Incomplete Follow-Up and Competing Risks as Sources of Bias in Vascular Surgical Investigations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Inclusion Criteria
2.2. Clinical Data Assessment
2.3. Ethics
2.4. Statistical Analysis
3. Results
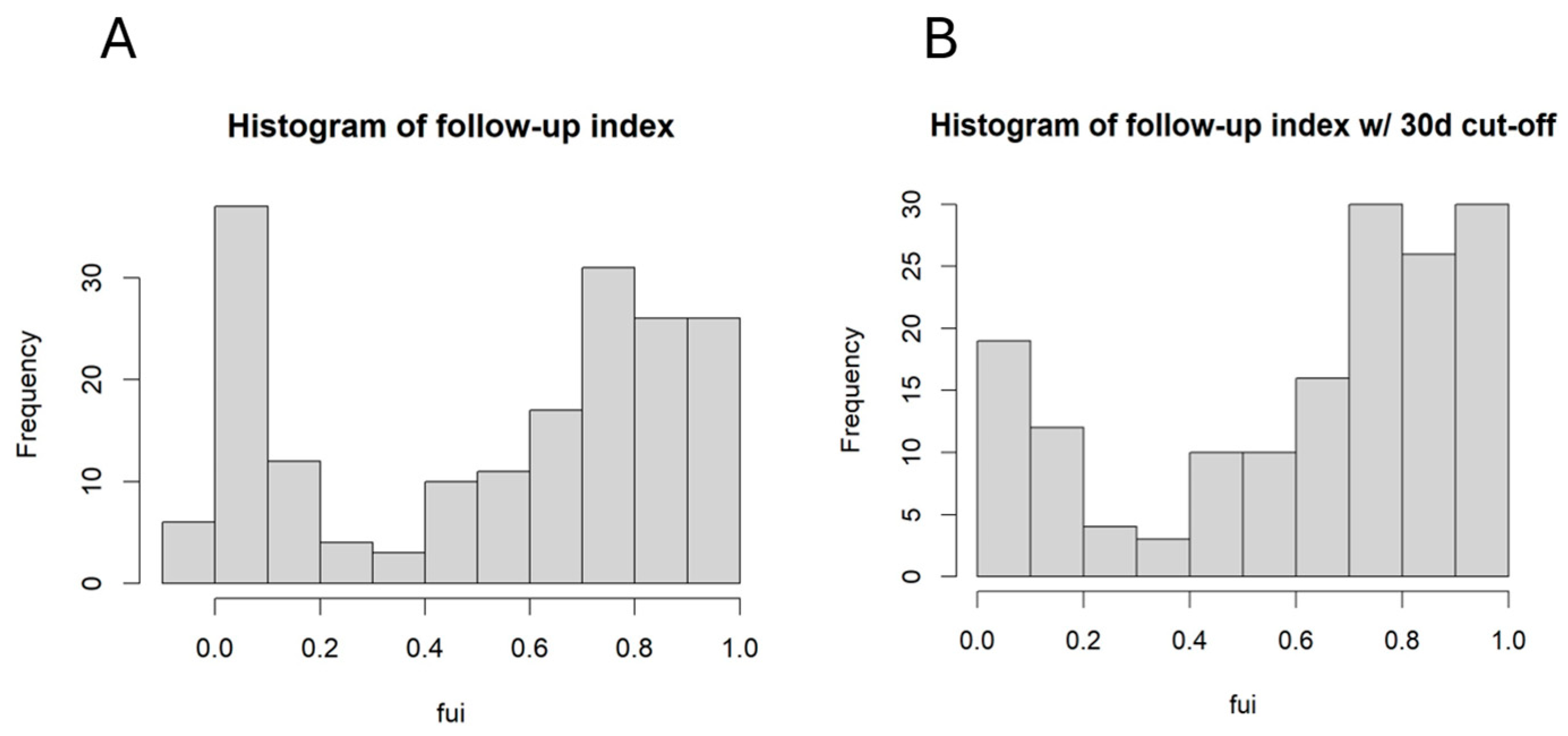
3.1. The Completeness of Follow-Up Depended on the Fontaine Stage
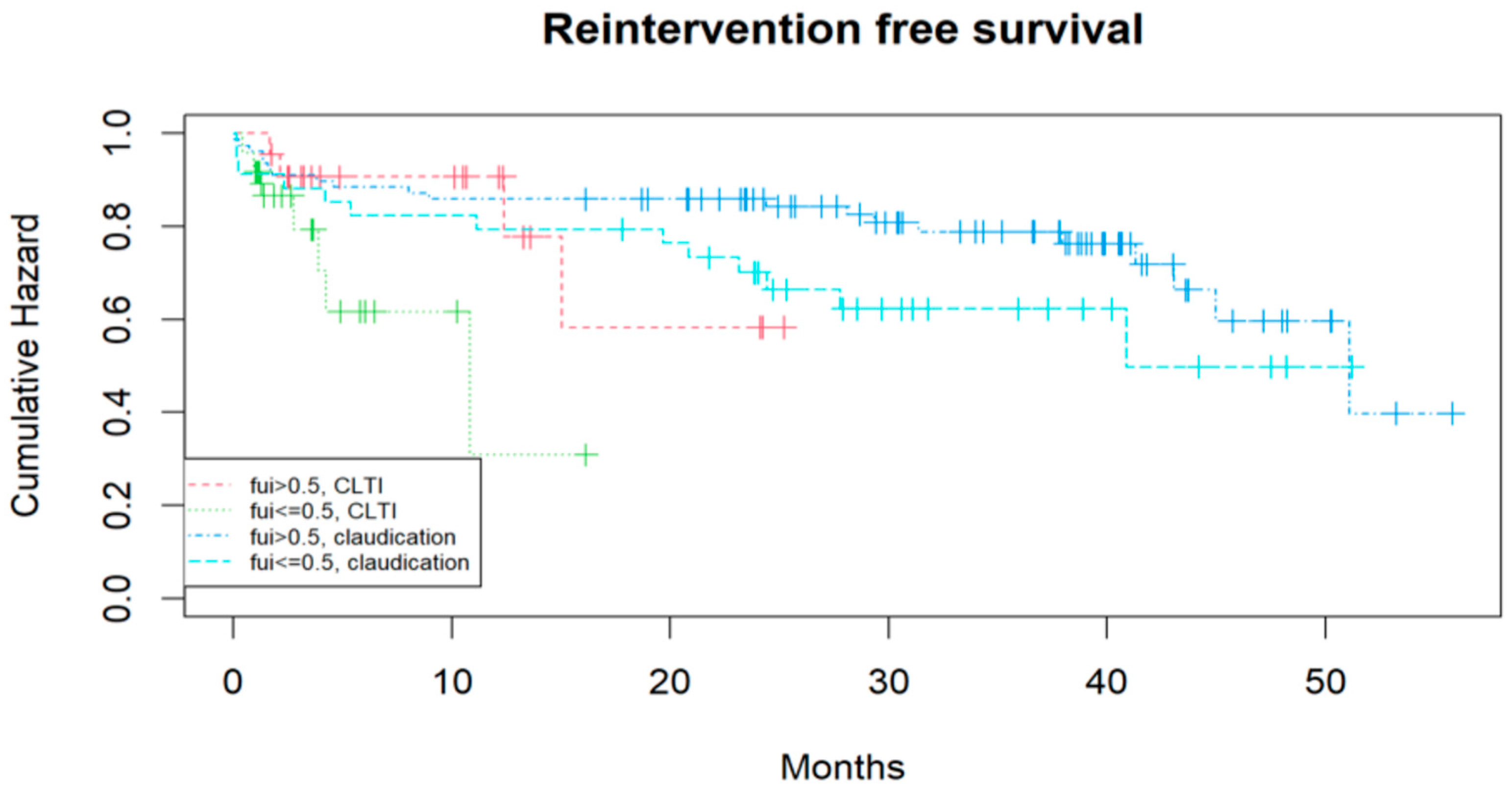
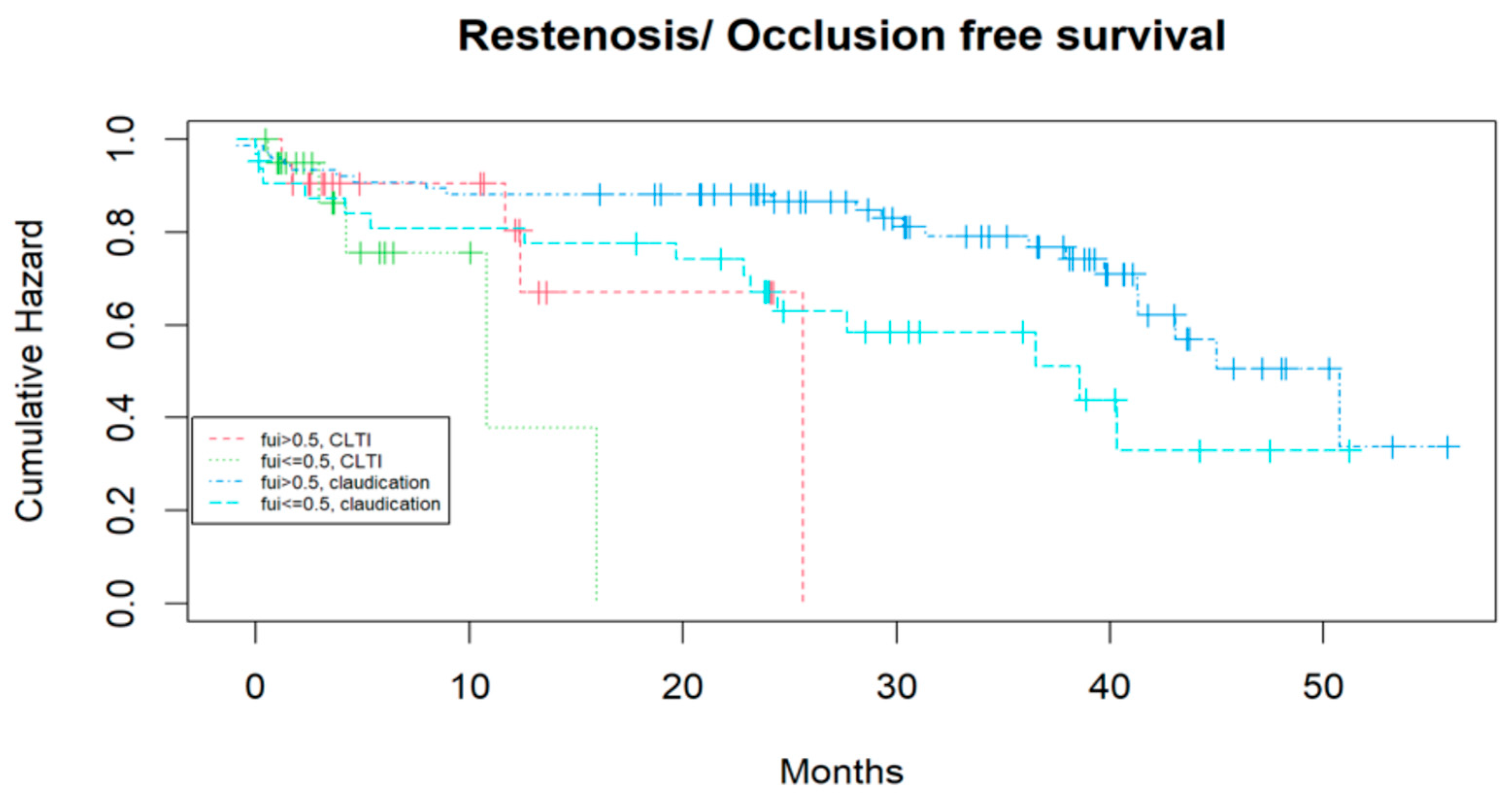
3.2. Identifying Prognostic Factors for the Mid-Term Outcome After Treatment Considering Competing Risks
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CLTI | chronic limb-threatening ischemia |
| RFS | reintervention-free survival |
| FUI | follow-up index |
| PAD | peripheral artery disease |
| MRA | magnetic resonance angiography |
| DSA | digital subtraction angiography |
| KMC | Kaplan–Meier curves |
| IC | intermittent claudication |
| CIHD | chronic ischemic heart disease |
