Early Use of Cryoprecipitate Versus Plasma and Clinical Outcomes in Major Spine Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Exposure and Endpoints
2.3. Data Collection
2.4. Anesthetic and Surgical Management
2.5. Statistical Analyses
3. Results
3.1. Patient Demographic and Baseline Characteristics
3.2. Intraoperative Blood Product Transfusion Patterns
3.3. Study Endpoints
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rocos, B.; Kato, S.; Oitment, C.; Smith, J.; Jentszch, T.; Martin, A.; Rienmuller, A.; Nielsen, C.; Shaffrey, C.I.; Lenke, L.G.; et al. Blood Management and Conservation During Adult Spine Deformity Surgery. Glob. Spine J. 2025, 15 (Suppl. S3), 95s–107s. [Google Scholar] [CrossRef] [PubMed]
- Bible, J.E.; Mirza, M.; Knaub, M.A. Blood-loss Management in Spine Surgery. J. Am. Acad. Orthop. Surg. 2018, 26, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.M.; Rendo, M.J.; Reddoch-Cardenas, K.M.; Burris, J.K.; Meledeo, M.A.; Cap, A.P. Recent advances in use of fresh frozen plasma, cryoprecipitate, immunoglobulins, and clotting factors for transfusion support in patients with hematologic disease. Semin. Hematol. 2020, 57, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Vlaar, A.P.; Toy, P.; Fung, M.; Looney, M.R.; Juffermans, N.P.; Bux, J.; Bolton-Maggs, P.; Peters, A.L.; Silliman, C.C.; Kor, D.J.; et al. A consensus redefinition of transfusion-related acute lung injury. Transfusion 2019, 59, 2465–2476. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, M.S.; Bora, V. Managing Fresh-Frozen Plasma Transfusion Adverse Effects: Allergic Reactions, TACO, and TRALI; StatPearls Publishing LLC.: Petersburg, FL, USA, 2025. [Google Scholar]
- Green, L.; Bolton-Maggs, P.; Beattie, C.; Cardigan, R.; Kallis, Y.; Stanworth, S.J.; Thachil, J.; Zahra, S. British Society of Haematology Guidelines on the spectrum of fresh frozen plasma and cryoprecipitate products: Their handling and use in various patient groups in the absence of major bleeding. Br. J. Haematol. 