Preliminary Experience with Extradural Clinoidectomy and Lamina Terminalis Fenestration in Anterior Communicating Artery Aneurysm Surgery: A Matched Case–Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Data Collection and Outcome Definitions
2.3. Statistical Analysis
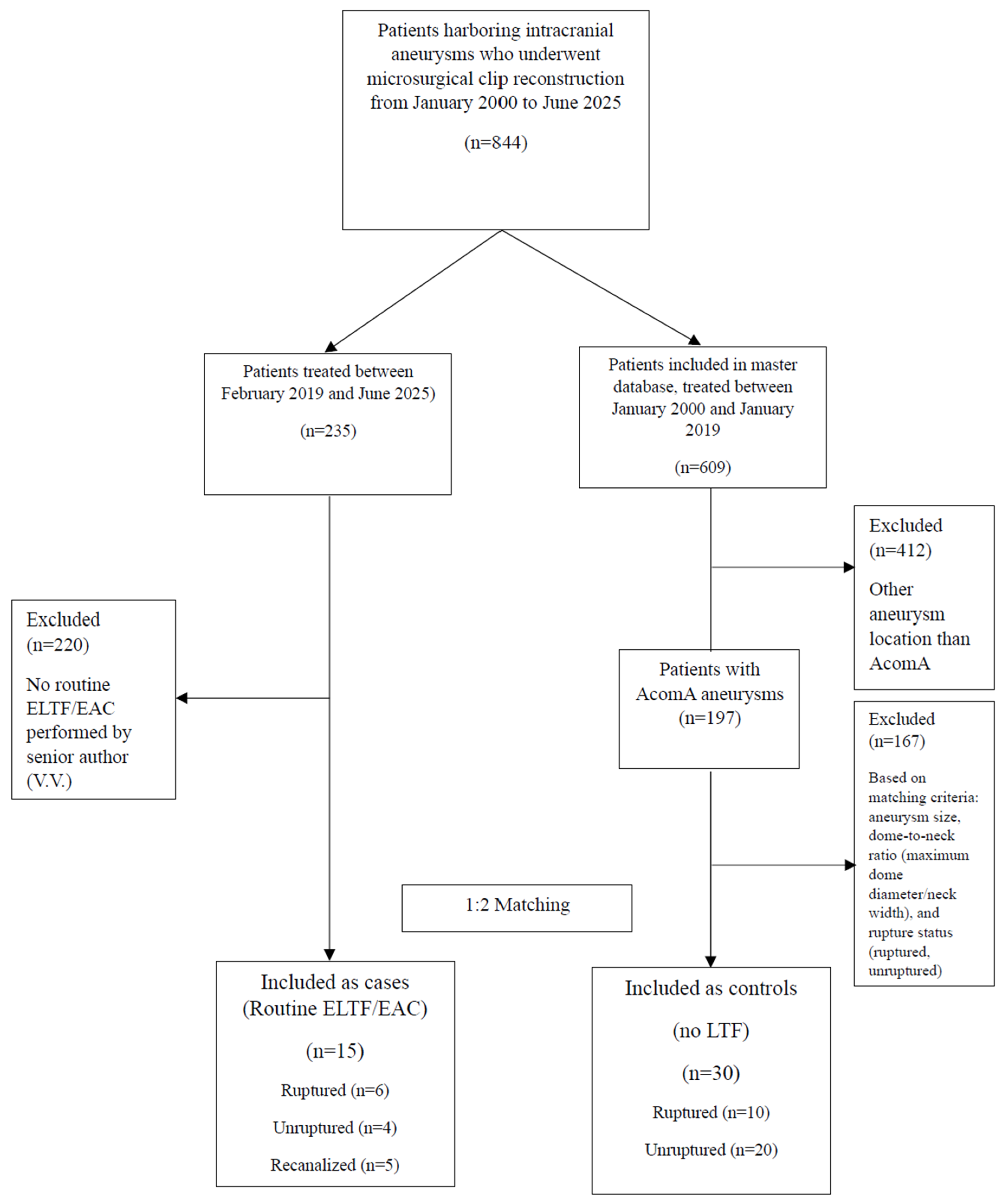
3. Results
3.1. Patient Inclusion
3.2. Baseline Characteristics
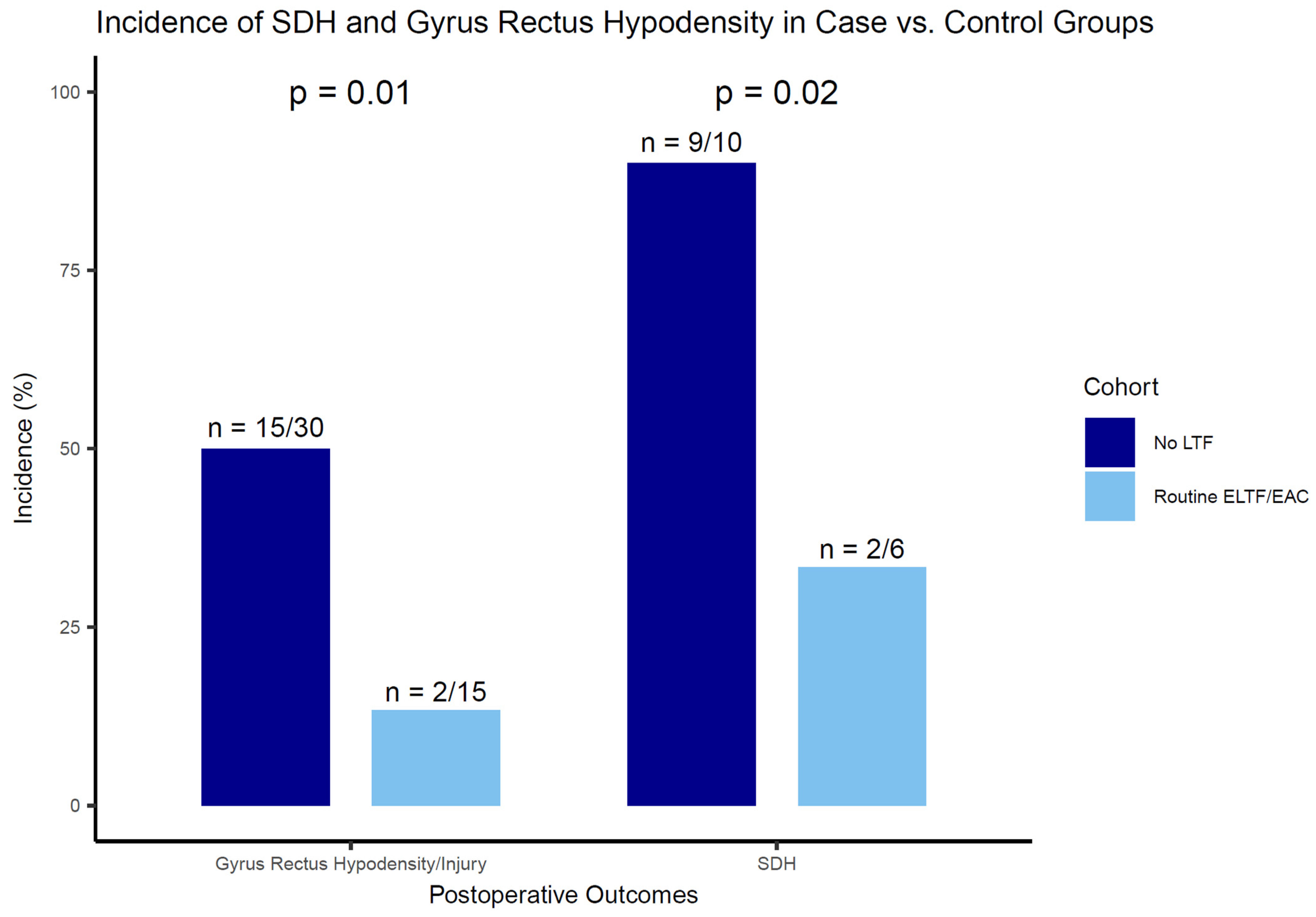
3.3. SDH and Gyrus Rectus Hypodensity Outcomes
3.4. Postoperative and Follow-Up Clinical Outcomes
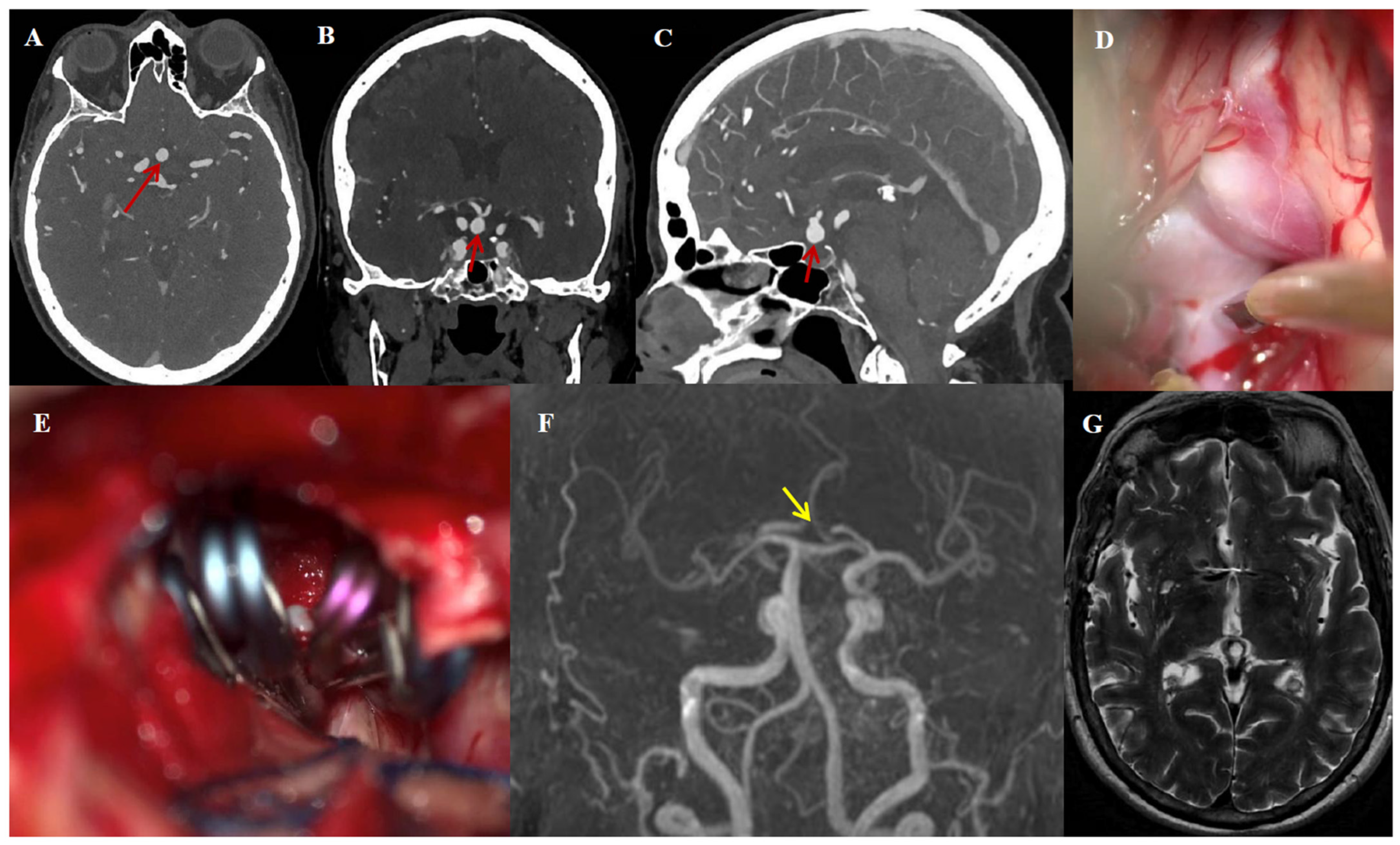
3.5. Illustrative Case #1
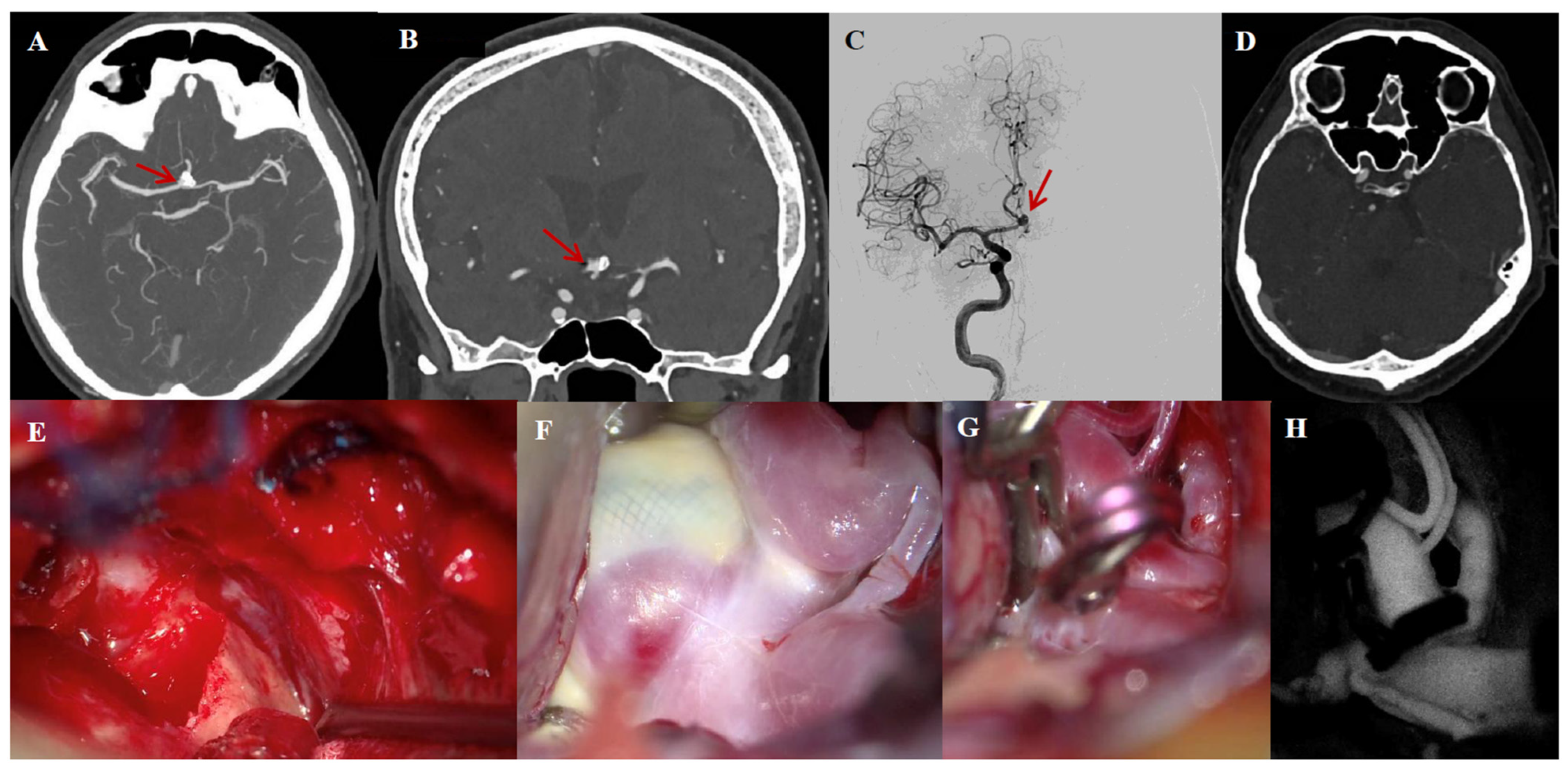
3.6. Illustrative Case #2
4. Discussion
4.1. Key Findings
4.2. Effect of ELTF on Postoperative SDH
4.3. Training, Microvascular Surgical Philosophy, and Skull Base Techniques
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| aSAH | Aneurysmal subarachnoid hemorrhage |
| AcomA | Anterior communicating artery |
| IA | Intracranial aneurysm |
| UIA | Unruptured intracranial aneurysm |
| ISAT | International Subarachnoid Aneurysm Trial |
| EAC | Extradural anterior clinoidectomy |
| ACP | Anterior clinoid process |
| CSF | Cerebrospinal fluid |
| ICA | Internal carotid artery |
| ELTF | Extradural lamina terminalis fenestration |
| LTF | Lamina terminalis fenestration |
| SDH | Shunt-dependent hydrocephalus |
| EVD | External ventricular drainage |
| VP | Ventriculoperitoneal |
| mRS | Modified Rankin score |
| WFNS | World Federation of Neurosurgical Societies |
| ICH | Intracerebral hemorrhage |
| UHR-PC-CTA | Ultra-High-Resolution Photon-Counting Computed Tomography Angiography |
| DCI | Delayed cerebral ischemia |
| NCCT | Non-contrast computed tomography |
| IQR | Interquartile range |
| PHASES | Population, Hypertension, Age, Size, Earlier SAH, and Site |
| TIA | Transient ischemic attack |
| MRA | Magnetic resonance angiography |
| WEB | Woven EndoBridge |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| OR | Odds ratio |
| CI | Confidence interval |
References
