (88.9) | 0.29 |
| 101.0 (100) | 11.0 (100.0) | 90.0 (100.0) | 1.0 |
| 35.0 (34.7) | 2.0 (18.2) | 32.0 (35.6) | 0.54 |
| PCSK9 inhibitor, n (%) | 0.0 | 0.0 (0.0) | 0.0 (0.0) | NA |
| Laboratory data at 1-year follow-up | ||||
| 140.3 ± 25.9 | 162.5 ± 7.92 | 136.3 ± 2.91 | 0.01 |
| 56.2 ± 12.1 | 54.5 ± 3.71 | 56.4 ± 1.30 | 0.65 |
| 99.3 ± 30.3 | 114.3 ± 8.02 | 85.8 ± 2.90 | 0.01 |
| 63.7 ± 18.7 | 76.9 ± 4.80 | 61.2 ± 1.70 | 0.02 |
| 139.7 ± 74.4 | 156.7 ± 22.5 | 137.8 ± 7.91 | 0.26 |
| 6.1 ± 0.63 | 6.3 ± 0.19 | 6.1 ± 0.06 | 0.22 |
| Overall | AN (n = 11) | Non-AN (n = 90) | p | |
|---|---|---|---|---|
| Target vessel | 0.11 | |||
| 1.0 (0.99) | 0.0 (0.0) | 1.0 (1.11) | |
| 68.0 (67.3) | 5.0 (45.5) | 63.0 (70.0) | |
| 7.0 (6.93) | 0.0 (0.0) | 7.0 (7.77) | |
| 25.0 (24.8) | 6.0 (54.5) | 19.0 (21.1) | |
| Stent | 0.46 | |||
| DP-DES, n (%) | 37.0 (36.6) | 5.0 (45.5) | 32.0 (35.6) | |
| BP-DES, n (%) | 64.0 (63.4) | 6.0 (54.5) | 58.0 (64.4) | |
| QCA | ||||
| Baseline | ||||
| Reference proximal diameter (mm) | 2.9 ± 0.63 | 2.6 ± 0.19 | 2.9 ± 0.06 | 0.36 |
| Reference distal diameter (mm) | 2.4 ± 0.51 | 2.3 ± 0.16 | 2.4 ± 0.05 | 0.38 |
| Minimal lumen diameter (mm) | 0.98 ± 0.36 | 0.79 ± 0.11 | 1.0 ± 0.03 | 0.07 |
| Diameter stenosis (%) | 59.8 ± 15.7 | 64.2 ± 4.92 | 59.3 ± 1.60 | 0.41 |
| After procedure | ||||
| Stent proximal diameter (mm) | 3.1 ± 0.48 | 3.2 ± 0.15 | 3.1 ± 0.05 | 0.39 |
| Stent distal diameter (mm) | 2.8 ± 0.47 | 2.9 ± 0.15 | 2.8 ± 0.05 | 0.16 |
| Minimal lumen diameter (mm) | 2.5 ± 0.44 | 2.7 ± 0.13 | 2.5 ± 0.04 | 0.03 |
| Diameter stenosis (%) | 15.9 ± 8.31 | 10.1 ± 2.62 | 16.5 ± 0.87 | 0.02 |
| Acute gain (mm) | 1.54 ± 0.60 | 1.92 ± 0.18 | 1.53 ± 0.06 | 0.01 |
| At 1-year follow-up | ||||
| Stent proximal diameter (mm) | 3.1 ± 0.52 | 3.1 ± 0.16 | 3.1 ± 0.05 | 0.72 |
| Stent distal diameter (mm) | 2.8 ± 0.54 | 2.8 ± 0.17 | 2.8 ± 0.05 | 0.74 |
| Minimal lumen diameter (mm) | 2.4 ± 0.63 | 1.9 ± 0.19 | 2.5 ± 0.06 | 0.13 |
| Diameter stenosis (%) | 19.4 ± 13.3 | 37.4 ± 3.72 | 17.4 ± 1.30 | 0.06 |
| Late loss (mm) | 0.08 ± 0.52 | 0.76 ± 0.14 | −0.01 ± 0.05 | 0.01 |
| Overall | AN (n = 11) | Non-AN (n = 90) | p | |
|---|---|---|---|---|
| Pre-PCI | ||||
| Etiology | 0.28 | |||
| Plaque rupture, n (%) | 80.0 (79.2) | 11.0 (100.0) | 69.0 (76.6) | |
| Erosion, n (%) | 3.0 (2.97) | 0.0 (0.0) | 3.0 (3.33) | |
| Calcified nodule, n (%) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | |
| Others, n (%) | 18.0 (17.8) | 0.0 (0.0) | 18.0 (20.0) | |
| Plaque morphologies | 0.43 | |||
| Lipid rich Plaque, n (%) | 83.0 (82.2) | 11.0 (100.0) | 72.0 (80.0) | |
| Fibrous Plaque, n (%) | 9.0 (8.91) | 0.0 (0.0) | 9.0 (10.0) | |
| Calcification, n (%) | 2.0 (1.98) | 0.0 (0.0) | 2.0 (2.22) | |
| Others, n (%) | 7.0 (6.93) | 0.0 (0.0) | 7.0 (7.77) | |
| Angiographic findings | ||||
| Pre TIMI flow grade 0/1 n (%) | 66.0 (65.3) | 3.0 (27.2) | 63.0 (70.0) | 0.01 |
| Final TIMI flow grade 3 n (%) | 95.0 (94.1) | 11.0 (100.0) | 84.0 (93.3) | 0.67 |
| Lesion length, mm | 25.0 ± 9.96 | 20.0 ± 2.92 | 25.6 ± 1.13 | 0.03 |
| Proximal reference lumen area, mm2 | 14.0 ± 6.20 | 13.9 ± 37.8 | 28.1 ± 13.4 | 0.70 |
| Distal reference lumen area, mm2 | 10.0 ± 4.92 | 11.5 ± 14.5 | 15.3 ± 5.10 | 0.35 |