2018, 181, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Hinton, J.V.; Xing, Z.; Fletcher, C.M.; Perry, L.A.; Karamesinis, A.; Shi, J.; Ramson, D.M.; Penny-Dimri, J.C.; Liu, Z.; Williams-Spence, J.; et al. Association of Perioperative Cryoprecipitate Transfusion and Mortality After Cardiac Surgery. Ann. Thorac. Surg. 2023, 116, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Hinton, J.V.; Xing, Z.; Fletcher, C.; Perry, L.A.; Karamesinis, A.; Shi, J.; Penny-Dimri, J.C.; Ramson, D.; Coulson, T.G.; Segal, R.; et al. Association of perioperative transfusion of fresh frozen plasma and outcomes after cardiac surgery. Acta Anaesthesiol. Scand. 2024, 68, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Purvis, T.E.; Wang, T.Y.; Sankey, E.W.; Frank, S.M.; Goodwin, C.R.; Sciubba, D.M. Defining Usage and Clinical Outcomes Following Perioperative Fresh Frozen Plasma and Platelet Administration in Spine Surgery Patients. Clin. Spine. Surg. 2019, 32, E246.e51. [Google Scholar]
- Naik, B.I.; Pajewski, T.N.; Bogdonoff, D.I.; Zuo, Z.; Clark, P.; Terkawi, A.S.; Durieux, M.E.; Shaffrey, C.I.; Nemergut, E.C. Rotational thromboelastometry-guided blood product management in major spine surgery. J. Neurosurg. Spine. 2015, 23, 239–249. [Google Scholar] [CrossRef] [PubMed]
- McQuilten, Z.K.; Andrianopoulos, N.; Wood, E.M.; Cole-Sinclair, M.F.; McNeil, J.J.; Cameron, P.A.; Reid, C.M.; Newcomb, A.E.; Smith, J.A.; Phillips, L.E. Transfusion practice varies widely in cardiac surgery: Results from a national registry. J. Thorac. Cardiovasc. Surg. 2014, 147, 1684–1690.e1. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.; Tobian, A.A.R.; Shaz, B.H. Noninfectious transfusion-associated adverse events and their mitigation strategies. Blood 2019, 133, 1831–1839. [Google Scholar] [CrossRef] [PubMed]
- Geck, M.J.; Singh, D.; Gunn, H.; Stokes, J.K.; Truumees, E. Relationship Between Preoperative Plasma Fibrinogen Concentration, Perioperative Bleeding, and Transfusions in Elective Adult Spinal Deformity Correction. Spine Deform. 2019, 7, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Rossaint, R.; Afshari, A.; Bouillon, B.; Cerny, V.; Cimpoesu, D.; Curry, N.; Duranteau, J.; Filipescu, D.; Grottke, O.; Grønlykke, L.; et al. The European guideline on management of major bleeding and coagulopathy following trauma: Sixth edition. Crit. Care 2023, 27, 80. [Google Scholar] [CrossRef]
| Overall (n = 189) | Cryoprecipitate (n = 120) | Plasma (n = 69) | |
|---|---|---|---|
| Age, years | 68.0 [59.0, 73.0] | 67.0 [60.0, 73.0] | 69.0 [58.0, 73.0] |
| Race, n (%) | |||