| Coeff | coefficient |
References
- von Allmen, R.S.; Tinner, C.; Schmidli, J.; Tevaearai, H.T.; Dick, F. Randomized controlled comparison of cross-sectional survey approaches to optimize follow-up completeness in clinical studies. PLoS ONE 2019, 14, e0213822. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C.; Fine, J.P. Fine, Accounting for competing risks in randomized controlled trials: A review and recommendations for improvement. Stat. Med. 2017, 36, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Becker, F.; Robert-Ebadi, H.; Ricco, J.-B.; Setacci, C.; Cao, P.; de Donato, G.; Eckstein, H.; De Rango, P.; Diehm, N.; Schmidli, J.; et al. Chapter I: Definitions, epidemiology, clinical presentation and prognosis. Eur. J. Vasc. Endovasc. Surg. 2011, 42 (Suppl. S2), S4–S12. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Eckstein, H.; De Rango, P.; Setacci, C.; Ricco, J.-B.; de Donato, G.; Becker, F.; Robert-Ebadi, H.; Diehm, N.; Schmidli, J.; et al. Chapter II: Diagnostic methods. Eur. J. Vasc. Endovasc. Surg. 2011, 42 (Suppl. S2), S13–S32. [Google Scholar] [CrossRef] [PubMed]
- Dick, F.; Ricco, J.-B.; Davies, A.; Cao, P.; Setacci, C.; de Donato, G.; Becker, F.; Robert-Ebadi, H.; Eckstein, H.; De Rango, P.; et al. Chapter VI: Follow-up after revascularisation. Eur. J. Vasc. Endovasc. Surg. 2011, 42 (Suppl. S2), S75–S90. [Google Scholar] [CrossRef] [PubMed]
- Diehm, N.; Schmidli, J.; Setacci, C.; Ricco, J.-B.; de Donato, G.; Becker, F.; Robert-Ebadi, H.; Cao, P.; Eckstein, H.; De Rango, P.; et al. Chapter III: Management of cardiovascular risk factors and medical therapy. Eur. J. Vasc. Endovasc. Surg. 2011, 42 (Suppl. S2), S33–S42. [Google Scholar] [CrossRef] [PubMed]
- Lepäntalo, M.; Apelqvist, J.; Setacci, C.; Ricco, J.-B.; de Donato, G.; Becker, F.; Robert-Ebadi, H.; Cao, P.; Eckstein, H.; De Rango, P.; et al. Chapter V: Diabetic foot. Eur. J. Vasc. Endovasc. Surg. 2011, 42 (Suppl. S2), S60–S74. [Google Scholar] [CrossRef] [PubMed]
- Setacci, C.; de Donato, G.; Teraa, M.; Moll, F.L.; Ricco, J.-B.; Becker, F.; Robert-Ebadi, H.; Cao, P.; Eckstein, H.-H.; De Rango, P.; et al. Chapter IV: Treatment of critical limb ischaemia. Eur. J. Vasc. Endovasc. Surg. 2011, 42 (Suppl. S2), S43–S59. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. Ann. Intern. Med. 2007, 147, W-163–W-194. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2010. [Google Scholar]
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer: NewYork, NY, USA, 2000; ISBN 0-387-98784-3. [Google Scholar]
- Sakar, D. Lattice: Multivariate Data Visualization with R.; Springer: New York, NY, USA, 2008. [Google Scholar]
- Gray, B. cmprsk: Subdistribution Analysis of Competing Risks, R Package Version 2.2-12. 2024. Available online: https://CRAN.R-project.org/package=cmprsk (accessed on 16 October 2025).