| Minimum lumen area, mm2 | 3.27 ± 2.50 | 3.46 ± 0.74 | 3.34 ± 0.27 | 0.52 |
| Procedural details | ||||
| Thrombus aspiration, n (%) | 63.0 (62.4) | 5.0 (45.5) | 58.0 (64.4) | 0.22 |
| Excimer laser coronary angioplasty (ELCA), n (%) | 19.0 (18.8) | 0.0 (0.0) | 19.0 (21.1) | 0.09 |
| Balloon | ||||
| Pre-POBA, n (%) | 75.0 (74.3) | 9.0 (81.8) | 66.0 (73.3) | 0.54 |
| Balloon pressure, atm | 11.5 ± 0.62 | 10.6 ± 1.3 | 11.1 ± 0.42 | 0.64 |
| Balloon size, mm | 2.41 ± 0.37 | 2.51 ± 0.17 | 2.30 ± 0.05 | 0.44 |
| Balloon length, mm | 14.8 ± 0.72 | 15.0 ± 0.55 | 14.9 ± 0.18 | 0.74 |
| Post POBA, n (%) | 65.0 (64.4) | 5.0 (45.5) | 60.0 (66.6) | 0.17 |
| Balloon pressure | 17.9 ± 0.82 | 15.5 ± 2.3 | 18.1 ± 0.75 | 0.28 |
| Balloon size, mm | 3.42 ± 0.72 | 3.41 ± 0.36 | 3.42 ± 0.11 | 0.74 |
| Balloon length, mm | 11.4± 0.75 | 10.7± 1.8 | 11.2 ± 0.62 | 0.85 |
| Post-PCI | ||||
| Quantitative analysis | ||||
| Lumen area at most protruding site, mm2 | 6.94 ± 2.3 | 7.87 ± 0.67 | 6.84 ± 0.24 | 0.28 |
| Stent area at most protruding site, mm2 | 7.76 ± 2.6 | 9.19 ± 0.76 | 7.74 ± 0.27 | 0.19 |
| Stent length, mm | 27.2 ± 12.3 | 23.5 ± 3.1 | 27.7 ± 1.1 | 0.19 |
| Stent diameter, mm | 3.12 ± 0.44 | 3.30 ± 0.13 | 3.14 ± 0.05 | 0.20 |
| Protrusion analysis | ||||
| Protrusion height mm | 0.41 ± 0.20 | 0.52 ± 0.05 | 0.39 ± 0.01 | 0.01 |
| Protrusion length mm | 3.10 ± 2.8 | 2.54 ± 0.99 | 3.50 ± 0.34 | 0.75 |
| Most Protrusion area, mm2 | 75.0 ± 65.3 | 80.8 ± 22.9 | 78.7 ± 8.1 | 0.19 |
| 1-year follow-up | ||||
| Minimal lumen area, mm2 | 5.87 ± 2.18 | 5.14 ± 0.67 | 6.14 ± 0.24 | 0.18 |
| Neointimal area, mm2 | 1.63 ± 1.30 | 2.80 ± 0.46 | 0.67 ± 0.16 | <0.001 |
| Stent area, mm2 | 7.72 ± 2.50 | 9.21 ± 0.73 | 7.40 ± 0.26 | 0.06 |
| % area stenosis, % | 22.2 ± 14.6 | 46.6 ± 3.62 | 19.1 ± 1.31 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aoki, Y.; Nakamura, N.; Torii, S.; Natsumeda, M.; Turcotte-Gosselin, F.; Shiozaki, M.; Hashimoto, K.; Suzuki, D.; Omura, R.; Aihara, K.; et al. Progression of Protruding Plaque in Acute Coronary Syndrome Diagnosed by Serial Optical Coherence Tomography. J. Clin. Med. 2025, 14, 7468. https://doi.org/10.3390/jcm14217468
Aoki Y, Nakamura N, Torii S, Natsumeda M, Turcotte-Gosselin F, Shiozaki M, Hashimoto K, Suzuki D, Omura R, Aihara K, et al. Progression of Protruding Plaque in Acute Coronary Syndrome Diagnosed by Serial Optical Coherence Tomography. Journal of Clinical Medicine. 2025; 14(21):7468. https://doi.org/10.3390/jcm14217468
Chicago/Turabian StyleAoki, Yuki, Norihito Nakamura, Sho Torii, Makoto Natsumeda, Frederic Turcotte-Gosselin, Manabu Shiozaki, Kaho Hashimoto, Daiki Suzuki, Ryosuke Omura, Kazuki Aihara, and et al. 2025. "Progression of Protruding Plaque in Acute Coronary Syndrome Diagnosed by Serial Optical Coherence Tomography" Journal of Clinical Medicine 14, no. 21: 7468. https://doi.org/10.3390/jcm14217468
APA StyleAoki, Y., Nakamura, N., Torii, S., Natsumeda, M., Turcotte-Gosselin, F., Shiozaki, M., Hashimoto, K., Suzuki, D., Omura, R., Aihara, K., Sakai, K., Nakano, M., Nakazawa, G., & Ikari, Y. (2025). Progression of Protruding Plaque in Acute Coronary Syndrome Diagnosed by Serial Optical Coherence Tomography. Journal of Clinical Medicine, 14(21), 7468. https://doi.org/10.3390/jcm14217468





