Vaginal Tumor Cell Exfoliation in Cervical and Endometrial Cancer: A Comparative Washing Cytology Study with Implications for Minimally Invasive Surgery
Abstract
1. Introduction
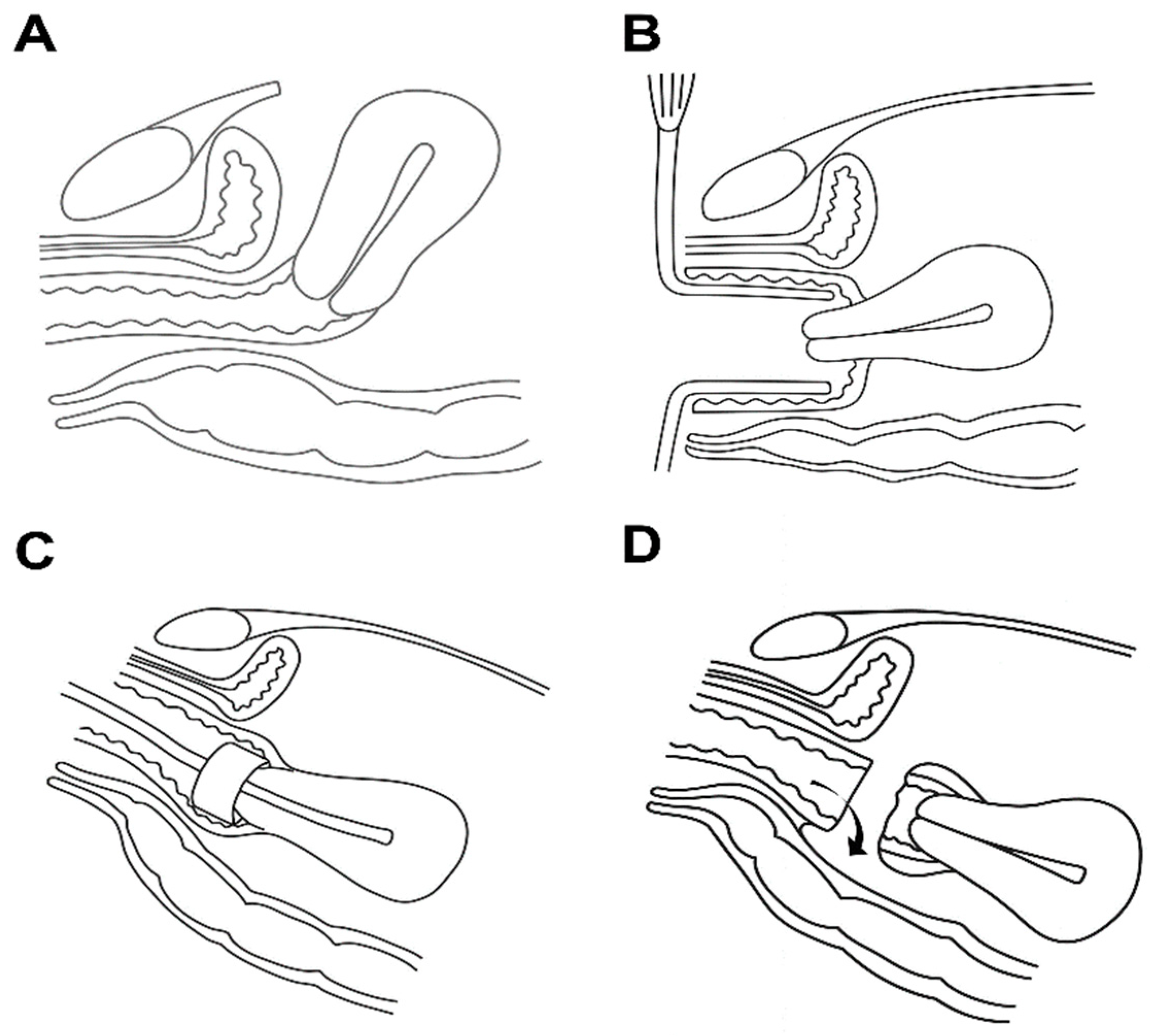
2. Materials and Methods
- Nondiagnostic—insufficient cellularity for interpretation;
- Negative for malignancy—no malignant cells identified, only reactive changes;
- Atypia of undetermined significance (AUS)—equivocal cytologic atypia;
- Suspicious for malignancy (SFM)—strongly suggestive of malignancy but not definitive;
- Malignant—unequivocal malignant cells present.
