Gait Recovery After Total Hip Arthroplasty with Subtrochanteric Osteotomy in Highly Dislocated Hips: A Retrospective Single-Center Cohort Study
Abstract
1. Introduction
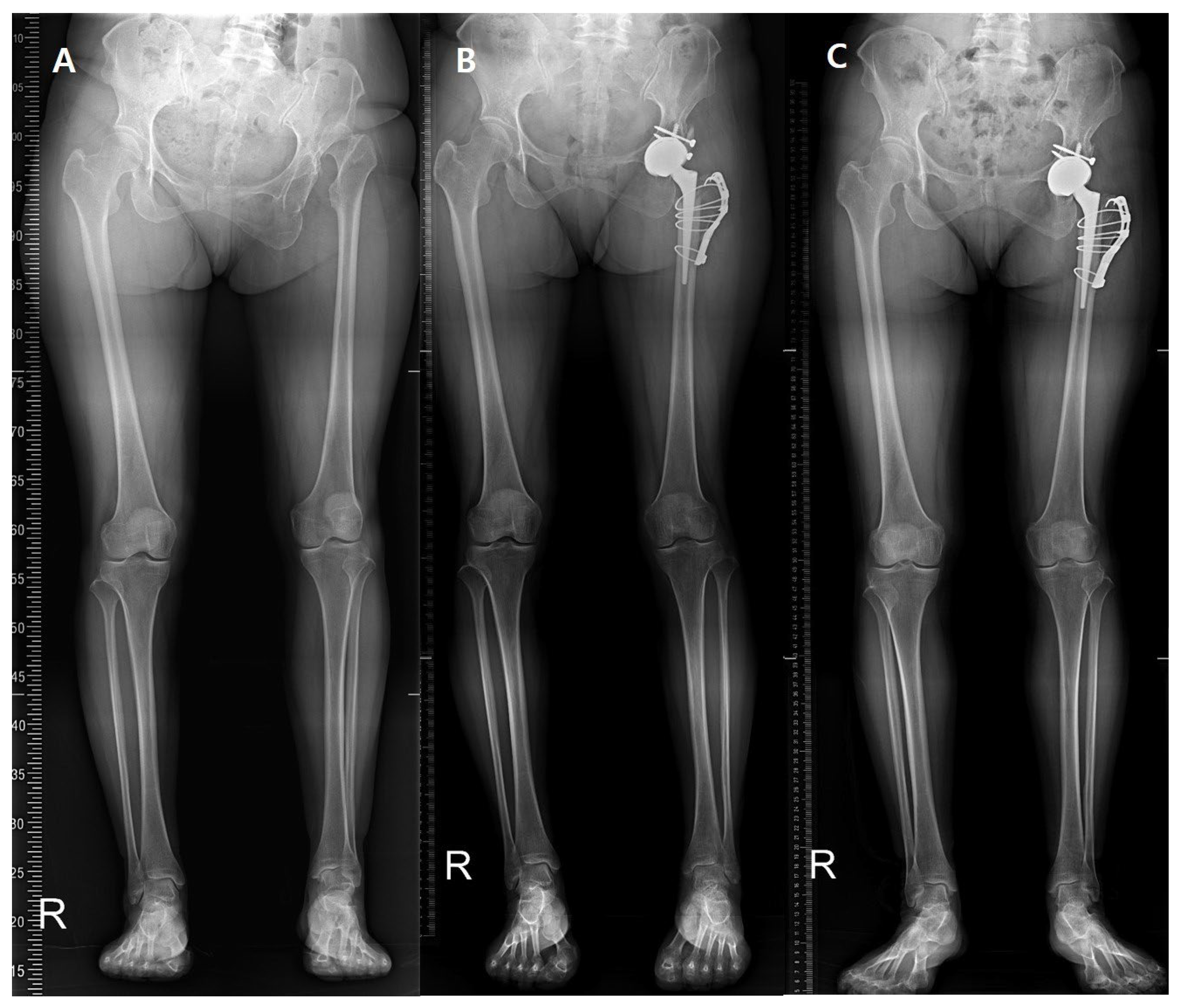
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Learmonth, I.D.; Young, C.; Rorabeck, C. The operation of the century: Total hip replacement. Lancet 2007, 370, 1508–1519. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.N.; Liu, J.L.; Jia, X.L.; Zhou, Q.; Yang, L.; Zhang, Y. Midterm Results of Total Hip Arthroplasty in Patients With High Hip Dislocation After Suppurative Hip Arthritis. J. Arthroplast. 2019, 34, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.Y.; Jin, J.Y.; Cheon, J.H.; Yoon, T.R.; Park, K.S. Survival Analysis of Total Hip Arthroplasty for High Hip Dislocation Secondary to Developmental Dysplasia or Septic Arthritis of the Hip. J. Arthroplast. 2021, 36, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Seo, H.S.; Kim, J.S. Outcomes after THA in patients with high hip dislocation after childhood sepsis. Clin. Orthop. Relat. Res. 2009, 467, 2371–2378. [Google Scholar] [CrossRef]
- Park, C.W.; Lim, S.J.; Cha, Y.T.; Park, Y.S. Total Hip Arthroplasty With Subtrochanteric Shortening Osteotomy in Patients With High Hip Dislocation Secondary to Childhood Septic Arthritis: A Matched Comparative Study With Crowe IV Developmental Dysplasia. J. Arthroplast. 2020, 35, 204–211. [Google Scholar] [CrossRef]
- Mu, W.; Yang, D.; Xu, B.; Mamtimin, A.; Guo, W.; Cao, L. Midterm Outcome of Cementless Total Hip Arthroplasty in Crowe IV-Hartofilakidis Type III Developmental Dysplasia of the Hip. J. Arthroplast. 2016, 31, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Nema, S.K.; Madegowda, A.; Chouhan, D.; Garg, A.K. Similar Outcomes between Monoblock and Modular Femoral Stems in Total Hip Arthroplasty with Shortening Osteotomy for Dysplastic Hips at Five Years: A Systematic Review with Meta-analysis. Hip Pelvis 2025, 37, 1–16. [Google Scholar] [CrossRef]
- Wamper, K.E.; Sierevelt, I.N.; Poolman, R.W.; Bhandari, M.; Haverkamp, D. The Harris hip score: Do ceiling effects limit its usefulness in orthopedics? Acta Orthop. 2010, 81, 703–707. [Google Scholar] [CrossRef]
- Clement, N.D.; Weir, D.; Holland, J.P.; Gerrand, C.H.; Deehan, D.J. An Overview and Predictors of Achieving the Postoperative Ceiling Effect of the WOMAC Score Following Total Knee Arthroplasty. J. Arthroplast. 2019, 34, 273–280. [Google Scholar] [CrossRef]
