Recurrence of Head and Neck Squamous Cell Carcinoma: Did the COVID-19 Pandemic Have an Impact on Therapeutic Management?
Abstract
1. Introduction
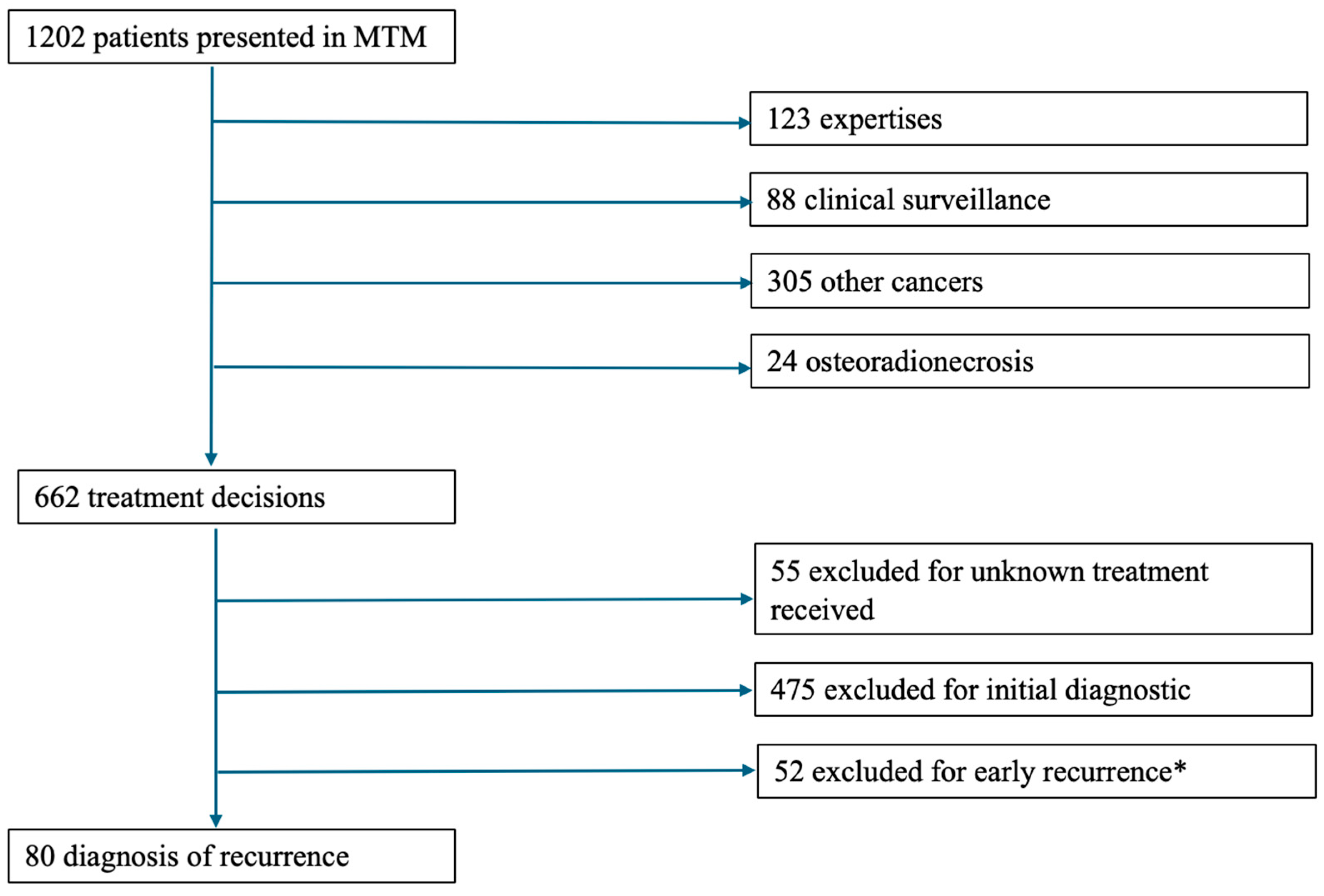
2. Materials and Methods
2.1. Study Design
- -
- Files recorded and discussed in Multidisciplinary Team meetings (MTM) at the University Hospital Centre between 1 January 2019 and 31 December 2021.
- -
- Prior diagnosis of HNSCC of the oral cavity, oropharynx, hypopharynx, or larynx.
- -
- Completion of prior treatment for the aforementioned carcinoma at least 6 months prior to the study period.