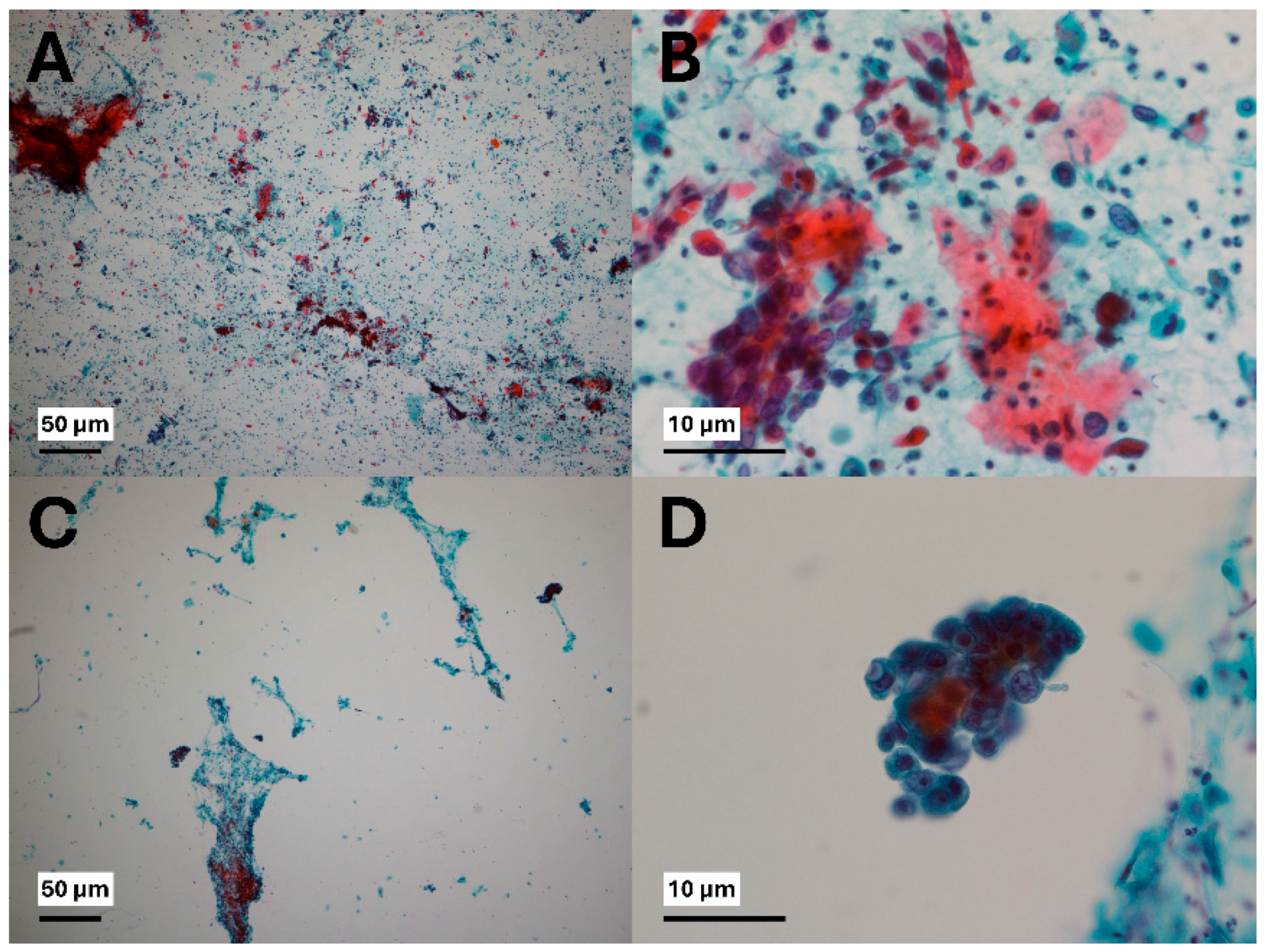
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bosch, F.X.; Manos, M.M.; Munoz, N.; Sherman, M.; Jansen, A.M.; Peto, J.; Schiffman, M.H.; Moreno, V.; Kurman, R.; Shah, K.V. Prevalence of human papillomavirus in cervical cancer: A worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J. Natl. Cancer Inst. 1995, 87, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Viveros-Carreno, D.; Fernandes, A.; Pareja, R. Updates on cervical cancer prevention. Int. J. Gynecol. Cancer 2023, 33, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Kaaks, R.; Lukanova, A.; Kurzer, M.S. Obesity, endogenous hormones, and endometrial cancer risk: A synthetic review. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1531–1543. [Google Scholar]
- Raglan, O.; Kalliala, I.; Markozannes, G.; Cividini, S.; Gunter, M.J.; Nautiyal, J.; Gabra, H.; Paraskevaidis, E.; Martin-Hirsch, P.; Tsilidis, K.K.; et al. Risk factors for endometrial cancer: An umbrella review of the literature. Int. J. Cancer 2019, 145, 1719–1730. [Google Scholar] [CrossRef]
- Janda, M.; Gebski, V.; Forder, P.; Jackson, D.; Williams, G.; Obermair, A.; Committee, L.T. Total laparoscopic versus open surgery for stage 1 endometrial cancer: The LACE randomized controlled trial. Contemp. Clin. Trials 2006, 27, 353–363. [Google Scholar] [CrossRef]
- Janda, M.; Gebski, V.; Davies, L.C.; Forder, P.; Brand, A.; Hogg, R.; Jobling, T.W.; Land, R.; Manolitsas, T.; Nascimento, M.; et al. Effect of Total Laparoscopic Hysterectomy vs. Total Abdominal Hysterectomy on Disease-Free Survival Among Women With Stage I Endometrial Cancer: A Randomized Clinical Trial. JAMA 2017, 317, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L.; Wu, K.Y.; Huang, K.G.; Lee, P.S.; Yen, C.F. Long-term survival outcomes of laparoscopically assisted radical hysterectomy in treating early-stage cervical cancer. Am. J. Obstet. Gynecol. 2010, 203, 165.e1–165.e7. [Google Scholar] [CrossRef]
- Nam, J.H.; Park, J.Y.; Kim, D.Y.; Kim, J.H.; Kim, Y.M.; Kim, Y.T. Laparoscopic versus open radical hysterectomy in early-stage cervical cancer: Long-term survival outcomes in a matched cohort study. Ann. Oncol. 2012, 23, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, R.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef]
- Hopkins, M.P.; Dulai, R.M.; Occhino, A.; Holda, S. The effects of carbon dioxide pneumoperitoneum on seeding of tumor in port sites in a rat model. Am. J. Obstet. Gynecol. 1999, 181, 1329–1333; discussion 1333–1334. [Google Scholar] [CrossRef]