- Bennett, D.; Ogonda, L.; Elliott, D.; Humphreys, L.; Beverland, D.E. Comparison of gait kinematics in patients receiving minimally invasive and traditional hip replacement surgery: A prospective blinded study. Gait Posture 2006, 23, 374–382. [Google Scholar] [CrossRef]
- Ewen, A.M.; Stewart, S.; St Clair Gibson, A.; Kashyap, S.N.; Caplan, N. Post-operative gait analysis in total hip replacement patients-a review of current literature and meta-analysis. Gait Posture 2012, 36, 1–6. [Google Scholar] [CrossRef]
- Bennett, D.; Humphreys, L.; O’Brien, S.; Kelly, C.; Orr, J.F.; Beverland, D.E. Gait kinematics of age-stratified hip replacement patients--a large scale, long-term follow-up study. Gait Posture 2008, 28, 194–200. [Google Scholar] [CrossRef]
- Beaulieu, M.L.; Lamontagne, M.; Beaulé, P.E. Lower limb biomechanics during gait do not return to normal following total hip arthroplasty. Gait Posture 2010, 32, 269–273. [Google Scholar] [CrossRef]
- Yoo, J.I.; Cha, Y.H.; Kim, K.J.; Kim, H.Y.; Choy, W.S.; Hwang, S.C. Gait analysis after total hip arthroplasty using direct anterior approach versus anterolateral approach: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2019, 20, 63. [Google Scholar] [CrossRef]
- Martinez, L.; Noé, N.; Beldame, J.; Matsoukis, J.; Poirier, T.; Brunel, H.; Van Driessche, S.; Lalevée, M.; Billuart, F. Quantitative gait analysis after total hip arthroplasty through a minimally invasive direct anterior approach: A case control study. Orthop. Traumatol. Surg. Res. 2022, 108, 103214. [Google Scholar] [CrossRef]
- Petersen, M.K.; Andersen, N.T.; Mogensen, P.; Voight, M.; Søballe, K. Gait analysis after total hip replacement with hip resurfacing implant or Mallory-head Exeter prosthesis: A randomised controlled trial. Int. Orthop. 2011, 35, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.J.; Lemaire, E.D. Temporal-spatial gait parameter models of very slow walking. Gait Posture 2018, 61, 125–129. [Google Scholar] [CrossRef]
- Makino, A.; Yamaguchi, K.; Sumi, D.; Ichikawa, M.; Ohno, M.; Nagano, A.; Goto, K. Ground reaction force and electromyograms of lower limb muscles during fast walking. Front. Sports Act. Living 2022, 4, 1055302. [Google Scholar] [CrossRef]