- -
- Availability of both planned and executed treatment plans in their records
- -
- First-time diagnosis of HNSCC diagnoses.
- -
- Tumor localization in the salivary glands, sinuses, or nasal cavities.
- -
- Minor patients (under 18 years of age).
- -
- Cutaneous squamous cell carcinoma.
- -
- Histological findings other than squamous cell carcinoma.
- -
- Files presented in MTM solely for expert discussion.
- -
- Unavailable treatment plans.
2.2. Data Collected
- -
- Patients’ specifications and medical history including gender, age, World Health Organization Performance Status (PS) index [29], cirrhosis, diabetes, chronic obstructive pulmonary disease (COPD), history of malnutrition, cardiovascular history, history of other cancers (current or remission), and past and/or current alcohol and tobacco use.
- -
- HNSCC characteristics: Site (oral cavity, oropharynx, hypopharynx, larynx, or cervical lymphadenopathy without an identified primary tumor site), Tumor Node Metastasis (TNM) status at time of diagnosis presented according to the eighth edition of the UICC (Union for International Cancer Control) 2017 TNM classification [30], histopathology, and human papillomavirus (HPV) status.
- -
- Initial Extension Assessment According to the Recommendations of the French Society of Otorhinolaryngology (SFORL) which included: pan endoscopy of the upper aerodigestive tract with the provision of a summary diagram and an operative report; Ear, Nose, and Throat (ENT) MRI; cervical-thoracic (CT) scan; Positron Emission Tomography (PET-CT scan); or another ultrasound/scan-guided biopsy.
- -
- Multidisciplinary team meeting (MTM): These meetings were held weekly and jointly by the University Hospital Center and the affiliated cancer center. Each meeting involved at least 3 medical or surgical specialties from among the following: ENT, maxillofacial or reconstructive surgeon, medical oncologists, radiation oncologists, radiologists, and pathologists. Before each meeting, the patient’s referring physician completed and verified a standardized form. During the meeting, the patient’s case was described, and each aspect of the extension assessment was analyzed. The form included the previous relevant data, attending physicians, and the date. In our center, the patient is not physically present at the MTM. After the meeting, the MTM coordinator summarized collective decision on the treatment protocol and validated it. The document summarizing the discussions and the MTM recommendation was placed in the patient’s electronic medical record. The collective treatment protocol decision was shared with the patient during the consultation conducted by the referring ENT physician after the meeting. During this consultation, the patient provided consent or refusal regarding the proposed treatment.
- -
- Various time intervals were calculated from the patient’s medical record: the diagnostic delay (time between the consultation that suspected recurrence and the MTM finalizing the treatment), and the time to treatment initiation (time between the MTM and the start of treatment).