- Austin, P.C.; Steyerberg, E.W.; Putter, H. Fine-Gray subdistribution hazard models to simultaneously estimate the absolute risk of different event types: Cumulative total failure probability may exceed 1. Stat. Med. 2021, 40, 4200–4212. [Google Scholar] [CrossRef] [PubMed]
- Farber, A.; Menard, M.T.; Conte, M.S.; Kaufman, J.A.; Powell, R.J.; Choudhry, N.K.; Hamza, T.H.; Assmann, S.F.; Creager, M.A.; Cziraky, M.J.; et al. Surgery or Endovascular Therapy for Chronic Limb-Threatening Ischemia. N. Engl. J. Med. 2022, 387, 2305–2316. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, A.W.; A Moakes, C.; Popplewell, M.; Meecham, L.; Bate, G.R.; Kelly, L.; Chetter, I.; Diamantopoulos, A.; Ganeshan, A.; Hall, J.; et al. A vein bypass first versus a best endovascular treatment first revascularisation strategy for patients with chronic limb threatening ischaemia who required an infra-popliteal, with or without an additional more proximal infra-inguinal revascularisation pr. Lancet 2023, 401, 1798–1809. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, A.W.; Hall, J.; Moakes, C.A.; Popplewell, M.; Meecham, L.; Bate, G.R.; Kelly, L.; Diamantopoulos, A.; Ganeshan, A.; Houlind, K.; et al. Editor’s Choice—Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL)-2 Trial: Analysis of the Timing and Causes of Death in Participants Randomised to an Infrapopliteal Vein Bypass or Best Endovascular Treatment First Revascularisation Strat. Eur. J. Vasc. Endovasc. Surg. 2025, 69, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Czihal, M.; Malyar, N.; Stausberg, J.; Hoffmann, U. Patient Characteristics in the Recording Courses of Vascular Diseases (Reccord) Registry: Comparison with the Voyager Pad Endovascular Cohort. J. Cardiovasc. Dev. Dis. 2023, 10, 115. [Google Scholar] [CrossRef] [PubMed]


| Co-Factor | All (N = 183) | FUI ≥ 0.5 (N = 112) | FUI < 0.5 (N = 48) | p |
|---|---|---|---|---|
| Age (years) | 69 | 67 | 71 | 0.09 § |
| Sex = female (%) | 33 | 34 | 38 | 0.88 # |
| DM (%) | 43 | 44 | 46 | 0.95 # |
| CIHD (%) | 34 | 33 | 38 | 0.97 # |
| RI w/o dialysis (%) | 14 | 13 | 13 | 0.72 # |
| RI w/ dialysis | 6 | 5 | 8 | 1 # |
| Endovascular (%) | 60 | 65 | 50 | 0.1 # |
| Open surgical (%) | 27 | 26 | 27 | 1 # |
| Emergency surgery (%) | 7 | 8 | 0 | 0.88 # |
| Re-operation during hospital stay | 7.6 | 4.5 | 18.8 | 0.008 # * |
| Bleeding | 10 | 10 | 6 | 0.8# |
| Major amputation within 12 months | 0.8 | 2.7 | 21.4 | 0.03 # * |
| Death within 12 months | 0.8 | 0.9 | 0 | 1 # |
| Co-Factor | Initial Model | Final Model | ||
|---|---|---|---|---|
| Estimate | p | Estimate | p | |
| (Intercept) | 0.78 | <0.001 | 0.79 | <0.001 ** |