- Etminan, N.; Chang, H.S.; Hackenberg, K.; de Rooij, N.K.; Vergouwen, M.D.I.; Rinkel, G.J.E.; Algra, A. Worldwide Incidence of Aneurysmal Subarachnoid Hemorrhage According to Region, Time Period, Blood Pressure, and Smoking Prevalence in the Population: A Systematic Review and Meta-analysis. JAMA Neurol. 2019, 76, 588–597. [Google Scholar] [CrossRef]
- GBD 2021 Global Subarachnoid Hemorrhage Risk Factors Collaborators. Global, Regional, and National Burden of Nontraumatic Subarachnoid Hemorrhage: The Global Burden of Disease Study 2021. JAMA Neurol. 2025, 82, 765. [Google Scholar] [CrossRef]
- Chen, J.; Li, M.; Zhu, X.; Chen, Y.; Zhang, C.; Shi, W.; Chen, Q.; Wang, Y. Anterior Communicating Artery Aneurysms: Anatomical Considerations and Microsurgical Strategies. Front. Neurol. 2020, 11, 1020. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, A.; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of Neurosurgical Clipping versus Endovascular Coiling in 2143 Patients with Ruptured Intracranial Aneurysms: A Randomised Trial. Lancet 2002, 360, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Aboukais, R.; Karnoub, M.A.; Haettel, P.; Bretzner, M.; Bourgeois, P.; Lejeune, J.P. Selective Clipping of Giant Anterior Communicating Artery Aneurysms Remains a Reliable Therapeutic Option. Clin. Neurol. Neurosurg. 2023, 232, 107868. [Google Scholar] [CrossRef]
- Dolenc, V.V. A Combined Epi-and Subdural Direct Approach to Carotid-Ophthalmic Artery Aneurysms. J. Neurosurg. 1985, 62, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, A.; Balasingam, V.; Shiokawa, Y.; McMenomey, S.O.; Delashaw, J.B. Extradural Anterior Clinoidectomy. Technical Note. J. Neurosurg. 2005, 102, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, F.C.; Bustamante, J.L.; Martin, C.; Targa Garcia, A.A.; Feldman, S.E.; Pastor, F.; Orellana, M.C.; Rubino, P.A.; Quilis Quesada, V. Intra-and Extradural Anterior Clinoidectomy: Anatomy Review and Surgical Technique Step by Step. Surg. Radiol. Anat. 2021, 43, 1291–1303. [Google Scholar] [CrossRef]