- -
- History during the remission duration defined by the date of completion of the first treatment and the consultation with a specialist confirming the recurrence: time of remission, prior treatment (surgery, radiotherapy, chemotherapy).
- -
- Treatment center was registered (reference center or other center), and 3-year survival.
2.3. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Treatment Delays
3.3. Treatment Performed
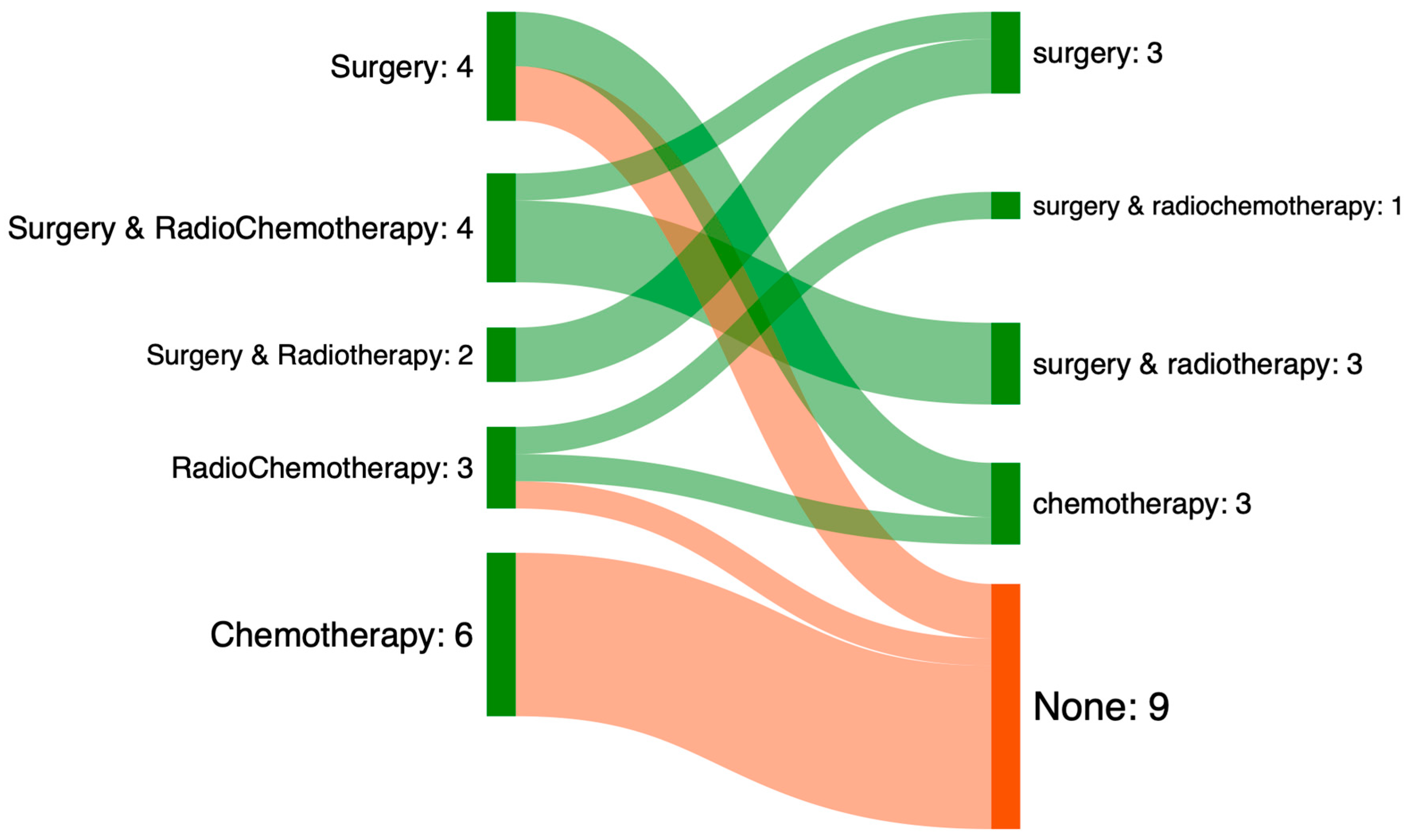
3.4. Mismatch Between Treatment Prescribed and Treatment Performed
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MTM | Multidisciplinary Team Meetings |
| CCF | Cancer Communication Folder |
| CNIL | Commission National Informatique et Liberté |
| PS | Performance Status |
| TNM | Tumor Node Metastasis |
| COPD | Chronic Obstructive Pulmonary Disease |
| UICC | Union International for Cancer Control |
| HPV | Human Papilloma Virus |
| SFORL | French Society of Oto-Rhino-Laryngology |
| ENT | Ear, Nose, and Throat |
| HNSCC | Head and Neck Squamous Cell Carcinoma |
References
- Peng, J.; Raverdy, N.; Ganry, O.; de La Roche-Saint-André, G.; Dubreuil, A.; Lorriaux, A. Descriptive epidemiology of upper aerodigestive tract cancers in the department of Somme. Bull. Cancer 2000, 87, 201–206. [Google Scholar] [PubMed]
- Pignon, J.-P.; le Maître, A.; Maillard, E.; Bourhis, J.; MACH-NC Collaborative. Group Meta-Analysis of Chemotherapy in Head and Neck Cancer (MACH-NC): An Update on 93 Randomised Trials and 17,346 Patients. Radiother. Oncol. 2009, 92, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Posner, M.R.; Hershock, D.M.; Blajman, C.R.; Mickiewicz, E.; Winquist, E.; Gorbounova, V.; Tjulandin, S.; Shin, D.M.; Cullen, K.; Ervin, T.J.; et al. Cisplatin and Fluorouracil Alone or with Docetaxel in Head and Neck Cancer. N. Engl. J. Med. 2007, 357, 1705–1715. [Google Scholar] [CrossRef]
- Brockstein, B.; Haraf, D.J.; Rademaker, A.W.; Kies, M.S.; Stenson, K.M.; Rosen, F.; Mittal, B.B.; Pelzer, H.; Fung, B.B.; Witt, M.-E.; et al. Patterns of Failure, Prognostic Factors and Survival in Locoregionally Advanced Head and Neck Cancer Treated with Concomitant Chemoradiotherapy: A 9-Year, 337-Patient, Multi-Institutional Experience. Ann. Oncol. 2004, 15, 1179–1186. [Google Scholar] [CrossRef]
- Mimeault, M.; Batra, S.K. New Advances on Critical Implications of Tumor- and Metastasis-Initiating Cells in Cancer Progression, Treatment Resistance and Disease Recurrence. Histol. Histopathol. 2010, 25, 1057–1073. [Google Scholar] [CrossRef]
- Salama, J.K.; Vokes, E.E. Concurrent Chemotherapy and Re-Irradiation for Locoregionally Recurrent Head and Neck Cancer. Semin. Oncol. 2008, 35, 251–261. [Google Scholar] [CrossRef]
- Bulbul, M.G.; Genovese, T.J.; Hagan, K.; Rege, S.; Qureshi, A.; Varvares, M.A. Salvage Surgery for Recurrent Squamous Cell Carcinoma of the Head and Neck: Systematic Review and Meta-Analysis. Head Neck 2022, 44, 275–285. [Google Scholar] [CrossRef]
