Interventions for Fontan Pathway Obstruction in Patients Following Total Cavopulmonary Connection
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Availability Statement
2.2. Ethical Statement
2.3. Patients and Data Collection
2.4. Surgical Techniques
2.5. Cardiac Catheterization and Intervention Techniques
2.6. Definition of Failing Fontan, Protein-Losing Enteropathy, and Plastic Bronchitis
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Perioperative Data
3.3. Incidence, Location of, and Treatment for TCPC Pathway Obstruction
3.4. Risk Factors for TCPC Pathway Intervention
4. Discussion
4.1. Summary of the Results
4.2. Incidence of TCPC Pathway Obstruction
4.3. Surgical vs. Interventional Therapy: Timing and Treatment Options
4.4. Left-PA Stenting
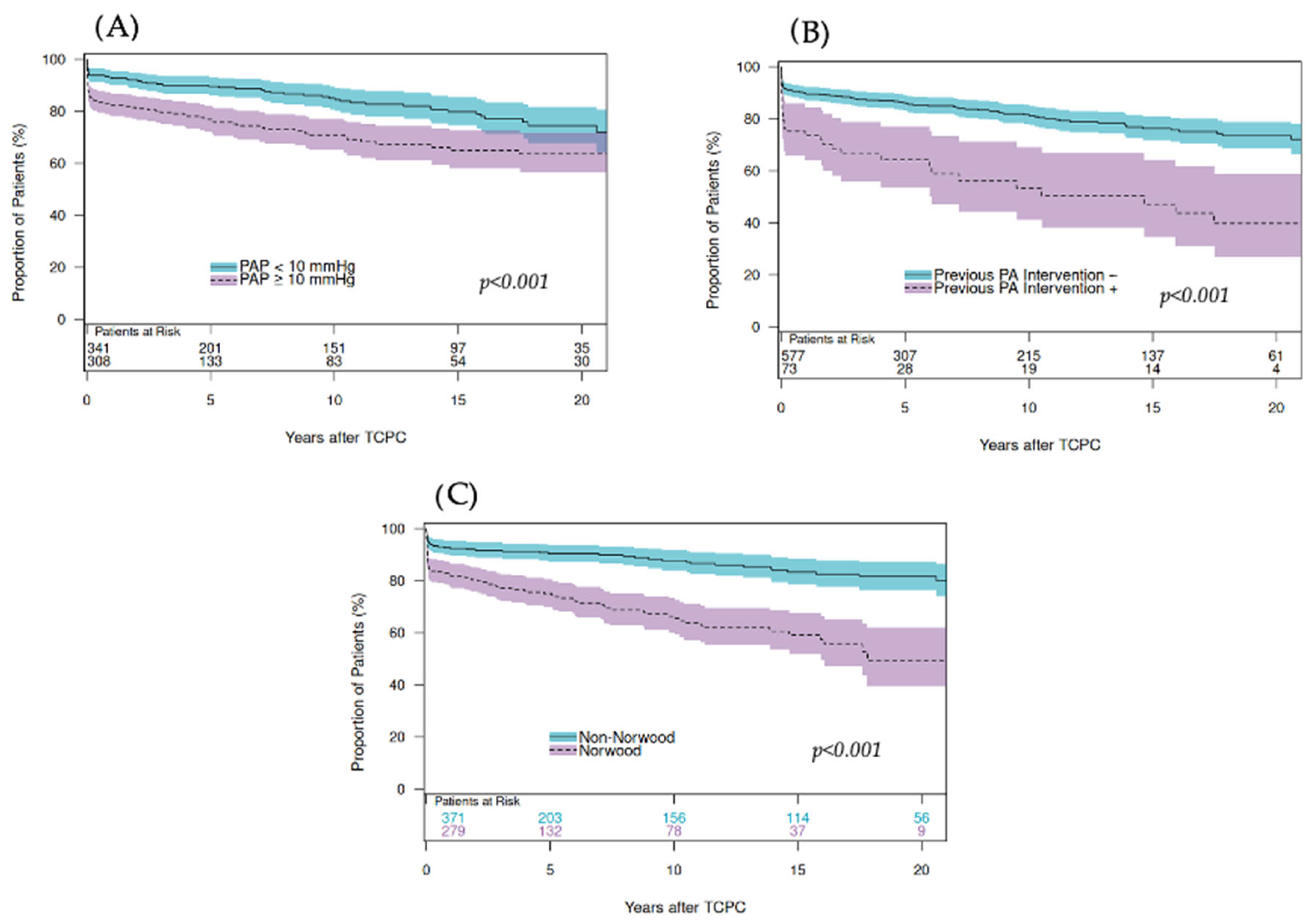
4.5. Impact of Fontan Pathway Obstruction on Late Outcomes
4.6. Future Prospective
4.7. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCPS | bidirectional cavopulmonary shunt |
| DKS | Damus–Kaye–Stansel |
| HR | hazard ratio |
| IQR | interquartile ranges |
| IVC | inferior vena cava |
| LAP | left-atrial pressure |
| NYHA | New York Heart Association |
| PA | pulmonary artery |
| PAP | pulmonary artery pressure |
| PB | plastic bronchitis |
| PDA | patent ductus arteriosus |
| PLE | protein-losing enteropathy |
| SVC | superior vena cava |
| TCPC | total cavopulmonary connection |
References
- Fontan, F.; Baudet, E. Surgical repair of tricuspid atresia. Thorax 1971, 26, 240–248. [Google Scholar] [CrossRef]
- Kreutzer, G.O.; Galindez, E.; Bono, H.; De Palma, C.; Laura, J.P. An operation for the correction of tricuspid atresia. J. Thorac. Cardiovasc. Surg. 1973, 66, 613–621. [Google Scholar] [CrossRef]
- de Leval, M.R.; Kilner, P.; Gewillig, M.; Bull, C. Total cavopulmonary connection: A logical alternative to atriopulmonary connection for complex Fontan operations. Experimental studies and early clinical experience. J. Thorac. Cardiovasc. Surg. 1988, 96, 682–695. [Google Scholar] [CrossRef]
- Marcelletti, C.; Corno, A.; Giannico, S.; Marino, B. Inferior vena cava-pulmonary artery extracardiac conduit: A new form of right heart bypass. J. Thorac. Cardiovasc. Surg. 1990, 100, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Amodeo, A.; Galletti, L.; Marianeschi, S.; Picardo, S.; Giannico, S.; Di Renzi, P.; Marcelletti, C. Extracardiac Fontan operation for complex cardiac anomalies: Seven years’ experience. J. Thorac. Cardiovasc. Surg. 1997, 114, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Dennis, M.; Zannino, D.; du Plessis, K.; Bullock, A.; Disney, P.J.S.; Radford, D.J.; Hornung, T.; Grigg, L.; Cordina, R.; D’udekem, Y.; et al. Clinical Outcomes in Adolescents and Adults After the Fontan Procedure. J. Am. Coll. Cardiol. 