- Abuzayed, B.; Tanriover, N.; Biceroglu, H.; Yuksel, O.; Tanriover, O.; Albayram, S.; Akar, Z. Pneumatization Degree of the Anterior Clinoid Process: A New Classification. Neurosurg. Rev. 2010, 33, 367–373; discussion 374. [Google Scholar] [CrossRef]
- da Costa, M.D.S.; de Oliveira Santos, B.F.; de Araujo Paz, D.; Rodrigues, T.P.; Abdala, N.; Centeno, R.S.; Cavalheiro, S.; Lawton, M.T.; Chaddad-Neto, F. Anatomical Variations of the Anterior Clinoid Process: A Study of 597 Skull Base Computerized Tomography Scans. Oper. Neurosurg. 2016, 12, 289–297. [Google Scholar] [CrossRef]
- Sadigh, Y.; Talbi, L.; Monchen, J.; Cozar, A.; Gori, K.; Bos, E.M.; Dammers, R.; Volovici, V. Effect of Routine Extradural Optic Canal Decompression Performed by Skull Base Trained Surgeons on Visual Outcomes in Patients with Anterior Skull Base Meningiomas. Acta Neurochir. 2025, 167, 170. [Google Scholar] [CrossRef]
- Krisht, A.F.; Basma, J.; Cai, L. Transcavernous Approach in Vascular Neurosurgery. Neurosurg. Clin. N. Am. 2022, 33, e1–e6. [Google Scholar] [CrossRef]
- Germanwala, A.V.; Huang, J.; Tamargo, R.J. Hydrocephalus after Aneurysmal Subarachnoid Hemorrhage. Neurosurg. Clin. N. Am. 2010, 21, 263–270. [Google Scholar] [CrossRef]
- Mao, J.; Zhu, Q.; Ma, Y.; Lan, Q.; Cheng, Y.; Liu, G. Fenestration of Lamina Terminalis During Anterior Circulation Aneurysm Clipping on Occurrence of Shunt-Dependent Hydrocephalus After Aneurysmal Subarachnoid Hemorrhage: Meta-Analysis. World Neurosurg. 2019, 129, e1–e5. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.Y.; Kim, J.H.; Cheong, J.H.; Bak, K.H.; Kim, C.H.; Yi, H.J.; Kim, K.M. Influence of Lamina Terminalis Fenestration on the Occurrence of the Shunt-Dependent Hydrocephalus in Anterior Communicating Artery Aneurysmal Subarachnoid Hemorrhage. J. Korean Med. Sci. 2006, 21, 113–118. [Google Scholar] [CrossRef]
- Black, P.M. Hydrocephalus and Vasospasm after Subarachnoid Hemorrhage from Ruptured Intracranial Aneurysms. Neurosurgery 1986, 18, 12–16. [Google Scholar] [CrossRef]