- Benson, R.; Giridhar, P.; Venkatesulu, B.P.; Mallick, S.; Raza, M.W.; Rath, G.K. Re-Irradiation for Head and Neck Squamous Cell Carcinoma. J. Egypt. Natl. Cancer Inst. 2017, 29, 1–9. [Google Scholar] [CrossRef]
- Daste, A.; Larroquette, M.; Gibson, N.; Lasserre, M.; Domblides, C. Immunotherapy for Head and Neck Squamous Cell Carcinoma: Current Status and Perspectives. Immunotherapy 2024, 16, 187–197. [Google Scholar] [CrossRef]
- Chang, J.-H.; Wu, C.-C.; Yuan, K.S.-P.; Wu, A.T.H.; Wu, S.-Y. Locoregionally Recurrent Head and Neck Squamous Cell Carcinoma: Incidence, Survival, Prognostic Factors, and Treatment Outcomes. Oncotarget 2017, 8, 55600–55612. [Google Scholar] [CrossRef]
- Machiels, J.-P.; René Leemans, C.; Golusinski, W.; Grau, C.; Licitra, L.; Gregoire, V.; EHNS Executive Board; ESMO Guidelines Committee; ESTRO Executive Board. Squamous Cell Carcinoma of the Oral Cavity, Larynx, Oropharynx and Hypopharynx: EHNS-ESMO-ESTRO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2020, 31, 1462–1475. [Google Scholar] [CrossRef]
- Spencer, S.A.; Harris, J.; Wheeler, R.H.; Machtay, M.; Schultz, C.; Spanos, W.; Rotman, M.; Meredith, R.; Ang, K.-K. Final Report of RTOG 9610, a Multi-Institutional Trial of Reirradiation and Chemotherapy for Unresectable Recurrent Squamous Cell Carcinoma of the Head and Neck. Head Neck 2008, 30, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Agra, I.M.G.; Carvalho, A.L.; Pontes, E.; Campos, O.D.; Ulbrich, F.S.; Magrin, J.; Kowalski, L.P. Postoperative Complications after En Bloc Salvage Surgery for Head and Neck Cancer. Arch. Otolaryngol. Head Neck Surg. 2003, 129, 1317–1321. [Google Scholar] [CrossRef] [PubMed]
- Saloura, V.; Cohen, E.E.W.; Licitra, L.; Billan, S.; Dinis, J.; Lisby, S.; Gauler, T.C. An Open-Label Single-Arm, Phase II Trial of Zalutumumab, a Human Monoclonal Anti-EGFR Antibody, in Patients with Platinum-Refractory Squamous Cell Carcinoma of the Head and Neck. Cancer Chemother. Pharmacol. 2014, 73, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Solé, G. Mode d’emploi des Réunions de Concertation Pluridisciplinaire (RCP)—Objectifs et principe de fonctionnement. Med. Sci. 2018, 34, 23–25. [Google Scholar] [CrossRef]
- Orgerie, M.-B.; Duchange, N.; Pélicier, N.; Chapet, S.; Dorval, É.; Rosset, P.; Lemarié, É.; Hervé, C.; Moutel, G. La Réunion de Concertation Pluridisciplinaire: Quelle Place Dans La Décision Médicale En Cancérologie? Bull. Cancer 2010, 97, 255–264. [Google Scholar] [CrossRef]
- Hervochon, R.; Atallah, S.; Levivien, S.; Teissier, N.; Baujat, B.; Tankere, F. Impact de l’épidémie de Coronavirus-19 Sur l’activité Chirurgicale En ORL. Ann. Fr. Oto-Rhino-Laryngol. Pathol. Cerv.-Fac. 2020, 137, 248–250. [Google Scholar] [CrossRef]
- Laccourreye, O.; Mirghani, H.; Evrard, D.; Bonnefont, P.; Brugel, L.; Tankere, F.; Coste, A.; Barry, B.; Baujat, B.; Atallah, S.; et al. Impact Du Premier Mois de Confinement Covid 19 Sur l’activité Chirurgicale Carcinologique Des Services Universitaires d’otorhinolaryngologie En Île-de-France. Ann. Fr. Oto-Rhino-Laryngol. Pathol. Cerv.-Fac. 2020, 137, 251–255. [Google Scholar] [CrossRef]