- Kong, T.W.; Lee, J.; Yum, S.H.; Kim, J.; Son, J.H.; Chang, S.J.; Ryu, H.S. Spillage and displacement of indocyanine green-stained tissues from uterine cervix to pelvic peritoneum: A proof of concept study for colpotomy approach in minimally invasive surgery. Taiwan J. Obstet. Gynecol. 2023, 62, 119–122. [Google Scholar] [CrossRef]
- Sadeghipour, A.; Babaheidarian, P. Making Formalin-Fixed, Paraffin Embedded Blocks. Methods Mol. Biol. 2019, 1897, 253–268. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Ren, W.H.; Wang, Q.; Jin, H.Z.; Guo, Y.Y.; Lin, D.M. A retrospective analysis of serous effusions based on the newly proposed international system for reporting serous fluid cytopathology: A report of 3633 cases in an oncological center. Diagn. Pathol. 2022, 17, 56. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chandra, A.; Cai, G. The International System for Reporting Serous Fluid Cytopathology-An Updated Review. J. Clin. Transl. Pathol. 2023, 3, 160–177. [Google Scholar] [CrossRef]
- Castilho da Silva, D.J.; Dos Santos, C.R.; Xavier-Junior, J.C.C. Risk of Malignancy in Effusions according to the International System for Serous Fluid Cytopathology: A Review. Acta Cytol. 2024, 68, 384–393. [Google Scholar] [CrossRef]
- Ramirez, P.T.; Robledo, K.P.; Frumovitz, M.; Pareja, R.; Ribeiro, R.; Lopez, A.; Yan, X.; Isla, D.; Moretti, R.; Bernardini, M.Q.; et al. LACC Trial: Final Analysis on Overall Survival Comparing Open Versus Minimally Invasive Radical Hysterectomy for Early-Stage Cervical Cancer. J. Clin. Oncol. 2024, 42, 2741–2746. [Google Scholar] [CrossRef]
- Walker, J.L.; Piedmonte, M.R.; Spirtos, N.M.; Eisenkop, S.M.; Schlaerth, J.B.; Mannel, R.S.; Barakat, R.; Pearl, M.L.; Sharma, S.K. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J. Clin. Oncol. 2012, 30, 695–700. [Google Scholar] [CrossRef]
- Fusegi, A.; Kanao, H.; Tsumura, S.; Murakami, A.; Abe, A.; Aoki, Y.; Nomura, H. Minimally invasive radical hysterectomy and the importance of avoiding cancer cell spillage for early-stage cervical cancer: A narrative review. J. Gynecol. Oncol. 2023, 34, e5. [Google Scholar] [CrossRef] [PubMed]
- Touhami, O.; Plante, M. Minimally Invasive Surgery for Cervical Cancer in Light of the LACC Trial: What Have We Learned? Curr. Oncol. 2022, 29, 1093–1106. [Google Scholar] [CrossRef] [PubMed]