- Mohr, G.; Ferguson, G.; Khan, M.; Malloy, D.; Watts, R.; Benoit, B.; Weir, B. Intraventricular Hemorrhage from Ruptured Aneurysm: Retrospective Analysis of 91 Cases. J. Neurosurg. 1983, 58, 482–487. [Google Scholar] [CrossRef]
- Vassilouthis, J.; Richardson, A.E. Ventricular Dilatation and Communicating Hydrocephalus Following Spontaneous Subarachnoid Hemorrhage. J. Neurosurg. 1979, 51, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, S.L.; Ilik, M.K.; Erdi, F.; Ustun, M.E. The Role of Fenestration of the Lamina Terminalis on Symptomatic Vasospasm After Aneurysmal Subarachnoid Hemorrhage: A Clinical Research. Turk. Neurosurg. 2016, 26, 714–719. [Google Scholar] [CrossRef]
- Volovici, V.; Verploegh, I.S.C.; Vos, J.C.; Delwel, E.J.; Bijvoet, H.W.; van Putten, E.H.; Schouten, J.W.; Avezaat, C.J.; Dirven, C.M.; Dammers, R. Can Young Vascular Neurosurgeons Become Proficient in Microsurgical Clip Reconstruction in the Endovascular Era? A Rotterdam Cohort Spanning 2 Decades with Propensity Score Matching for Complexity. World Neurosurg. 2020, 144, e780–e788. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Ivan, M.E.; Safaee, M.M.; Martirosyan, N.L.; Rodríguez-Hernández, A.; Sullinger, B.; Kuruppu, P.; Habdank-Kolaczkowski, J.; Lawton, M.T. Anatomical Triangles Defining Routes to Anterior Communicating Artery Aneurysms: The Junctional and Precommunicating Triangles and the Role of Dome Projection. J. Neurosurg. 2019, 132, 1517–1528. [Google Scholar] [CrossRef]
- Bonita, R.; Beaglehole, R. Recovery of Motor Function after Stroke. Stroke 1988, 19, 1497–1500. [Google Scholar] [CrossRef]
- Sano, H.; Inamasu, J.; Kato, Y.; Satoh, A.; Murayama, Y. Modified World Federation of Neurosurgical Societies Subarachnoid Hemorrhage Grading System. Surg. Neurol. Int. 2016, 7 (Suppl. S18), S502–S503. [Google Scholar] [CrossRef]
- Frontera, J.A.; Claassen, J.; Schmidt, J.M.; Wartenberg, K.E.; Temes, R.; Connolly, E.S.; Macdonald, R.L.; Mayer, S.A. Prediction of Symptomatic Vasospasm after Subarachnoid Hemorrhage: The Modified Fisher Scale. Neurosurgery 2006, 59, 21–27. [Google Scholar] [CrossRef]