- Potier, A.-L.; Leroy, M.; Mortuaire, G.; Rysman, B.; Morisse, M.; Mouawad, F. Impact of the 2nd, 3rd and 4th Waves of the COVID-19 Pandemic on Wait Times in Head and Neck Cancer: A Retrospective Study in a French Expert Center. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2024, 141, 268–274. [Google Scholar] [CrossRef]
- Fakhry, N.; Schultz, P.; Morinière, S.; Breuskin, I.; Bozec, A.; Vergez, S.; de Garbory, L.; Hartl, D.; Temam, S.; Lescanne, E.; et al. Consensus Français Sur La Pratique de La Chirurgie Oncologique ORL Pendant La Pandémie de COVID-19. Ann. Fr. Oto-Rhino-Laryngol. Pathol. Cerv.-Fac. 2020, 137, 150–151. [Google Scholar] [CrossRef]
- Reliquet, B.; Thibault, T.; Elhomsy, P.; Chbihi, D.; Folia, M.; Guigou, C. Alignment Between Treatment Decision and Treatment Administration for Squamous Cell Carcinoma of the Upper Aerodigestive Tract Before, During, and After the COVID-19 Pandemic: A Retrospective Analysis. J. Clin. Med. 2025, 14, 2613. [Google Scholar] [CrossRef] [PubMed]
- Bortot, L.; Targato, G.; Noto, C.; Giavarra, M.; Palmero, L.; Zara, D.; Bertoli, E.; Dri, A.; Andreetta, C.; Pascoletti, G.; et al. Multidisciplinary Team Meeting Proposal and Final Therapeutic Choice in Early Breast Cancer: Is There an Agreement? Front. Oncol. 2022, 12, 885992. [Google Scholar] [CrossRef]
- Pons-Tostivint, E.; Daubisse-Marliac, L.; Grosclaude, P.; Oum Sack, E.; Goddard, J.; Morel, C.; Dunet, C.; Sibrac, L.; Lagadic, C.; Bauvin, E.; et al. Multidisciplinary Team Meeting and EUSOMA Quality Indicators in Breast Cancer Care: A French Regional Multicenter Study. Breast 2019, 46, 170–177. [Google Scholar] [CrossRef]
- Vinod, S.K.; Wellege, N.T.; Kim, S.; Duggan, K.J.; Ibrahim, M.; Shafiq, J. Translation of Oncology Multidisciplinary Team Meeting (MDM) Recommendations into Clinical Practice. BMC Health Serv. Res. 2021, 21, 461. [Google Scholar] [CrossRef]
- Lein, A.; Liu, D.T.; Haas, M.; Salkic, A.; Ibrisevic, A.; Uscuplic, S.; Harcinovic, A.; Brkic, T.; Thurner, T.; Brkic, F.F. Impact of the COVID-19 Pandemic on Management of Surgically Treated Laryngeal Squamous Cell Carcinoma. Biomol. Biomed. 2024, 24, 188–195. [Google Scholar] [CrossRef]
- Ferrazzo, K.L.; Danesi, C.C.; Martins, N.M.B.; Antoniazzi, R.P. Impact of the COVID-19 Pandemic on the Severity of Newly Diagnosed Cases of Head and Neck Cancer in Southern Brazil. An. Acad. Bras. Cienc. 2024, 96, e20230462. [Google Scholar] [CrossRef]
- Chbihi, D.; Corda, M.; Thibault, T.; Baude, J.; Guigou, C.; Folia, M. Assessment of Professional Practices in the Care Pathway of Patients with Upper Aerodigestive Tract Cancer in a University Hospital. J. Clin. Med. 2024, 13, 6623. [Google Scholar] [CrossRef]
- Premier Vaccin Contre la COVID19 Disponible en France: COMIRNATY, en Pratique. Available online: https://www.vidal.fr/actualites/26442-premier-vaccin-contre-la-covid-19-disponible-en-france-comirnaty-en-pratique.html (accessed on 21 January 2025).