- Nica, A.; Kim, S.R.; Gien, L.T.; Covens, A.; Bernardini, M.Q.; Bouchard-Fortier, G.; Kupets, R.; May, T.; Vicus, D.; Laframboise, S.; et al. Survival after minimally invasive surgery in early cervical cancer: Is the intra-uterine manipulator to blame? Int. J. Gynecol. Cancer 2020, 30, 1864–1870. [Google Scholar] [CrossRef]
- Padilla-Iserte, P.; Lago, V.; Tauste, C.; Diaz-Feijoo, B.; Gil-Moreno, A.; Oliver, R.; Coronado, P.; Martin-Salamanca, M.B.; Pantoja-Garrido, M.; Marcos-Sanmartin, J.; et al. Impact of uterine manipulator on oncological outcome in endometrial cancer surgery. Am. J. Obstet. Gynecol. 2021, 224, 65.e1–65.e11. [Google Scholar] [CrossRef]
- Zorzato, P.C.; Uccella, S.; Biancotto, G.; Bosco, M.; Festi, A.; Franchi, M.; Garzon, S. Intrauterine manipulator during hysterectomy for endometrial cancer: A systematic review and meta-analysis of oncologic outcomes. Am. J. Obstet. Gynecol. 2024, 230, 185–198.e184. [Google Scholar] [CrossRef] [PubMed]
- Volz, J.; Koster, S.; Spacek, Z.; Paweletz, N. The influence of pneumoperitoneum used in laparoscopic surgery on an intraabdominal tumor growth. Cancer 1999, 86, 770–774. [Google Scholar] [CrossRef]
- Kanao, H.; Aoki, Y.; Takeshima, N. Unexpected result of minimally invasive surgery for cervical cancer. J. Gynecol. Oncol. 2018, 29, e73. [Google Scholar] [CrossRef] [PubMed]
- Kong, T.W.; Chang, S.J.; Piao, X.; Paek, J.; Lee, Y.; Lee, E.J.; Chun, M.; Ryu, H.S. Patterns of recurrence and survival after abdominal versus laparoscopic/robotic radical hysterectomy in patients with early cervical cancer. J. Obstet. Gynaecol. Res. 2016, 42, 77–86. [Google Scholar] [CrossRef]
- Kim, J.M.; Chong, G.O.; Park, N.J.; Choi, Y.E.; Lee, J.; Lee, Y.H.; Hong, D.G.; Park, J.Y. Survival Impact of Residual Cancer Cells in Intraoperative Peritoneal Washes following Radical Hysterectomy for Cervical Cancer. J. Clin. Med. 2022, 11, 2659. [Google Scholar] [CrossRef]
- Klapdor, R.; Hertel, H.; Hillemanns, P.; Rottger, M.; Soergel, P.; Kuehnle, E.; Jentschke, M. Peritoneal contamination with ICG-stained cervical secretion as surrogate for potential cervical cancer tumor cell dissemination: A proof-of-principle study for laparoscopic hysterectomy. Acta Obstet. Gynecol. Scand. 2019, 98, 1398–1403. [Google Scholar] [CrossRef]
- Kong, T.W.; Son, J.H.; Paek, J.; Chang, S.J.; Ryu, H.S. Selection criteria and colpotomic approach for safe minimally invasive radical hysterectomy in early-stage cervical cancer. J. Gynecol. Oncol. 2020, 31, e7. [Google Scholar] [CrossRef] [PubMed]