2018, 71, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Giannico, S.; Trezzi, M.; Cantarutti, N.; Cafiero, G.; Ravà, L.; Adorisio, R.; Brancaccio, G.; Albanese, S.; Drago, F.; Carotti, A.; et al. Late outcome of extracardiac Fontan patients: 32 years of follow-up. Eur. J. Cardio-Thoracic Surg. 2022, 62, ezac301. [Google Scholar] [CrossRef]
- Schilling, C.; Dalziel, K.; Nunn, R.; Du Plessis, K.; Shi, W.Y.; Celermajer, D.; Winlaw, D.; Weintraub, R.G.; Grigg, L.E.; Radford, D.J.; et al. The Fontan epidemic: Population projections from the Australia and New Zealand Fontan Registry. Int. J. Cardiol. 2016, 219, 14–19. [Google Scholar] [CrossRef]
- Hagler, D.J.; Miranda, W.R.; Haggerty, B.J.; Anderson, J.H.; Johnson, J.N.; Cetta, F.; Said, S.M.; Taggart, N.W. Fate of the Fontan connection: Mechanisms of stenosis and management. Congenit. Heart Dis. 2019, 14, 571–581. [Google Scholar] [CrossRef]
- Van Puyvelde, J.; Rega, F.; Budts, W.; Van De Bruaene, A.; Cools, B.; Gewillig, M.; Eyskens, B.; Heying, R.; Salaets, T.; Meyns, B. Defining the causes for Fontan circulatory failure in total cavopulmonary connection patients. Interdiscip. Cardiovasc. Thorac. Surg. 2024, 39, ivae188. [Google Scholar] [CrossRef]
- McGovern, E.; Alsaied, T.; Szugye, N.; Pradhan, S.; Batlivala, S.P.; Lubert, A.; Hirsch, R. The Fontan Pathway: Change in Dimension and Catheter-Based Intervention over Time. Pediatr. Cardiol. 2021, 42, 1740–1748. [Google Scholar] [CrossRef]
- Van Brakel, T.J.; Schoof, P.H.; de Roo, F.; Nikkels, P.G.; Evens, F.C.; Haas, F. High incidence of Dacron conduit stenosis for extracardiac Fontan procedure. J. Thorac. Cardiovasc. Surg. 2014, 147, 1568–1572. [Google Scholar] [CrossRef] [PubMed]
- Udink Ten Cate, F.E.A.; Trieschmann, U.; Germund, I.; Hannes, T.; Emmel, M.; Bennink, G.; Sreeram, N. Stenting the Fontan pathway in paediatric patients with obstructed extracardiac conduits. Heart 2017, 103, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Rychik, J.; Atz, A.M.; Celermajer, D.S.; Deal, B.J.; Gatzoulis, M.A.; Gewillig, M.H.; Hsia, T.-Y.; Hsu, D.T.; Kovacs, A.H.; McCrindle, B.W.; et al. Evaluation and Management of the Child and Adult With Fontan Circulation: A Scientific Statement From the American Heart Association. Circulation 2019, 140, e234–e284. [Google Scholar] [CrossRef] [PubMed]
- Mets, J.M.; Bergersen, L.; Mayer, J.E., Jr.; Marshall, A.C.; McElhinney, D.B. Outcomes of stent implantation for obstruction of intracardiac lateral tunnel Fontan pathways. Circ. Cardiovasc. Interv. 2013, 6, 92–100. [Google Scholar] [CrossRef]