- Chow, R.; Chiu, N.; Bruera, E.; Krishnan, M.; Chiu, L.; Lam, H.; DeAngelis, C.; Pulenzas, N.; Vuong, S.; Chow, E. Inter-Rater Reliability in Performance Status Assessment among Health Care Professionals: A Systematic Review. Ann. Palliat. Med. 2016, 5, 83–92. [Google Scholar] [CrossRef]
- Huang, S.H.; O’Sullivan, B. Overview of the 8th Edition TNM Classification for Head and Neck Cancer. Curr. Treat. Options Oncol. 2017, 18, 40. [Google Scholar] [CrossRef] [PubMed]
- Mickey, R.M.; Greenland, S. The Impact of Confounder Selection Criteria on Effect Estimation. Am. J. Epidemiol. 1989, 129, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Harrel, F.E., Jr. Regression Modelling Strategies with Applications to Linear Models, Logistic Regression, and Survival Analysis; Springer: Berlin/Heidelberg, Germany, 2001; p. 568. ISBN 0-387-95232-2. [Google Scholar]
- Strojan, P.; Corry, J.; Eisbruch, A.; Vermorken, J.B.; Mendenhall, W.M.; Lee, A.W.M.; Haigentz, M.; Beitler, J.J.; de Bree, R.; Takes, R.P.; et al. Recurrent and Second Primary Squamous Cell Carcinoma of the Head and Neck: When and How to Reirradiate. Head Neck 2015, 37, 134–150. [Google Scholar] [CrossRef]
- Williamson, A.; Jashek-Ahmed, F.; Hardman, J.; Paleri, V. Functional and Quality-of-Life Outcomes Following Salvage Surgery for Recurrent Squamous Cell Carcinoma of the Head and Neck: A Systematic Review and Meta-Analysis. Eur. Arch. Otorhinolaryngol. 2023, 280, 4597–4618. [Google Scholar] [CrossRef]
- Hsieh, J.C.-H.; Wang, H.-M.; Wu, M.-H.; Chang, K.-P.; Chang, P.-H.; Liao, C.-T.; Liau, C.-T. Review of Emerging Biomarkers in Head and Neck Squamous Cell Carcinoma in the Era of Immunotherapy and Targeted Therapy. Head Neck 2019, 41 (Suppl. S1), 19–45. [Google Scholar] [CrossRef]
- McDonald, M.W.; Zolali-Meybodi, O.; Lehnert, S.J.; Estabrook, N.C.; Liu, Y.; Cohen-Gadol, A.A.; Moore, M.G. Reirradiation of Recurrent and Second Primary Head and Neck Cancer with Proton Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 808–819. [Google Scholar] [CrossRef]
- Ruhstaller, T.; Roe, H.; Thürlimann, B.; Nicoll, J.J. The Multidisciplinary Meeting: An Indispensable Aid to Communication between Different Specialities. Eur. J. Cancer 2006, 42, 2459–2462. [Google Scholar] [CrossRef] [PubMed]
- Massoubre, J.; Lapeyre, M.; Pastourel, R.; Dupuch, V.; Biau, J.; Dillies, A.-F.; Mom, T.; Pereira, B.; Gilain, L.; Saroul, N. Will the Presence of the Patient at Multidisciplinary Meetings Influence the Decision in Head and Neck Oncology Management? Acta Otolaryngol. 2018, 138, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Boysen, M.; Lövdal, O.; Winther, F.; Tausjö, J. The Value of Follow-up in Patients Treated for Squamous Cell Carcinoma of the Head and Neck. Eur. J. Cancer 1992, 28, 426–430. [Google Scholar] [CrossRef]