- Jansen, I.G.H.; Mulder, M.J.H.L.; Goldhoorn, R.B.; MR CLEAN Registry investigators. Endovascular Treatment for Acute Ischaemic Stroke in Routine Clinical Practice: Prospective, Observational Cohort Study (MR CLEAN Registry). BMJ 2018, 360, k949. [Google Scholar] [CrossRef] [PubMed]
- Kosteljanetz, M. CSF Dynamics in Patients with Subarachnoid and/or Intraventricular Hemorrhage. J. Neurosurg. 1984, 60, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Torvik, A.; Bhatia, R.; Murthy, V.S. Transitory Block of the Arachnoid Granulations Following Subarachnoid Haemorrhage: A Postmortem Study. Acta Neurochir. 1978, 41, 137–146. [Google Scholar] [CrossRef]
- Auer, L.M.; Mokry, M. Disturbed Cerebrospinal Fluid Circulation After Subarachnoid Hemorrhage and Acute Aneurysm Surgery. Neurosurgery 1990, 26, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Gjerris, F.; Boesen, F.; Schmidt, K.; Harmsen, A.; Lester, J.; Børgesen, S.E.; Sørensen, P.S. Resistance to Cerebrospinal Fluid Outflow and Intracranial Pressure in Patients with Hydrocephalus After Subarachnoid Haemorrhage. Acta Neurochir. 1987, 88, 79–86. [Google Scholar] [CrossRef]
- Grant, J.A.; McLone, D.G. Third Ventriculostomy: A Review. Surg. Neurol. 1997, 47, 210–212. [Google Scholar] [CrossRef]
- Joakimsen, O.; Mathiesen, E.B.; Monstad, P.; Selseth, B. CSF Hydrodynamics After Subarachnoid Hemorrhage. Acta Neurol. Scand. 1987, 75, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Volovici, V.; Suarez, J.I. Long-Term Outcomes After Subarachnoid Hemorrhage—The Road to Recovery Is Longer Than Expected. JAMA Netw. Open 2025, 8, e251686. [Google Scholar] [CrossRef] [PubMed]
- Tjoumakaris, S.I.; Hanel, R.; Mocco, J.; Sultan, M.A.A.; Froehler, M.; Lieber, B.B.; Coon, A.; Tateshima, S.; Altschul, D.J.; Narayanan, S.; et al. ARISE I Consensus Review on the Management of Intracranial Aneurysms. Stroke 2024, 55, 1428–1437. [Google Scholar] [CrossRef] [PubMed]
- Volovici, V.; Cenzato, M.; Meling, T.R.; EXPERVASC. The European Expertise Network for Open Microvascular Surgery. Lancet Neurol. 2024, 23, 1187. [Google Scholar] [CrossRef]