- Schreiber, C.; Hörer, J.; Vogt, M.; Cleuziou, J.; Prodan, Z.; Lange, R. Nonfenestrated extracardiac total cavopulmonary connection in 132 consecutive patients. Ann. Thorac. Surg. 2007, 84, 894–899. [Google Scholar] [CrossRef]
- Ono, M.; Kasnar-Samprec, J.; Hager, A.; Cleuziou, J.; Burri, M.; Langenbach, C.; Callegari, A.; Strbad, C.; Vogt, M.; Hörer, J.; et al. Clinical outcome following total cavopulmonary connection: A 20-year single-center experience. Eur. J. Cardio-Thoracic Surg. 2016, 50, 632–641. [Google Scholar] [CrossRef]
- Nakata, S.; Imai, Y.; Takanashi, Y.; Kurosawa, H.; Tezuka, K.; Nakazawa, M.; Ando, M.; Takao, A. A new method for the quantitative standardization of cross-sectional areas of the pulmonary arteries in congenital heart diseases with decreased pulmonary blood flow. J. Thorac. Cardiovasc. Surg. 1984, 88, 610–619. [Google Scholar] [CrossRef]
- Glatz, A.C.; Petit, C.J.; Goldstein, B.H.; Kelleman, M.S.; McCracken, C.E.; McDonnell, A.; Buckey, T.; Mascio, C.E.; Shashidharan, S.; Ligon, R.A.; et al. Comparison Between Patent Ductus Arteriosus Stent and Modified Blalock-Taussig Shunt as Palliation for Infants With Ductal-Dependent Pulmonary Blood Flow: Insights From the Congenital Catheterization Research Collaborative. Circulation 2018, 137, 589–601. [Google Scholar] [CrossRef]
- Winzig, A.; Matsubara, M.; Palm, J.; Schaeffer, T.; Osawa, T.; Lemmen, T.; Niedermaier, C.; Heinisch, P.P.; Georgiev, S.; Piber, N.; et al. Impact of previous left pulmonary artery stent on the outcome of a total cavopulmonary connection. Eur. J. Cardio-Thoracic Surg. 2025, 67, ezaf157. [Google Scholar] [CrossRef]
- Kramer, P.; Schleiger, A.; Schafstedde, M.; Danne, F.; Nordmeyer, J.; Berger, F.; Ovroutski, S. A Multimodal Score Accurately Classifies Fontan Failure and Late Mortality in Adult Fontan Patients. Front. Cardiovasc. Med. 2022, 9, 767503. [Google Scholar] [CrossRef]
- Carrillo, S.A.; Best, C.; Hersey, D.; Texter, K.; McConnell, P.I.; Boe, B.; Galantowicz, M. Preemptive stenting of the left pulmonary artery during comprehensive stage 2 procedure does not influence Fontan candidacy. JTCVS Open 2023, 13, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Krings, G.J.; van der Stelt, F.; Molenschot, M.C.; Breur, J.M. Oval stenting in left pulmonary artery stenosis: A novel double balloon technique to prevent airway compression in single ventricle. EuroIntervention 2020, 15, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Ewert, P.; Eicken, A.; Tanase, D.; Georgiev, S.; Will, A.; Pankalla, C.; Nagdyman, N.; Meierhofer, C.; Hörer, J. Transcatheter implantation of covered stents serving as extravascular conduits-Proof of a CT-based approach in three cases. Catheter. Cardiovasc. Interv. 2022, 99, 2054–2063. [Google Scholar] [CrossRef]