- Deura, I.; Kanamori, R.; Nagasawa, Y.; Kuji, S.; Ohara, T.; Tozawa, A.; Shimada, M.; Suzuki, N. A simple technique of vaginal cuff closure to prevent tumor cell spillage in laparoscopic radical hysterectomy for uterine cervical cancer. Asian J. Endosc. Surg. 2021, 14, 665–668. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Li, G.; Li, L.; Geng, P.; Song, H. Vaginal Stump Ligation for Cervical Cancer. Iran. J. Public. Health 2017, 46, 1332–1337. [Google Scholar]
- Yuan, P.; Liu, Z.; Qi, J.; Yang, X.; Hu, T.; Tan, H. Laparoscopic Radical Hysterectomy with Enclosed Colpotomy and without the Use of Uterine Manipulator for Early-Stage Cervical Cancer. J. Minim. Invasive Gynecol. 2019, 26, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Fusegi, A.; Kanao, H.; Ishizuka, N.; Nomura, H.; Tanaka, Y.; Omi, M.; Aoki, Y.; Kurita, T.; Yunokawa, M.; Omatsu, K.; et al. Oncologic Outcomes of Laparoscopic Radical Hysterectomy Using the No-Look No-Touch Technique for Early Stage Cervical Cancer: A Propensity Score-Adjusted Analysis. Cancers 2021, 13, 6097. [Google Scholar] [CrossRef] [PubMed]
- Kohler, C.; Hertel, H.; Herrmann, J.; Marnitz, S.; Mallmann, P.; Favero, G.; Plaikner, A.; Martus, P.; Gajda, M.; Schneider, A. Laparoscopic radical hysterectomy with transvaginal closure of vaginal cuff—A multicenter analysis. Int. J. Gynecol. Cancer 2019, 29, 845–850. [Google Scholar] [CrossRef]
- Feghali, E.J.; Lagana, A.S.; Daccache, A.; Bitar, R.; Garzon, S.; Uccella, S.; Petousis, S.; Sleiman, Z. Endobag use in laparoscopic gynecological surgeries: A systematic review. Minim. Invasive Ther. Allied Technol. 2022, 31, 698–703. [Google Scholar] [CrossRef] [PubMed]
| Variables | Cervical Cancer (n = 36) | Endometrial Cancer (n = 46) | ||
|---|---|---|---|---|
| Age (years) (Mean ± SD) | 55.4 ± 13.9 | 55.7 ± 11.8 | ||
| BMI (Mean ± SD) | 23.3 ± 3.9 | 25.1 ± 4.5 | ||
| Parity (number) | Nullipara | 6 | 9 | |
| Primipara | 10 | 4 | ||
| Multipara | 20 | 33 | ||
| Stage (number) (Cervical cancer 2018 FIGO stage Endometrial cancer 2009 FIGO stage) | I | 15 | 35 | |
| II | 10 | 3 | ||
| III | 9 | 6 | ||
| IV | 2 | 2 | ||
| Metastasis (number) | Pelvic LN | 8 | 8 | |
| Para-aortic LN | 4 | 6 | ||
| Distant | 1 | 2 | ||
| Histology (number) | SCC | 26 | Endometrioid | 38 |
| Adenoca | 8 | Serous | 5 | |