- Deneuve, S.; Babin, E.; Lacau-St-Guily, J.; Baujat, B.; Bensadoun, R.-J.; Bozec, A.; Chevalier, D.; Choussy, O.; Cuny, F.; Fakhry, N.; et al. Recommandation de La SFORL (Version Courte) Sur l’organisation Du Parcours de Soins En ORL: Processus de Décision Thérapeutique. Ann. Fr. Oto-Rhino-Laryngol. Pathol. Cerv.-Fac. 2015, 132, 201–203. [Google Scholar] [CrossRef]
- de Visscher, A.V.; Manni, J.J. Routine Long-Term Follow-up in Patients Treated with Curative Intent for Squamous Cell Carcinoma of the Larynx, Pharynx, and Oral Cavity. Does It Make Sense? Arch. Otolaryngol. Head Neck Surg. 1994, 120, 934–939. [Google Scholar] [CrossRef]
- Sun, K.; Tan, J.Y.; Thomson, P.J.; Choi, S.-W. Influence of Time between Surgery and Adjuvant Radiotherapy on Prognosis for Patients with Head and Neck Squamous Cell Carcinoma: A Systematic Review. Head Neck 2023, 45, 2108–2119. [Google Scholar] [CrossRef]
| 2019 N = 32 (100%) | 2020 N = 28 (100%) | 2021 N = 20 (100%) | p-Value 2 | |
|---|---|---|---|---|
| Age (years) 1 | 65 | 68 | 68 | 0.5 |
| Gender (male) | 28 (88) | 23 (82) | 17 (85) | >0.9 |
| Performance Status | 0.056 | |||
| 0 | 9 (28) | 8 (29) | 2 (10) | |
| 1 | 20 (62) | 11 (39) | 11 (55) | |
| 2 | 2 (6) | 5 (18) | 5 (25) | |
| 3 | 3 (10) | 2 (7) | 1 (5) | |
| 4 | 0 | 1 (4) | 1 (5) | |
| Medical history: | ||||
| COPD | 10 (31) | 5 (18) | 1 (5) | 0.068 |
| Hepatopathy | 4 (12) | 1 (4) | 0 (0) | 0.2 |
| Cardiovascular disease | 20 (63) | 14 (50) | 11 (55) | 0.6 |
| Diabetis | 7 (21) | 7 (25) | 6 (30) | 0.8 |
| Undernutrition | 18 (56) | 23 (82) | 12 (60) | 0.090 |
| Toxic habit: | ||||
| Active smoking | 72 (34) | 7 (25) | 5 (25) | 0.8 |
| Pack-Years (PY) 1 | 41 | 43 | 40 | |
| Alcoholism | 5 (16) | 11 (39) | 5 (25) | 0.6 |
| 3 years survival | 11 (32) | 8 (28) | 8 (40) | 0.8 |
| 2019 N = 32 (100%) | 2020 N = 28 (100%) | 2021 N = 20 (100%) | p-Value | |
|---|---|---|---|---|
| Primary Tumor Site | <0.001 | |||
| Oral Cavity | 12 (38) | 23 (43) | 6 (30) | |
| Oropharynx | 5 (16) | 9 (32) | 8 (40) | |
| Larynx | 5 (16) | 6 (21) | 6 (30) | |
| Hypopharynx | 10 (31) | 1 (4) | 0 | |
| HPV 16 positive status | 2 (6) | 1 (4) | 4 (20) | 0.13 |
| Initial staging | ||||
| T1/T2 | 13 (41) | 15 (54) | 11 (55) | 0.5 |
| T3/T4 | 19 (59) | 13 (46) | 9 (45) | |
| N+ | 16 (50) | 2 (7) | 5 (25) | 0.02 |
| M+ | 0 | 1 (4) | 0 | 0.12 |
| First Treatment | 0.003 | |||
| Surgery | 6 (19) | 13 (46) | 2 (10) | |
| RT | 5 (16) | 7 (25) | 3 (15) | |
| Surgery + RT | 1 (3) | 4 (14) | 4 (20) | |
| Surgery + RTCT | 5 (16) | 0 | 5 (25) | |
| RTCT | 15 (47) | 4 (14) | 6 (30) |
| 2019 N = 32 (100%) | 2020 N = 28 (100%) | 2021 N = 20 (100%) | p-Value | |
|---|---|---|---|---|
| Time to recurrence (month) | 22 (9; 46) | 63 (25; 131) | 48 (30; 102) | <0.001 |
| Time between MTM and treatment (days). | 30 (21; 45) | 16 (13; 25) | 24 (17; 27) | 0.002 |
| Circumstances of discovery *: | 0.23 | |||
| Clinical monitoring | 16 (50) | 8 (29) | 5 (25) | |
| Scheduled radiology | 5 (16) | 4 (14) | 2 (10) | |
| Patient symptoms | 11 (34) | 15 (54) | 13 (65) |
| 2019 N = 32 (100%) | 2020 N = 28 (100%) | 2021 N = 20 (100%) | |
|---|---|---|---|
| Surgery | 7 (22) | 9 (32) | 4 (20) |