- Euringer, C.; Kido, T.; Ruf, B.; Burri, M.; Heinisch, P.P.; Vodiskar, J.; Strbad, M.; Cleuziou, J.; Dilber, D.; Hager, A.; et al. Management of failing bidirectional cavopulmonary shunt: Impact of additional systemic to pulmonary artery shunt with classic Glenn physiology. J. Thorac. Cardiovasc. Surg. Open 2022, 11, 373–387. [Google Scholar]
- Noonan, P.; Kudumula, V.; Anderson, B.; Ramchandani, B.; Miller, P.; Dhillon, R.; Mehta, C.; Stumper, O. Stenting of the left pulmonary artery after palliation of hypoplastic left heart syndrome. Catheter. Cardiovasc. Interv. 2016, 88, 225–232. [Google Scholar] [CrossRef]
- Euringer, C.; Schaeffer, T.; Heinisch, P.P.; Burri, M.; Georgiev, S.; Lemmer, J.; Ewert, P.; Hager, A.; Hörer, J.; Ono, M. Changes in pulmonary artery index and its relation to outcome after stage II palliation in patients with hypoplastic left heart syndrome. Eur. J. Cardio-Thoracic Surg. 2023, 63, ezad077. [Google Scholar] [CrossRef]
- Kisamori, E.; Venna, A.; Chaudhry, H.E.; Desai, M.; Tongut, A.; Mehta, R.; Clauss, S.; Yerebakan, C.; D’uDekem, Y. Alarming rate of liver cirrhosis after the small conduit extracardiac Fontan: A comparative analysis with the lateral tunnel. J. Thorac. Cardiovasc. Surg. 2024, 168, 1221–1227. [Google Scholar] [CrossRef]
- Kavin, U.; Shahrier, A.; Bandisode, V.M.; Chowdhury, S.M.; Rhodes, J.F.; Gaydos, S.S. Fontan Conduit Stent-Angioplasty and Progression of Fontan-Associated Liver Disease. Pediatr. Cardiol. 2025, 46, 372–378. [Google Scholar] [CrossRef]
- Patel, N.D.; Saadat, E.; Sullivan, P.M.; Cheng, A.L.; Takao, C.; Berman, D.P. Transcatheter Stenting to Restore Cross-Sectional Area of Extracardiac Fontan Conduits. Catheter. Cardiovasc. Interv. 2025, 105, 1134–1141. [Google Scholar] [CrossRef]
- Downing, T.E.; Allen, K.Y.; Goldberg, D.J.; Rogers, L.S.; Ravishankar, C.; Rychik, J.; Fuller, S.; Montenegro, L.M.; Steven, J.M.; Gillespie, M.J.; et al. Surgical and Catheter-Based Reinterventions Are Common in Long-Term Survivors of the Fontan Operation. Circ. Cardiovasc. Interv. 2017, 10, e004924. [Google Scholar] [CrossRef]
- Agasthi, P.; Jain, C.C.; Egbe, A.C.; Hagler, D.J.; Cabalka, A.K.; Taggart, N.W.; Anderson, J.H.; Cetta, F.; Connolly, H.M.; Burchill, L.J.; et al. Clinical Outcomes of Percutaneous Fontan Stenting in Adults. Can. J. Cardiol. 2023, 39, 1358–1365. [Google Scholar] [CrossRef]
- Mendelsohn, A.M.; Bove, E.L.; Lupinetti, F.M.; Crowley, D.C.; Lloyd, T.R.; Beekman, R.H. Central pulmonary artery growth patterns after the bidirectional Glenn procedure. J. Thorac. Cardiovasc. Surg. 1994, 107, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Comentale, G.; Cucchi, M.; Serrao, A.; Careddu, L.; Napoleone, C.P.; Gargiulo, G.; Oppido, G. Impact of preoperative left pulmonary artery stenting on the Fontan procedure: A retrospective multicentre study. Eur. J. Cardio-Thoracic Surg. 2024, 65, ezae035. [Google Scholar] [CrossRef] [PubMed]