| Others | 2 | Carcinosarcoma | 3 | |
| Grade (number) | Well | 3 | Grade 1 | 14 |
| Moderate | 31 | Grade 2 | 19 | |
| Poor | 2 | Grade 3 | 13 | |
| Treatment (number) | Surgery | 16 | Surgery | 26 |
| Surgery + adjuvant CCRT | 1 | Surgery + RT | 9 | |
| CCRT | 18 | Surgery + CTx | 9 | |
| Surgery + CTx | 1 | Surgery + CTx + RTx | 2 | |
| Tumor marker (Mean ± SD) | SCC Ag(ng/mL) | 9.5 ± 17.1 | CA 19-9(U/mL) | 21.2 ± 37.7 |
| CA 125(U/mL) | 17.7 ± 12.2. | CA 125(U/mL) | 61.2 ± 17.6 | |
| Surgery type (number) | MIS | 8 | 25 | |
| Open | 10 | 21 | ||
| Result | Cervical Cancer | Endometrial Cancer | p-Value | |
|---|---|---|---|---|
| All stage Cervical cancer (n = 36) Endometrial cancer (n = 46) | Negative | 24 | 43 | 0.002 |
| Positive | 12 | 3 | ||
| Early stage (stage I or II) Cervical cancer (n = 25) Endometrial cancer (n = 38) | Negative | 19 | 38 | 0.003 * |
| Positive | 6 | 0 |
| Value | OR | 95% CI | p-Value |
|---|---|---|---|
| Age (years) <50 vs. ≥50 | 0.21 | 0.04–1.20 | 0.078 |
| Cancer type Cervical cancer vs. Endometrial cancer | 14.24 | 1.83–110.89 | 0.011 |
| Stage Stage III or IV vs. stage I or II | 9.53 | 2.08–43.61 | 0.004 |
| Grade Poor vs. Well or moderate | 0.21 | 0.06–5.30 | 0.597 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, J.M.; Choi, Y.S.; Lee, S.-J.; Jeong, Y.Y. Vaginal Tumor Cell Exfoliation in Cervical and Endometrial Cancer: A Comparative Washing Cytology Study with Implications for Minimally Invasive Surgery. J. Clin. Med. 2025, 14, 7383. https://doi.org/10.3390/jcm14207383
Ryu JM, Choi YS, Lee S-J, Jeong YY. Vaginal Tumor Cell Exfoliation in Cervical and Endometrial Cancer: A Comparative Washing Cytology Study with Implications for Minimally Invasive Surgery. Journal of Clinical Medicine. 2025; 14(20):7383. https://doi.org/10.3390/jcm14207383
Chicago/Turabian StyleRyu, Jung Min, Youn Seok Choi, Sun-Jae Lee, and Yoon Young Jeong. 2025. "Vaginal Tumor Cell Exfoliation in Cervical and Endometrial Cancer: A Comparative Washing Cytology Study with Implications for Minimally Invasive Surgery" Journal of Clinical Medicine 14, no. 20: 7383. https://doi.org/10.3390/jcm14207383
APA StyleRyu, J. M., Choi, Y. S., Lee, S.-J., & Jeong, Y. Y. (2025). Vaginal Tumor Cell Exfoliation in Cervical and Endometrial Cancer: A Comparative Washing Cytology Study with Implications for Minimally Invasive Surgery. Journal of Clinical Medicine, 14(20), 7383. https://doi.org/10.3390/jcm14207383