| Radiotherapy | 1 (3) | 2 (7) | 2 (10) |
| Radiochemoterapy | 2 (6) | 3 (11) | 2 (10) |
| Surgery and adjuvant Radiotherapy | 3 (9) | 7 (25) | 3 (15) |
| Surgery and adjuvant Radiochemotherapy | 8 (25) | 3 (11) | 0 |
| Exclusive Chemoterapy | 9 (28) | 1 (4) | 6 (30) |
| No treatment realised | 2 (6) | 3 (11) | 3 (15) |
| Characteristic | Missmatch MTM N = 19 1 | Match MTM N = 61 1 | p-Value 2 |
|---|---|---|---|
| Age | 66 (60, 71) | 69 (59, 77) | 0.5 |
| Gender (men) | 15 (79%) | 53 (87%) | 0.5 |
| PS ≥ 2 | 7 (37%) | 11 (18%) | 0.12 |
| COPD | 3 (16%) | 13 (21%) | 0.7 |
| Hepatopathy | 1 (5.3%) | 4 (6.6%) | >0.9 |
| Cardiovascular disease | 9 (47%) | 36 (59%) | 0.4 |
| Diabetes | 4 (21%) | 16 (26%) | 0.8 |
| Undernutrition | 15 (79%) | 38 (62%) | 0.3 |
| Previous cancer history | 5 (26%) | 15 (25%) | >0.9 |
| Active smoking | 13 (68%) | 45 (75%) | 0.6 |
| Alcoholism | 9 (47%) | 19 (32%) | 0.3 |
| Discovery of synchronous cancer | 3 (16%) | 4 (6.6%) | 0.3 |
| T > 2 | 11 (58%) | 30 (49%) | 0.6 |
| N+ | 5 (26%) | 15 (25%) | >0.9 |
| M+ | 0 (0%) | 8 (13%) | 0.2 |
| Time between MTM and treatment (days) | 23 (14, 30) | 24 (15, 38) | 0.6 |
| Time between end of first treatment and received diagnosis | 76 (12, 131) | 38 (18, 66) | 0.4 |
| 3 years survival | 3 (17%) | 24 (39%) | 0.094 |
| Year | 0.4 | ||
| 2019 | 6 (32%) | 26 (43%) | |
| 2020 | 6 (32%) | 22 (36%) | |
| 2021 | 7 (37%) | 13 (21%) |
| Characteristic | OR | 95% CI 1 | p-Value |
|---|---|---|---|
| PS ≥ 2 | 1.17 | 0.92, 1.49 | 0.2 |
| Undernutrition | 1.01 | 0.82, 1.26 | >0.9 |
| Alcoholism | 1.15 | 0.94, 1.41 | 0.2 |
| Discovery of synchronous cancer | 1.36 | 0.95, 1.96 | 0.10 |
| T > 2 | 1.05 | 0.87, 1.27 | 0.6 |
| N+ | 1.04 | 0.83, 1.29 | 0.7 |
| M+ | 0.70 | 0.48, 1.01 | 0.060 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reliquet, B.; Thibault, T.; Elhomsy, P.; Chbihi, D.; Folia, M.; Guigou, C. Recurrence of Head and Neck Squamous Cell Carcinoma: Did the COVID-19 Pandemic Have an Impact on Therapeutic Management? J. Clin. Med. 2025, 14, 7406. https://doi.org/10.3390/jcm14207406
Reliquet B, Thibault T, Elhomsy P, Chbihi D, Folia M, Guigou C. Recurrence of Head and Neck Squamous Cell Carcinoma: Did the COVID-19 Pandemic Have an Impact on Therapeutic Management? Journal of Clinical Medicine. 2025; 14(20):7406. https://doi.org/10.3390/jcm14207406
Chicago/Turabian StyleReliquet, Benjamin, Thomas Thibault, Paul Elhomsy, Dounia Chbihi, Mireille Folia, and Caroline Guigou. 2025. "Recurrence of Head and Neck Squamous Cell Carcinoma: Did the COVID-19 Pandemic Have an Impact on Therapeutic Management?" Journal of Clinical Medicine 14, no. 20: 7406. https://doi.org/10.3390/jcm14207406
APA StyleReliquet, B., Thibault, T., Elhomsy, P., Chbihi, D., Folia, M., & Guigou, C. (2025). Recurrence of Head and Neck Squamous Cell Carcinoma: Did the COVID-19 Pandemic Have an Impact on Therapeutic Management? Journal of Clinical Medicine, 14(20), 7406. https://doi.org/10.3390/jcm14207406