| Asian | 2 (1.1%) | 0 (0%) | 2 (2.9%) |
| Black or African American | 28 (14.8%) | 19 (15.8%) | 9 (13.0%) |
| Caucasian/White | 154 (81.5%) | 97 (80.8%) | 57 (82.6%) |
| Other | 5 (2.6%) | 4 (3.3%) | 1 (1.4%) |
| Female, n (%) | 129 (68.3%) | 83 (69.2%) | 46 (66.7%) |
| Weight, kg | 77.4 [64.4, 90.7] | 76.6 [64.3, 88.8] | 78.3 [67.1, 91.5] |
| BMI | 27.5 [23.9, 32.4] | 27.6 [24.1, 32.9] | 27.5 [23.7, 31.4] |
| Diabetes, n (%) | 23 (12.2%) | 14 (11.7%) | 9 (13.0%) |
| Hypertension, n (%) | 83 (43.9%) | 57 (47.5%) | 26 (37.7%) |
| Smoking status, n (%) | 7 (3.7%) | 4 (3.3%) | 3 (4.3%) |
| ASA status, n (%) | |||
| 1 or 2 | 35 (18.5%) | 22 (18.3%) | 13 (18.8%) |
| 3 or 4 | 154 (81.5%) | 98 (81.7%) | 56 (81.2%) |
| Preoperative Laboratory studies * | |||
| Hemoglobin, g/dL | 12.4 [11.3, 13.5] | 12.5 [11.7, 13.5] | 12.0 [10.7, 13.8] |
| Platelet count (×109/L) | 237 [198, 278] | 244 [201, 279] | 227 [190, 274] |
| INR | 1.00 [1.00, 1.10] | 1.00 [1.00, 1.10] | 1.10 [1.00, 1.15] |
| Emergency Status, n (%) | 2 (1.1%) | 1 (0.8%) | 1 (1.4%) |
| Surgical Service, n (%) | |||
| Neurosurgery | 124 (65.6%) | 95 (79.2%) | 29 (42.0%) |
| Orthopedics | 65 (34.4%) | 25 (20.8%) | 40 (58.0%) |
| Location, n (%) | |||
| University Hospital | 162 (85.7%) | 119 (99.2%) | 43 (62.3%) |
| Community Hospital | 27 (14.3%) | 1 (0.8%) | 26 (37.7%) |
| Case length, minutes | 528 [451, 639] | 548 [472, 632] | 498 [395, 644] |
| Procedure by CPT codes ** | |||
| Anterior arthrodesis/instrumentation 2–3 levels (22808, 22845) | 5 (2.6%) | 1 (0.8%) | 4 (5.8%) |
| Anterior arthrodesis/instrumentation 4–7 levels (22810, 22846) | 4 (2.1%) | 2 (1.7%) | 2 (2.9%) |
| Posterior arthrodesis/instrumentation up to 6 levels (22800, 22842) | 67 (35.4%) | 33 (27.5%) | 34 (49.3%) |
| Posterior arthrodesis/instrumentation 7–12 levels (22802, 22843) | 151 (79.9%) | 116 (96.7%) | 35 (50.7%) |
| Posterior arthrodesis/instrumentation 13+ levels (22804, 22844) | 46 (24.3%) | 30 (25%) | 16 (23.2%) |
| Posterior or lateral osteotomy (22206, 22207, 22208) | 47 (24.9%) | 36 (30%) | 11 (15.9%) |
| PRBCs, n (%) | 189 (100%) | 120 (100%) | 69 (100%) |
| N of PRBC units | 4 [2, 5] | 3 [2, 5] | 4 [2, 6] |
| Cryoprecipitate, n (%) | 151 (79.9%) | 120 (100%) | 31 (44.9%) |
| No. of Cryoprecipitate units | 2 [1, 2] | 2 [1, 2] | 1 [1, 2] |