- Park, K.S.; Yoon, T.R.; Song, E.K.; Seon, J.K.; Lee, K.B. Total hip arthroplasty in high dislocated and severely dysplastic septic hip sequelae. J. Arthroplast. 2012, 27, 1331–1336.e1331. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.F.; Mani, V.J.; Ranawat, C.S. Total hip replacement in congenital dislocation and dysplasia of the hip. J. Bone Jt. Surg. Am. 1979, 61, 15–23. [Google Scholar] [CrossRef]
- Hartofilakidis, G.; Stamos, K.; Ioannidis, T.T. Low friction arthroplasty for old untreated congenital dislocation of the hip. J. Bone Jt. Surg. Br. 1988, 70, 182–186. [Google Scholar] [CrossRef]
- Harris, W.H. Traumatic arthritis of the hip after dislocation and acetabular fractures: Treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J. Bone Jt. Surg. Am. 1969, 51, 737–755. [Google Scholar] [CrossRef]
- Broadhurst, C.; Rhodes, A.M.L.; Harper, P.; Perry, D.C.; Clarke, N.M.P.; Aarvold, A. What is the incidence of late detection of developmental dysplasia of the hip in England?: A 26-year national study of children diagnosed after the age of one. Bone Jt. J. 2019, 101-b, 281–287. [Google Scholar] [CrossRef]
- Samora, J.B.; Klingele, K. Septic arthritis of the neonatal hip: Acute management and late reconstruction. J. Am. Acad. Orthop. Surg. 2013, 21, 632–641. [Google Scholar] [CrossRef]
- Okano, T.; Enokida, M.; Otsuki, R.; Hagino, H.; Teshima, R. Recent trends in adult-onset septic arthritis of the knee and hip: Retrospective analysis of patients treated during the past 50 years. J. Infect. Chemother. 2011, 17, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, A.I.; Subesinghe, S.; Bharucha, T.; Ibrahim, F.; Kleymann, A.; Galloway, J.B. A population study of the reported incidence of native joint septic arthritis in the United Kingdom between 1998 and 2013. Rheumatology 2016, 55, 2176–2180. [Google Scholar] [CrossRef] [PubMed]
- Krych, A.J.; Howard, J.L.; Trousdale, R.T.; Cabanela, M.E.; Berry, D.J. Total hip arthroplasty with shortening subtrochanteric osteotomy in Crowe type-IV developmental dysplasia: Surgical technique. J. Bone Jt. Surg. Am. 2010, 92 Pt 2 (Suppl. 1), 176–187. [Google Scholar] [CrossRef] [PubMed]
- Tateda, K.; Nagoya, S.; Suzuki, D.; Kosukegawa, I.; Yamashita, T. Acetabular Morphology in Patients with Developmental Dysplasia of the Hip with High Dislocation. Hip Pelvis 2021, 33, 25–32. [Google Scholar] [CrossRef]