- Nassar, M.S.; Bertaud, S.; Goreczny, S.; Greil, G.; Austin, C.B.; Salih, C.; Anderson, D.; Hussain, T. Technical and anatomical factors affecting the size of the branch pulmonary arteries following first-stage Norwood palliation for hypoplastic left heart syndrome. Interact. Cardiovasc. Thorac. Surg. 2015, 20, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Moszura, T.; Mazurek-Kula, A.; Dryzek, P.; Sysa, A. Bronchial compression as adverse effect of left pulmonary artery stenting in a patient with hypoplastic left heart syndrome. Pediatr. Cardiol. 2010, 31, 530–533. [Google Scholar] [CrossRef]
- Li, Y.; Williams, R.J.; Dombrowski, N.D.; Watters, K.; Daly, K.P.; Irace, A.; Visner, G.A.; Rahbar, R.; Fynn-Thompson, F. Current Evaluation and Management of Plastic Bronchitis in the Pediatric Population. Int. J. Pediatr. Otorhinolaryngol. 2020, 130, 109799. [Google Scholar] [CrossRef]
- Tanase, D.; Ewert, P.; Eicken, A. Plastic bronchitis: Symptomatic improvement after pulmonary arterial stenting in four patients with Fontan circulation. Cardiol. Young 2015, 25, 151–153. [Google Scholar] [CrossRef]
- Ruda, C.; Piber, N.; Schaeffer, T.; Matsubara, M.; Palm, J.; Lemmen, T.; Heinisch, P.P.; Georgiev, S.; Hager, A.; Ewert, P.; et al. Interventions for Fontan Pathway Obstruction in Patients Following Total Cavopulmonary Connection. In Proceedings of the AEPC 58th Annual Meeting, Hamburg, Germany, 28–31 May 2025. [Google Scholar]


| Variables: N(%) or Median (IQR) | Total Cases | TCPC Intervention (−) | TCPC Intervention (+) | p-Value |
|---|---|---|---|---|
| Number of patients | 650 | 514 (79.1) | 136 (20.9) | |
| Age at TCPC (years) | 2.3 (1.8–3.3) | 2.3 (1.8–3.5) | 2.2 (1.8–2.7) | 0.003 |
| Weight at TCPC (kg) | 12.0 (10.7–14.0) | 12.0 (10.8–14.4) | 11.3 (10.5–13.3) | 0.092 |
| Primary diagnosis | ||||
| HLHS | 176 (27.1) | 121 (23.5) | 55 (40.4) | <0.001 |
| UVH | 134 (20.6) | 115(22.4) | 19 (14.0) | 0.031 |
| TA | 104 (16.0) | 90 (17.5) | 14 (10.3) | 0.041 |
| DILV | 96 (14.8) | 72 (14.0) | 24 (17.6) | 0.287 |
| PAIVS | 34 (5.2) | 31 (6.0) | 3 (2.2) | 0.075 |
| ccTGA | 32 (4.9) | 27 (5.3) | 5 (3.7) | 0.450 |
| UAVSD | 27 (4.2) | 19 (3.7) | 8 (5.9) | 0.256 |
| Others | 48 (7.4) | 38 (7.4) | 10 (7.4) | 0.987 |
| Dominant right ventricle (RV) | 344 (52.9) | 258 (50.2) | 86 (63.2) | 0.007 |
| Associated cardiac anomaly | ||||
| TGA | 215 (33.1) | 178 (34.6) | 37 (27.2) | 0.102 |
| DORV | 83 (12.8) | 70 (13.6) | 13 (9.6) | 0.207 |