| Plasma, No. (%) | 89 (47.1%) | 20 (16.7%) | 69 (100%) |
| No. of Plasma units | 2 [1, 3] | 2 [1, 2.3] | 2 [1, 3] |
| Plasma and Cryoprecipitate, n (%) | 51 (27.0%) | 20 (16.7%) | 31 (44.9%) |
| Apheresis platelet, n (%) | 64 (33.9%) | 40 (33.3%) | 24 (34.8%) |
| No. of Apheresis platelet units | 1 [1, 2] | 1 [1, 2] | 2 [1, 2] |
| Cell saver intake, n (%) | 120 (63.5%) | 88 (73.3%) | 32 (46.4%) |
| Cell saver volume, mL | 581 [375, 932] | 572 [377, 932] | 652 [375, 914] |
| Estimated Blood Loss (mL) | 2300 [1500, 3650] | 2400 [1500, 3700] | 2050 [1500, 3500] |
| Tranexamic Acid, n (%) | 133 (70.4%) | 104 (86.7%) | 29 (42.0%) |
| Total dose, g | 3.41 [1.97, 4.75] | 3.70 [2.45, 4.82] | 2.45 [1.42, 4.22] |
| Intraoperative ROTEM, n (%) | 159 (84.1%) | 117 (97.5%) | 42 (60.9%) |
| First FIBTEM A10 | 15.0 [12.0, 18.0] | 15.0 [12.0, 18.0] | 15.0 [12.0, 19.8] |
| Lowest FIBTEM A10 | 10.0 [8.0, 12.0] | 10.0 [8.0, 12.0] | 10.0 [6.0, 13.5] |
| First EXTEM A10 | 57.0 [51.0, 61.5] | 57.0 [51.0, 61.0] | 56.5 [52.3, 63.0] |
| Lowest EXTEM A10 | 48.0 [42.0, 52.0] | 48.0 [43.0, 51.0] | 46.0 [39.0, 53.8] |
| EXTEM Clotting Time | 62.0 [56.0, 68.0] | 62.0 [55.0, 66.0] | 67.0 [59.0, 74.8] |
| Highest EXTEM CT | 69.0 [64.0, 77.0] | 68.0 [62.0, 74.0] | 74.5 [69.0, 85.0] |
| Univariable | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
| Endpoints | NCryo | Cryoprecipitate | Nplasma | Plasma | Estimate (95% CI) | p | Adjusted Estimate (95% CI) | p |
| Total blood products transfused | 105 | 4 [2, 8] | 65 | 5 [3, 9] | −1 [−2, 1] | 0.253 | - | - |
| Hospital LOS (days) * | 120 | 7.6 [5.5, 10.5] | 69 | 7.4 [5.5, 12.5] | 0.96 (0.80, 1.15) | 0.648 | 0.84 (0.68, 1.04) | 0.109 |
| ICU-LOS (hours) * | 96 | 46.3 [27.8, 95.7] | 49 | 74.4 [36.3, 157] | 0.73 (0.53, 1.00) | 0.048 | 0.72 (0.50, 1.04) | 0.078 |
| Discharge Disposition ** | 120 | 67 | ||||||
| Home | 49 (40.8%) | 22 (32.8%) | ref | - | ref | - | ||
| Home with assistance/long-term facility | 71 (59.2%) | 45 (67.2%) | 0.71 (0.37, 1.32) | 0.281 | 0.41 (0.16, 0.97) | 0.049 | ||
| 1-year Mortality ** | 120 | 69 | ||||||
| Alive | 114 (95.0%) | 59 (85.5%) | ref | - | ref | - | ||
| Dead | 6 (5.0%) | 10 (14.5%) | 0.31 (0.10, 0.88) | 0.031 | 0.49 (0.13, 1.88) | 0.288 | ||
| Univariable | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