- Komotar, R.J.; Hahn, D.K.; Kim, G.H.; Starke, R.M.; Garrett, M.C.; Merkow, M.B.; Otten, M.L.; Sciacca, R.R.; Connolly, E.S., Jr. Efficacy of Lamina Terminalis Fenestration in Reducing Shunt-Dependent Hydrocephalus Following Aneurysmal Subarachnoid Hemorrhage: A Systematic Review. J. Neurosurg. 2009, 111, 147–154. [Google Scholar] [CrossRef]
| No. (%) | All (n = 15) | Ruptured (n = 6) | Unruptured (n = 4) | Recanalized (n = 5) * | Missings (%) | |
|---|---|---|---|---|---|---|
| Age at treatment, median (IQR) | 56 (52–65) | 57 (51–72) | 60 (54–66.5) | 55 (45.5–65.5) | 0 | |
| Sex, female | 8 (53) | 4 (67) | 2 (50) | 2 (40) | 0 | |
| History of hypertension | 9 (60) | 3 (50) | 4 (100) | 2 (40) | 0 | |
| History of smoking | 11 (73) | 2 (33) | 4 (100) | 5 (100) | 0 | |
| Previous aSAH | - | 0 (0) | 0 (0) | 4 (80) | 0 | |
| Other intracranial aneurysms | 5 (33) | 2 (33) | 1 (25) | 2 (40) | 0 | |
| Modified Fisher grade | 1 | - | 2 (33) | - | 1 (20) | 0 |
| 2 | - | 0 (0) | - | 0 (0) | 0 | |
| 3 | - | 3 (50) | - | 0 (0) | 0 | |
| 4 | - | 1 (17) | - | 0 (0) | 0 | |
| WFNS grade | 1 | - | 4 (67) | - | 1 (20) | 0 |
| 2 | - | 0 (0) | - | 0 (0) | 0 | |
| 3 | - | 0 (0) | - | 0 (0) | 0 | |
| 4 | - | 0 (0) | - | 0 (0) | 0 | |
| 5 | - | 2 (33) | - | 0 (0) | 0 | |
| ICH | - | 0 (0) | - | 0 (0) | 0 | |
| Previous treatment | - | - | - | Coiling (n = 4, 80%) WEB (n = 1, 20%) | 0 | |
| Baseline mRS | 0 | 6 (40) | 3 (50) | 2 (50) | 1 (20) | 0 |
| 1 | 6 (40) | 1 (17) | 2 (50) | 3 (60) | ||
| 2 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| 3 | 1 (7) | 0 (0) | 0 (0) | 1 (20) | ||
| 4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| 5 | 2 (13) | 2 (33) | 0 (0) | 0 (0) | ||
| No. (%) | All (n = 15) | Ruptured (n = 6) | Unruptured (n = 4) | Recanalized * (n = 5) | Missings (%) | |
|---|---|---|---|---|---|---|
| AcomA aneurysm projection | Anterior | 5 (33) | 1 (17) | 2 (50) | 2 (40) | 0 |
| Superior | 5 (33) | 3 (50) | 0 (0) | 2 (40) | ||
| Inferior | 5 (33) | 2 (33) | 2 (50) | 1 (20) | ||