| CoA | 83 (12.8) | 58 (11.3) | 25 (18.4) | 0.027 |
| Dextrocardia/situs inversus | 58 (8.9) | 47 (9.1) | 11 (8.1) | 0.701 |
| Heterotaxy | 49 (7.5) | 38 (7.34 | 11 (8.1) | 0.785 |
| TAPVC/PAPVC | 42 (6.5) | 29 (5.6) | 13 (9.6) | 0.098 |
| Systemic venous return anomaly | 61 (9.4) | 53 (10.3) | 8 (5.9) | 0.115 |
| Palliation and pre-Fontan condition | ||||
| Norwood/DKS | 279 (42.7) | 191 (37.2) | 88 (64.7) | <0.001 |
| AP Shunt | 190 (29.2) | 162 (31.5) | 28 (20.6) | 0.013 |
| PAB | 93 (14.3) | 81 (15.8) | 12 (8.8) | 0.040 |
| PDA stent | 39 (6.0) | 26 (5.1) | 13 (9.6) | 0.049 |
| Prior BCPS | 601 (92.5) | 474 (92.2) | 127 (93.4) | 0.647 |
| Age at BCPS (months) | 5.1 (3.6–9.6) | 5.5 (3.7–11.3) | 4.5 (3.3–6.5) | <0.001 |
| Weight at BCPS (kg) | 5.7 (4.8–7.1) | 5.8 (4.9–7.4) | 5.3 (4.5–6.2) | 0.009 |
| Variables: | Total Cases | TCPC Intervention (−) | TCPC Intervention (+) | p-Value |
|---|---|---|---|---|
| Number of patients | 650 | 514 (79.1) | 136 (20.9) | |
| Hemoglobin (g/dl) | 15.4 (14.2–16.7) | 15.4 (14.2–16.7) | 15.5 (141.-16.9) | 0.759 |
| Hemodynamic | ||||
| PAP (mmHg) | 9 (8–12) | 9 (8–11) | 10 (8–12) | <0.001 |
| LAP (mmHg) | 5 (4–7) | 5 (4–7) | 6 (4–8) | 0.011 |
| TPG (mmHg) | 4 (3–5) | 4 (3–5) | 4 (3–5) | 0.054 |
| SVP (mmHg) | 83 (77–93) | 83 (76–91) | 86 (79–96) | 0.012 |
| EDP (mmHg) | 8 (6–9) | 7 (6–9) | 8 (6–10) | 0.110 |
| MAP (mmHg) | 58 (52–64) | 58 (51–64) | 59 (52–65) | 0.454 |
| SO2 (%) | 83 (80–86) | 83 (80–86) | 82 (80–86) | 0.237 |
| PA size and balance | ||||
| PAI (mm2/m2) | 167 (132–215) | 174 (139–222) | 150 (114–188) | 0.002 |
| Right PAI (mm2/m2) | 104 (77–137) | 107 (81–138) | 94 (73–127) | 0.179 |
| Left PAI (mm2/m2) | 60 (43–87) | 65 (46–90) | 50 (37–64) | <0.001 |
| Left-to-right ratio | 0.60 (0.39–0.85) | 0.61 (0.40–0.90) | 0.50 (0.34–0.79) | 0.102 |
| Symmetry index | 0.58 (0.39–0.77) | 0.60 (0.40–0.77) | 0.50 (0.34–0.73) | 0.011 |
| Data are shown by N (%) or median (IQR) | ||||
| Variables | Total Cases | TCPC Intervention (−) | TCPC Intervention (+) | p-Value |
|---|---|---|---|---|
| Number of patients | 650 | 514 (79.1) | 136 (20.9) | |
| Operative data | ||||
| Type of TCPC | ||||
| Intracardial | 50 (7.7) | 43 (8.4) | 7 (5.1) | 0.210 |
| Extra-cardiac | 600 (92.3) | 471 (91.6) | 129 (94.9) | |
| Conduit diameter (mm) | ||||
| 14 | 1 (0.2) | 1 (0.2) | 0 (0.0) | 0.217 |
| 16 | 9 (1.5) | 7 (1.5) | 2 (1.6) | |
| 18 | 518 (86.3) | 399 (84.7) | 119 (92.2) | |
| 20 | 57 (9.5) | 50 (10.6) | 7 (5.4) | |
| 22 | 15 (2.5) | 14 (3.0) | 1 (0.8) | |
| CPB time (minutes) | 67 (48–101) | 65 (47–99) | 74 (56–109) | 0.008 |
| Aortic cross clamp (AXC) | 166 (25.5) | 136 (26.5) | 30 (22.1) | 0.295 |