| Endpoints | NCryo | Cryoprecipitate | Nplasma | Plasma | Estimate (95% CI) | p | Adjusted Estimate (95% CI) | p |
| Total blood products transfused | 85 | 4 [2, 7] | 34 | 3 [2, 5] | 1 [−0.5, 2] | 0.378 | - | - |
| Hospital LOS (days) * | 100 | 7.6 [5.5, 10.5] | 38 | 6.5 [4.6, 8.3] | 1.09 (0.88, 1.35) | 0.415 | 0.87 (0.64, 1.19) | 0.388 |
| ICU-LOS (hours) * | 80 | 44.9 [26.1, 99.0] | 19 | 68.2 [39.4, 126] | 0.86 (0.56, 1.30) | 0.465 | 0.83 (0.46 1.49) | 0.530 |
| Discharge Disposition ** | 100 | 37 | ||||||
| Home | 39 (39.0%) | 12 (32.4%) | ref | - | ref | - | ||
| Home with assistance/long-term facility | 61 (61.0%) | 25 (67.6%) | 0.75 (0.33, 1.64) | 0.481 | 0.16 (0.03, 0.69) | 0.025 | ||
| 1-year Mortality ** | 100 | 38 | ||||||
| Alive | 94 (94.0%) | 32 (84.2%) | ref | - | ref | - | ||
| Dead | 6 (6.0%) | 6 (15.8%) | 0.34 (0.10, 1.16) | 0.079 | 0.52 (0.10, 3.22) | 0.447 | ||
| Overall (n = 189) | Cryoprecipitate (n = 120) | Plasma (n = 69) | |
|---|---|---|---|
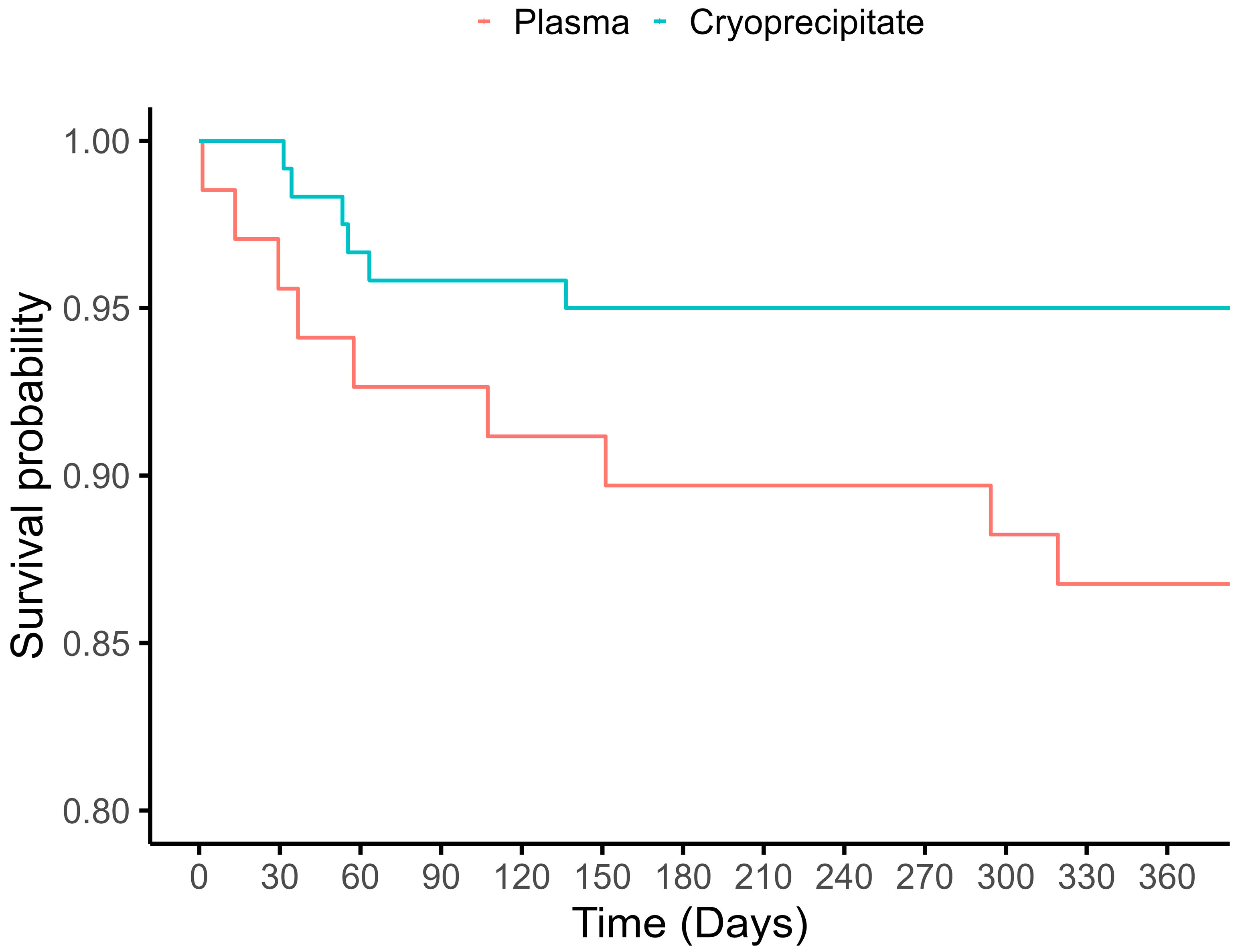
| 1-year mortality | 16 (8.5%) | 6 (5.0%) | 10 (14.5%) |
| Causes of death | |||
| Infection/sepsis | 8 (4.2%) | 3 (2.5%) | 5 (7.2%) |
| Cancer | 5 (2.6%) | 3 (2.5%) | 2 (2.9%) |
| Gastrointestinal bleed | 1 (0.5%) | 0 (0%) | 1 (1.4%) |
| Unknown | 2 (1.1%) | 0 (0%) | 2 (2.9%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Depuru, A.; La, J.O.; Treggiari, M.M.; Guinn, N.R. Early Use of Cryoprecipitate Versus Plasma and Clinical Outcomes in Major Spine Surgery. J. Clin. Med. 2025, 14, 7441. https://doi.org/10.3390/jcm14207441
Depuru A, La JO, Treggiari MM, Guinn NR. Early Use of Cryoprecipitate Versus Plasma and Clinical Outcomes in Major Spine Surgery. Journal of Clinical Medicine. 2025; 14(20):7441. https://doi.org/10.3390/jcm14207441
Chicago/Turabian StyleDepuru, Aparna, Jong Ok La, Miriam M. Treggiari, and Nicole R. Guinn. 2025. "Early Use of Cryoprecipitate Versus Plasma and Clinical Outcomes in Major Spine Surgery" Journal of Clinical Medicine 14, no. 20: 7441. https://doi.org/10.3390/jcm14207441
APA StyleDepuru, A., La, J. O., Treggiari, M. M., & Guinn, N. R. (2025). Early Use of Cryoprecipitate Versus Plasma and Clinical Outcomes in Major Spine Surgery. Journal of Clinical Medicine, 14(20), 7441. https://doi.org/10.3390/jcm14207441