| Aneurysm size | Very small (<6 mm) | 7 (47) | 3 (50) | 0 (0) | 4 (80) | 0 |
| Small (6–10 mm) | 5 (33) | 2 (33) | 2 (50) | 1 (20) | ||
| Large (11–25 mm) | 3 (20) | 1 (17) | 2 (50) | 0 (0) | ||
| Dome-to-neck ratio, median (IQR) | 2 (1.4–2.7) | 1.8 (1.4–2.7) | 2.1 (1.5–3.1) | - | 6 (40) | |
| Pneumatized anterior clinoid | 1 (7) | 0 (0) | 0 (0) | 1 (20) | 0 | |
| Clinoid bar | 1 (7) | 1 (17) | 0 (0) | 0 (0) | 0 | |
| Carotico-clinoid foramen | 2 (17) | 0 (0) | 0 (0) | 2 (40) | 0 | |
| ELTF (only in aSAH) | 5 (33) | 5 (83) | - | - | 2 (28) | |
| EAC | 15 (100) | 6 (100) | 4 (100) | 5 (100) | 0 | |
| First-in-line endovascular management attempted | 2 (13) | 2 (33) | 0 (0) | 0 (0) | 0 | |
| Number of temporary clippings, median (IQR) | 3 (2–5) | - | 3 (2–6) | - | 7 (47) | |
| Total duration of temporary clipping, median (min, IQR) | 12 (7–26) | - | 12 (7–35) | - | 8 (53) | |
| No. (%) | All (n = 15) | Ruptured (n = 6) | Unruptured (n = 4) | Recanalized (n = 5) * | Missings (%) | |
|---|---|---|---|---|---|---|
| Postoperative hypoperfusion gyrus rectus in NCCT | 2 (13) | 2 (33) | 0 (0) | 0 (0) | 0 | |
| Postoperative SDH | 2 (13) | 2 (33) | 0 (0) | 0 (0) | 0 | |
| Vasospasm | 4 (27) | 4 (67) | 0 (0) | 0 (0) | 0 | |
| DCI | 2 (13) | 2 (33) | 0 (0) | 0 (0) | 0 | |
| Postoperative ischemia due to vessel occlusion | 1 (7) | 0 (0) | 1 (25) | 0 (0) | 0 | |
| Aneurysm complete occlusion | 15 (100) | 6 (100) | 4 (100) | 5 (100) | 0 | |
| Discharge mRS | 0 | 1 (7) | 0 (0) | 0 (0) | 1 (20) | 0 |
| 1 | 6 (40) | 3 (50) | 1 (25) | 2 (40) | ||
| 2 | 3 (20) | 1 (17) | 2 (50) | 0 (0) | ||
| 3 | 3 (20) | 0 (0) | 1 (25) | 2 (40) | ||
| 4 | 1 (7) | 1 (17) | 0 (0) | 0 (0) | ||
| 5 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| 6 | 1 (7) | 1 (17) | 0 (0) | 0 (0) | ||
| First FU time, median (mo, IQR) | 2 (1–3) | - | 2 (1–2) | 3 (1–4) | 5 (33) | |
| First FU mRS | 0 | 2 (20) | 0 (0) | 1 (33) | 2 (40) | 5 (33) |
| 1 | 4 (40) | 2 (100) | 2 (67) | 1 (20) | ||
| 2 | 2 (20) | 0 (0) | 0 (0) | 0 (0) | ||
| 3 | 2 (20) | 0 (0) | 0 (0) | 2 (40) | ||