| AXC time (minutes) | 46 (26–73) | 47 (26–73) | 41 (24–73) | 0.901 |
| Fenestration at TCPC | 60 (9.2) | 45 (8.8) | 15 (11.0) | 0.415 |
| Concomitant procedure | 175 (26.9) | 138 (26.8) | 37 (27.2) | 0.933 |
| DKS | 17 (2.6) | 12 (2.3) | 5 (3.7) | 0.383 |
| AVV procedure | 79 (12.2) | 63 (12.3) | 16 (11.8) | 0.876 |
| PA reconstruction | 60 (9.2) | 44 (8.6) | 16 (11.8) | 0.251 |
| Atrioseptectomy | 31 (4.8) | 28 (5.4) | 3 (2.2) | 0.115 |
| SAS/VSD enlargement | 14 (2.2) | 12 (2.3) | 2 (1.5) | 0.537 |
| Pacemaker implant | 13 (2.0) | 10 (1.9) | 3 (2.2) | 0.847 |
| Postoperative data | ||||
| ICU stay (days) | 6 (4–9) | 6 (4–8) | 7 (4–11) | 0.020 |
| Hospital stay (days) | 20 (14–27) | 18 (14–26) | 24 (18–35) | <0.001 |
| Complications | ||||
| Pleural effusion | 318 (49.4) | 228 (44.9) | 90 (66.2) | <0.001 |
| Chylothorax | 139 (21.7) | 92 (18.2) | 47 (34.6) | <0.001 |
| Ascites | 124 (19.2) | 81 (15.9) | 43 (31.6) | <0.001 |
| Secondary fenestration | 11 (1.7) | 7 (1.4) | 4 (2.9) | 0.204 |
| Follow-up data | ||||
| PLE | 31 (5.0) | 10 (2.0) | 21 (21.2) | <0.001 |
| PB | 15 (2.4) | 4 (0.8) | 11 (8.3) | <0.001 |
| Failing Fontan | 76 (12.0) | 38 (7.6) | 38 (28.6) | <0.001 |
| Variables | N (%) |
|---|---|
| Number of patients | 136 |
| Surgical interventions | 10 (7.4) |
| LPA patch | 4 (2.9) |
| RPA patch | 1 (0.7) |
| Conduit revision | 8 (5.9) |
| SVC patch | 2 (1.5) |
| Catheter interventions | 128 (94.1) |
| LPA intervention | 107 (78.7) |
| LPA balloon | 43 (31.6) |
| LPA stent | 98 (72.1) |
| RPA interventions | 8 (5.9) |
| RPA balloon | 4 (2.9) |
| RPA stent | 7 (5.1) |
| Conduit interventions | 27 (19.9) |
| Conduit balloon | 7 (5.1) |
| Conduit stent | 24 (17.6) |
| IVC interventions | 20 (14.7) |
| IVC balloon | 5 (3.7) |
| IVC stent | 19 (14.0) |
| SVC interventions | 4 (2.9) |
| SVC balloon | 2 (1.5) |
| SVC stent | 3 (2.2) |
| Both surgical and catheter intervention were needed in 2 patients | |
| Risk Factors for Interventions for TCPC Pathway Obstruction | ||||||
|---|---|---|---|---|---|---|
| Variables | Univariate | Multivariate | ||||
| p-Value | HR | 95% CI | p-Value | HR | 95% CI | |
| HLHS | <0.001 | 2.238 | 1.582–3.166 | |||
| Dominant RV | 0.001 | 1.795 | 1.263–2.550 | |||
| Norwood/DKS | <0.001 | 3.031 | 2.121–4.332 | 0.003 | 2.228 | 1.303–3.811 |
| PDA stent | <0.001 | 3.125 | 1.736–6.627 | 0.009 | 2.574 | 1.262–5.250 |
| Pre-TCPC PA intervention | <0.001 | 2.864 | 1.926–4.260 | <0.001 | 2.514 | 1.457–4.337 |
| PAP pre TCPC | <0.001 | 1.084 | 1.035–1.135 | 0.004 | 1.161 | 1.049–1.286 |
| LAP pre TCPC | 0.002 | 1.105 | 1.037–1.177 | |||
| EDP pre TCPC | 0.005 | 1.090 | 1.026–1.157 | |||
| PA index pre-TCPC | 0.031 | 0.996 | 0.992–1.000 | |||