- Jiang, X.; Napier, C.; Hannigan, B.; Eng, J.J.; Menon, C. Estimating Vertical Ground Reaction Force during Walking Using a Single Inertial Sensor. Sensors 2020, 20, 4345. [Google Scholar] [CrossRef]
- Wannop, J.W.; Worobets, J.T.; Stefanyshyn, D.J. Normalization of ground reaction forces, joint moments, and free moments in human locomotion. J. Appl. Biomech. 2012, 28, 665–676. [Google Scholar] [CrossRef]
- Mei, Q.; Fernandez, J.; Fu, W.; Feng, N.; Gu, Y. A comparative biomechanical analysis of habitually unshod and shod runners based on a foot morphological difference. Hum. Mov. Sci. 2015, 42, 38–53. [Google Scholar] [CrossRef] [PubMed]
- McCrory, J.L.; White, S.C.; Lifeso, R.M. Vertical ground reaction forces: Objective measures of gait following hip arthroplasty. Gait Posture 2001, 14, 104–109. [Google Scholar] [CrossRef] [PubMed]


| Parameters | |
|---|---|
| Age (years) | 48.9 (28 to 66) |
| Female/male | 6/4 |
| Body mass index (kg/m2) | 29.8 (24.5 to 38.2) |
| Preoperative status | |
| DDH (Crowe type) | 5 (IV) |
| SSH (Hartofilakidis type) | 5 (III) |
| Follow-up (years) | 7.1 (3 to 10) |
| Pre-Operative (N = 10) | Post-Operative 1-Year (N = 10) | Post-Operative 5-Year (N = 5) | p Value (Pre- vs. 1-Year) | p Value (Pre- vs. 5-Year) | p Value (1- vs. 5-Year) | |
|---|---|---|---|---|---|---|
| Spatio-temporal | ||||||
| Cadence (steps/min) | 114.7 (100.1–128.7) | 112.6 (93.8–127.3) | 110.6 (93.4–117.8) | 0.462 | 0.742 | 0.852 |
| Speed (cm/s) | 103.9 (82.4–139.1) | 96.4 (68.6–126.5) | 98.8 (88.6–109.8) | 0.277 | 0.835 | 0.765 |
| Stride length (cm) | 108.5 (83.7–132.6) | 102.3 (88.0–119.0) | 107.9 (93.7–116.6) | 0.277 | 0.640 | 0.752 |
| Step length (cm) | 52.4 (37.4–69.4) | 50.2 (42.0–59.5) | 53.3 (43.6–60.1) | 0.458 | 0.437 | 0.956 |
| Step time (s) | 0.53 (0.47–0.60) | 0.54 (0.47–0.64) | 0.55 (0.51–0.64) | 0.541 | 0.771 | 0.764 |
| Single support (% cycle) | 35.6 (27.2–40.6) | 37.2 (30.2–39.9) | 37.4 (35.8–39.8) | 0.281 | 0.262 | 0.922 |
| IDS (% cycle) | 10.1 (6.2–14.7) | 11.6 (8.1–14.2) | 10.7 (8.2–12.3) | 0.171 | 0.988 | 0.654 |
| TDS (% cycle) | 8.6 (4.3–12.6) | 11.3 (5.8–14.0) | 13.2 (11.0–15.7) | 0.020 | 0.024 | 0.953 |
| Stance phase (% cycle) | 54.4 (49.5–59.7) | 60.1 (56.5–67.4) | 61.4 (59.1–62.9) | 0.001 | 0.003 | 0.945 |
| Swing phase (% cycle) | 45.6 (40.3–50.5) | 39.9 (32.6–43.5) | 38.6 (37.1–40.9) | 0.001 | 0.003 | 0.965 |