| Age | −0.004 | 0.08 | −0.004 | 0.09 |
| Female sex | 0.06 | 0.27 | ||
| Endovascular | 0.17 | 0.005 * | 0.16 | 0.007 ** |
| Open surgery | 0.21 | 0.005 * | 0.20 | 0.007 ** |
| CLTI | −0.12 | 0.02 * | −0.12 | 0.026 * |
| Intra-hospital re-operation | −0.29 | 0.003 * | −0.29 | 0.002 ** |
| Co-Factor | Exp (Coeff) | 2.5% CI | 97.5% CI | p |
|---|---|---|---|---|
| Age | 0.983 | 0.95 | 1.02 | 0.31 |
| FUI | 0.47 | 0.15 | 1.53 | 0.21 |
| CLTI | 2.32 | 1.18 | 4.55 | 0.015 * |
| Intervention | 1.20 | 0.56 | 2.56 | 0.64 |
| Profound femoral artery | 0.78 | 0.25 | 2.49 | 0.68 |
| Common femoral artery | 0.48 | 0.17 | 1.34 | 0.16 |
| Spf. femoral artery | 0.57 | 0.23 | 1.41 | 0.23 |
| Fibular artery | 1.52 | 0.71 | 3.23 | 0.28 |
| Anterior tibial artery | 0.48 | 0.23 | 0.99 | 0.05. |
| Posterior tibial artery | 1.22 | 0.60 | 2.48 | 0.57 |
| Co-Factor | Exp (Coeff) | 2.5% CI | 97.5% CI | p |
|---|---|---|---|---|
| Age | 0.94 | 0.85 | 1.04 | 0.24 |
| FUI | 0.015 | 8.28 × 10−4 | 0.265 | 0.004 ** |
| CLTI | 5.2 × 107 | 8.47 × 106 | 3.21 × 108 | <0.001 ** |
| Intervention | 2.44 | 0.63 | 9.45 | 0.2 |
| Profound femoral artery | 0.15 | 0.008 | 2.57 | 0.19 |
| Common femoral artery | 3.74 × 107 | 2.72 × 106 | 5.14 × 108 | <0.001 ** |
| Spf. femoral artery | 1.12 | 0.27 | 4.67 | 0.88 |
| Fibular artery | 0.19 | 0.24 | 1.46 | 0.11 |
| Anterior tibial artery | 0.75 | 0.16 | 3.57 | 0.72 |
| Posterior tibial artery | 2.16 × 107 | 2.49 × 106 | 1.88 × 108 | <0.001 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udelnow, A.; Smorodin, S.; Sinicin, E.; Tautenhahn, J.; Herold, J.; Barth, U.; Halloul, Z. Incomplete Follow-Up and Competing Risks as Sources of Bias in Vascular Surgical Investigations. J. Clin. Med. 2025, 14, 7419. https://doi.org/10.3390/jcm14207419
Udelnow A, Smorodin S, Sinicin E, Tautenhahn J, Herold J, Barth U, Halloul Z. Incomplete Follow-Up and Competing Risks as Sources of Bias in Vascular Surgical Investigations. Journal of Clinical Medicine. 2025; 14(20):7419. https://doi.org/10.3390/jcm14207419
Chicago/Turabian StyleUdelnow, Andrej, Semion Smorodin, Efim Sinicin, Joerg Tautenhahn, Joerg Herold, Udo Barth, and Zuhir Halloul. 2025. "Incomplete Follow-Up and Competing Risks as Sources of Bias in Vascular Surgical Investigations" Journal of Clinical Medicine 14, no. 20: 7419. https://doi.org/10.3390/jcm14207419
APA StyleUdelnow, A., Smorodin, S., Sinicin, E., Tautenhahn, J., Herold, J., Barth, U., & Halloul, Z. (2025). Incomplete Follow-Up and Competing Risks as Sources of Bias in Vascular Surgical Investigations. Journal of Clinical Medicine, 14(20), 7419. https://doi.org/10.3390/jcm14207419