| LPA index | 0.006 | 0.989 | 0.981–0.997 | |||
| Age at TCPC | 0.005 | 0.903 | 0.840–0.970 | |||
| Risk factors for interventions for left-PA stenosis | ||||||
| Variables | Univariate | Multivariate | ||||
| p-value | HR | 95% CI | p-value | HR | 95% CI | |
| HLHS | <0.001 | 2.734 | 1.874–3.990 | |||
| Dominant RV | <0.001 | 2.063 | 1.383–3.077 | |||
| Norwood/DKS | <0.001 | 4.408 | 2.876–6.757 | <0.001 | 3.476 | 1.852-6.525 |
| PDA stent | <0.001 | 3.934 | 2.164–7.153 | <0.001 | 4.049 | 1.931-8.489 |
| Pre-TCPC PA intervention | <0.001 | 2.925 | 1.893–4.518 | 0.005 | 2.323 | 1.284-4.204 |
| PAP pre TCPC | 0.041 | 1.058 | 1.002–1.116 | 0.010 | 1.158 | 1.035-1.295 |
| LAP pre TCPC | 0.010 | 1.098 | 1.022–1.180 | |||
| EDP pre TCPC | 0.035 | 1.075 | 1.005–1.150 | |||
| PA index pre-TCPC | 0.002 | 0.993 | 0.989–0.997 | |||
| LPA index | <0.001 | 0.984 | 0.975–0.993 | |||
| Age at TCPC | 0.003 | 0.858 | 0.776–0.948 | |||
| Risk factors for interventions for IVC/conduit obstruction | ||||||
| Variables | Univariate | Multivariate | ||||
| p-value | HR | 95% CI | p-value | HR | 95% CI | |
| DILV | 0.037 | 2.074 | 1.046–4.110 | |||
| Dominant RV | 0.044 | 0.527 | 0.282–0.983 | |||
| PAP pre-TCPC | 0.021 | 1.086 | 1.013–1.166 | 0.021 | 1.085 | 1.012–1.164 |
| Weight at TCPC | 0.047 | 1.025 | 1.000–1.051 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piber, N.; Ruda, C.; Schaeffer, T.; Matsubara, M.; Palm, J.; Lemmen, T.; Heinisch, P.P.; Georgiev, S.; Hager, A.; Ewert, P.; et al. Interventions for Fontan Pathway Obstruction in Patients Following Total Cavopulmonary Connection. J. Clin. Med. 2025, 14, 7447. https://doi.org/10.3390/jcm14207447
Piber N, Ruda C, Schaeffer T, Matsubara M, Palm J, Lemmen T, Heinisch PP, Georgiev S, Hager A, Ewert P, et al. Interventions for Fontan Pathway Obstruction in Patients Following Total Cavopulmonary Connection. Journal of Clinical Medicine. 2025; 14(20):7447. https://doi.org/10.3390/jcm14207447
Chicago/Turabian StylePiber, Nicole, Christina Ruda, Thibault Schaeffer, Muneaki Matsubara, Jonas Palm, Teresa Lemmen, Paul Philipp Heinisch, Stanimir Georgiev, Alfred Hager, Peter Ewert, and et al. 2025. "Interventions for Fontan Pathway Obstruction in Patients Following Total Cavopulmonary Connection" Journal of Clinical Medicine 14, no. 20: 7447. https://doi.org/10.3390/jcm14207447
APA StylePiber, N., Ruda, C., Schaeffer, T., Matsubara, M., Palm, J., Lemmen, T., Heinisch, P. P., Georgiev, S., Hager, A., Ewert, P., Krane, M., Hörer, J., & Ono, M. (2025). Interventions for Fontan Pathway Obstruction in Patients Following Total Cavopulmonary Connection. Journal of Clinical Medicine, 14(20), 7447. https://doi.org/10.3390/jcm14207447