| 4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| 5 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Last FU time, median (mo, IQR) | 11 (9–13) | - | - | 10 (7.5–12.5) | 8 (53) | |
| Last FU mRS | 0 | 1 (14) | 0 (0) | 0 (0) | 1 (25) | 8 (53) |
| 1 | 4 (57) | 2 (100) | 1 (100) | 1 (25) | ||
| 2 | 1 (14) | 0 (0) | 0 (0) | 1 (25) | ||
| 3 | 1 (14) | 0 (0) | 0 (0) | 1 (25) | ||
| 4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| 5 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| FU aneurysm recurrence | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Procedure-related morbidity | 1 (10) | 0 (0) | 0 (0) | 1 (20) | 5 (33) | |
| Procedure-related mortality | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadigh, Y.; Schouten, J.W.; van Putten, E.H.P.; Dammers, R.; Volovici, V. Preliminary Experience with Extradural Clinoidectomy and Lamina Terminalis Fenestration in Anterior Communicating Artery Aneurysm Surgery: A Matched Case–Control Study. J. Clin. Med. 2025, 14, 7413. https://doi.org/10.3390/jcm14207413
Sadigh Y, Schouten JW, van Putten EHP, Dammers R, Volovici V. Preliminary Experience with Extradural Clinoidectomy and Lamina Terminalis Fenestration in Anterior Communicating Artery Aneurysm Surgery: A Matched Case–Control Study. Journal of Clinical Medicine. 2025; 14(20):7413. https://doi.org/10.3390/jcm14207413
Chicago/Turabian StyleSadigh, Yasmin, Joost W. Schouten, Erik H. P. van Putten, Ruben Dammers, and Victor Volovici. 2025. "Preliminary Experience with Extradural Clinoidectomy and Lamina Terminalis Fenestration in Anterior Communicating Artery Aneurysm Surgery: A Matched Case–Control Study" Journal of Clinical Medicine 14, no. 20: 7413. https://doi.org/10.3390/jcm14207413
APA StyleSadigh, Y., Schouten, J. W., van Putten, E. H. P., Dammers, R., & Volovici, V. (2025). Preliminary Experience with Extradural Clinoidectomy and Lamina Terminalis Fenestration in Anterior Communicating Artery Aneurysm Surgery: A Matched Case–Control Study. Journal of Clinical Medicine, 14(20), 7413. https://doi.org/10.3390/jcm14207413