| Vertical GRF | ||||||
| 1st peak force (N/BW) | 0.96 (0.69–1.30) | 1.11 (0.95–1.31) | 0.91 (0.78–0.99) | 0.045 | 0.240 | 0.856 |
| 2nd peak force (N/BW) | 0.87 (0.59–1.12) | 1.10 (1.00–1.30) | 1.01 (0.97–1.03) | 0.001 | 0.307 | 0.621 |
| Dynamic ROM | ||||||
| Sagittal | 25.8 (11.9–50.4) | 30.8 (19.3–53.3) | 37.0 (30.1–53.8) | 0.257 | 0.051 | 0.652 |
| Transverse | 13.6 (5.5–24.2) | 11.3 (5.7–16.8) | 11.4 (8.1–16.6) | 0.286 | 0.099 | 0.564 |
| Coronal | 10.0 (5.6–16.3) | 7.4 (3.4 –14.3) | 8.2 (5.2–11.0) | 0.115 | 0.671 | 0.620 |
| Pre-Operative (N = 10) | Post-Operative 1-Year (N = 10) | Post-Operative 5-Year (N = 5) | p Value (Pre- vs. 1-Year) | p Value (Pre- vs. 5-Year) | p Value (1- vs. 5-Year) | |
|---|---|---|---|---|---|---|
| 1st peak force (N/BW) | 1.05 (0.73–1.39) | 1.23 (1.05–1.70) | 0.96 (0.91–1.03) | 0.102 | 0.120 | 0.850 |
| 2nd peak force (N/BW) | 0.97 (0.77–1.25) | 1.20 (1.04–1.43) | 1.03 (0.98–1.09) | 0.017 | 0.652 | 0.782 |
| Pre-Operative | Post-Operative | p Value | |
|---|---|---|---|
| HHS | 57.2 (43–67) | 79.6 (61–88) | 0.001 |
| LLD (mm) | 43.6 (18.2–71.6) | 9.8 (2.1–22.1) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.-J.; Lee, G.-W.; Lee, C.Y.; Park, K.-S. Gait Recovery After Total Hip Arthroplasty with Subtrochanteric Osteotomy in Highly Dislocated Hips: A Retrospective Single-Center Cohort Study. J. Clin. Med. 2025, 14, 7446. https://doi.org/10.3390/jcm14207446
Park C-J, Lee G-W, Lee CY, Park K-S. Gait Recovery After Total Hip Arthroplasty with Subtrochanteric Osteotomy in Highly Dislocated Hips: A Retrospective Single-Center Cohort Study. Journal of Clinical Medicine. 2025; 14(20):7446. https://doi.org/10.3390/jcm14207446
Chicago/Turabian StylePark, Chan-Jin, Gun-Woo Lee, Chan Young Lee, and Kyung-Soon Park. 2025. "Gait Recovery After Total Hip Arthroplasty with Subtrochanteric Osteotomy in Highly Dislocated Hips: A Retrospective Single-Center Cohort Study" Journal of Clinical Medicine 14, no. 20: 7446. https://doi.org/10.3390/jcm14207446
APA StylePark, C.-J., Lee, G.-W., Lee, C. Y., & Park, K.-S. (2025). Gait Recovery After Total Hip Arthroplasty with Subtrochanteric Osteotomy in Highly Dislocated Hips: A Retrospective Single-Center Cohort Study. Journal of Clinical Medicine, 14(20), 7446. https://doi.org/10.3390/jcm14207446






