Diagnostic Value of NO-Related Biomarkers (ADMA, NO, eNOS) in Stable COPD and Acute Exacerbation of COPD
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Study Design and Population
2.3. Definition of AECOPD
2.4. Definition of Stable COPD
2.5. Inclusion Criteria
2.6. Exclusion Criteria
2.7. Clinical and Demographic Assessment
2.8. Pulmonary Function Tests
2.9. Assessment of Blood Tests
2.10. Statistical Analysis
3. Results
3.1. Characteristics of the Study Populations
3.2. The Comparison of Demographic and Clinical Findings
3.3. The Comparison of Laboratory Findings, Serum ADMA, NO, and eNOS Levels
3.4. Correlation Analysis Between the Parameters
3.5. The Comparisons by Gender and Exacerbation Frequency
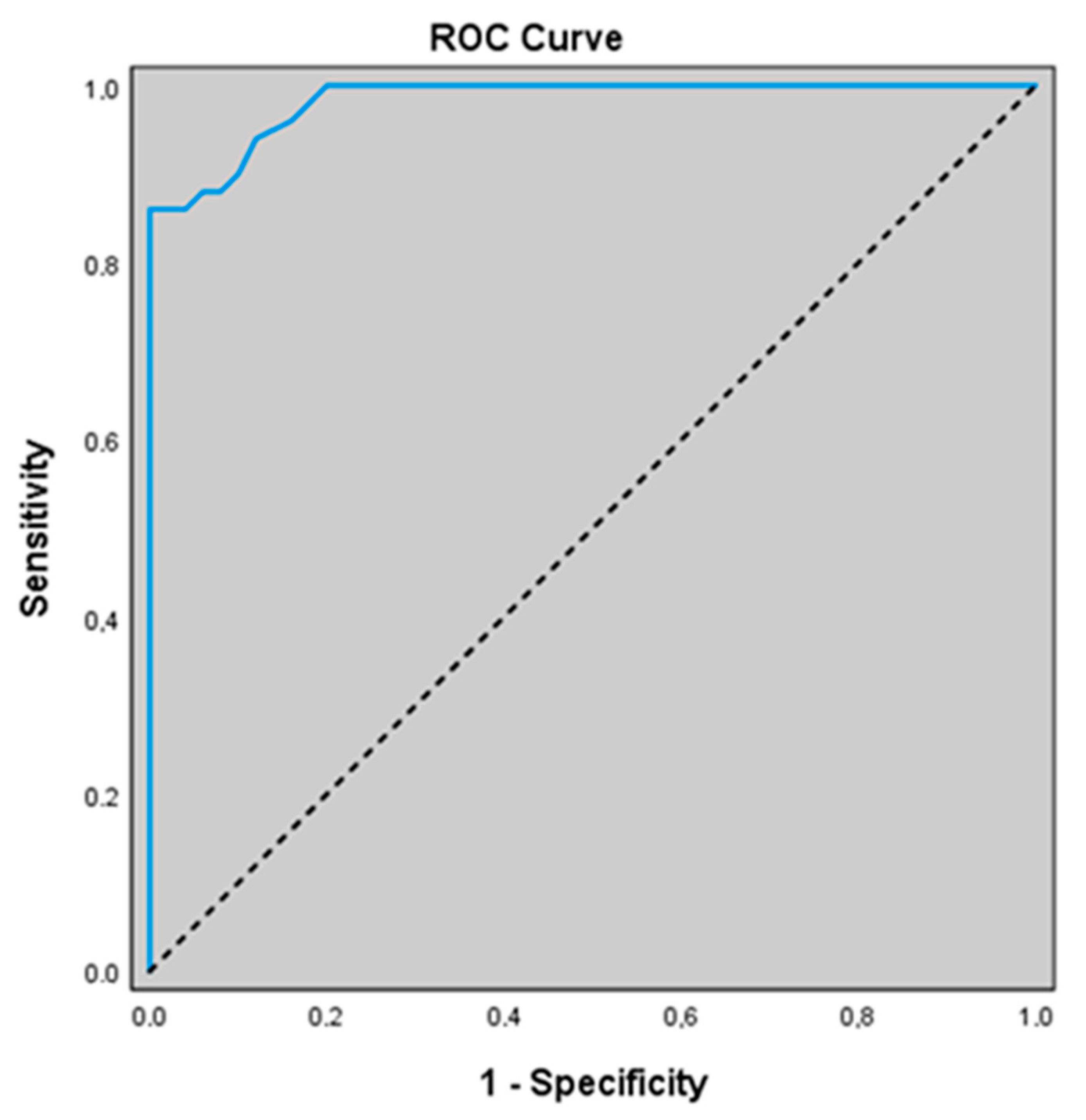
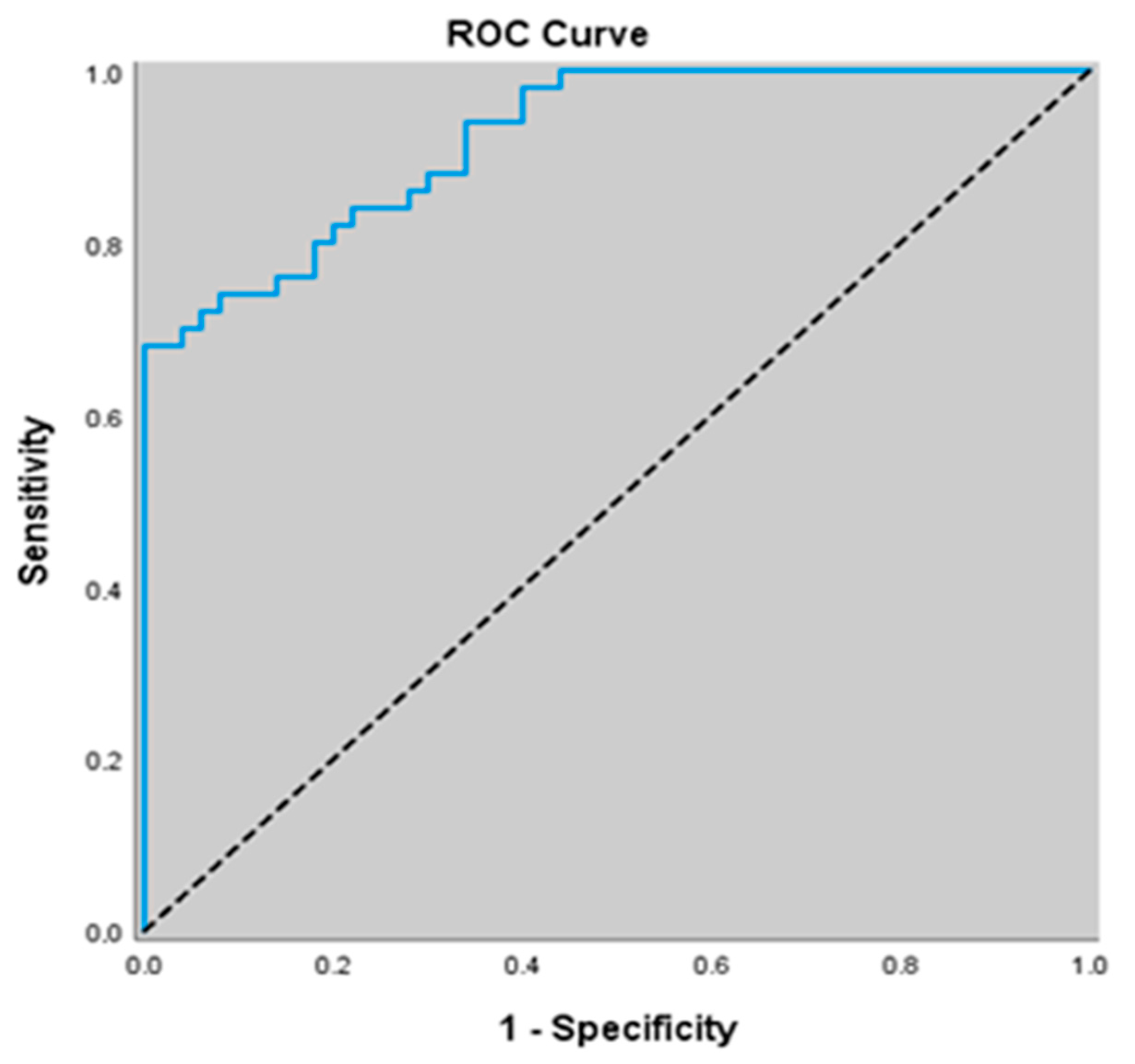
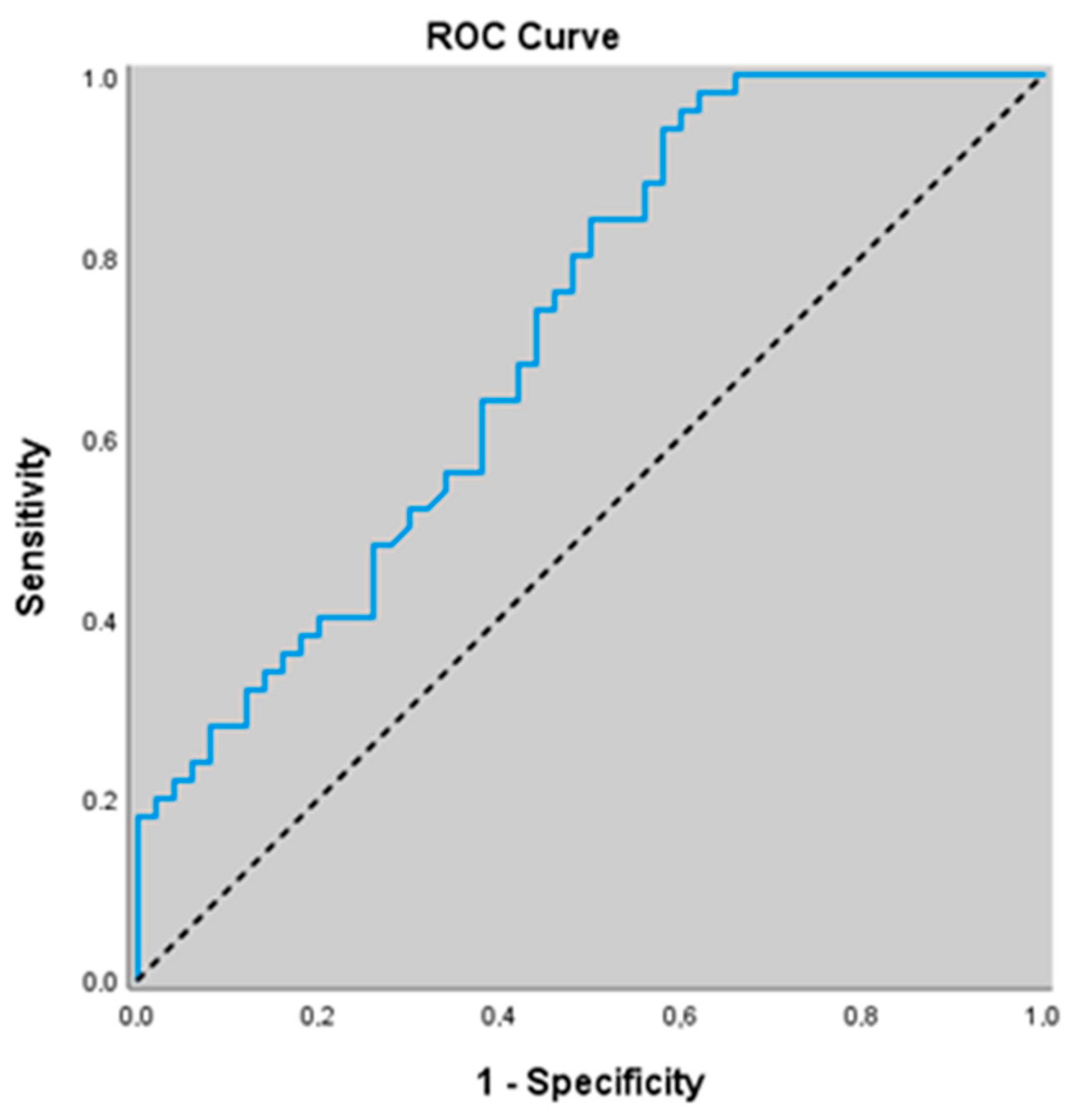
3.6. Diagnostic Value of NO-Related Biomarkers for Predicting Acute Exacerbation of COPD
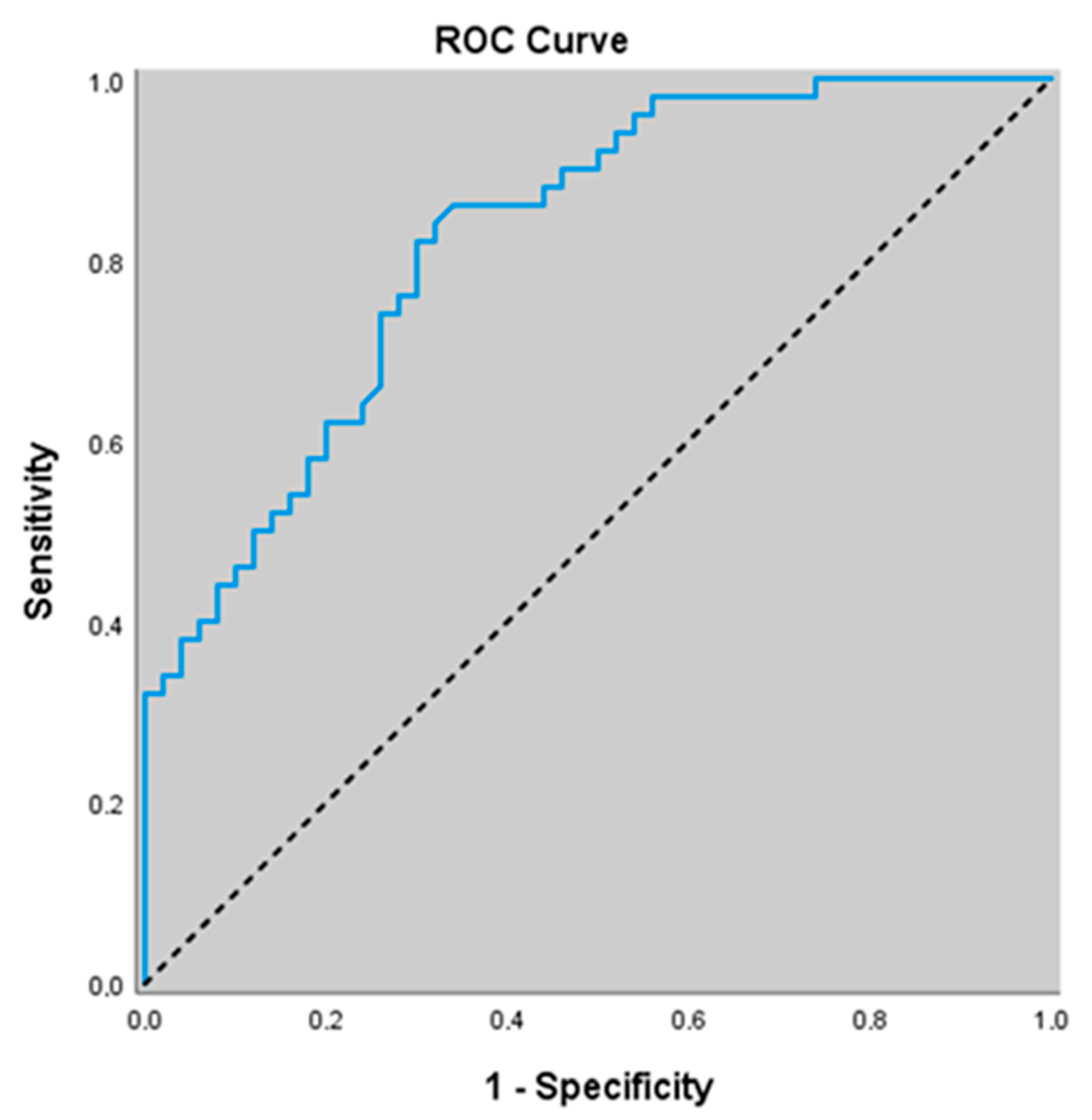
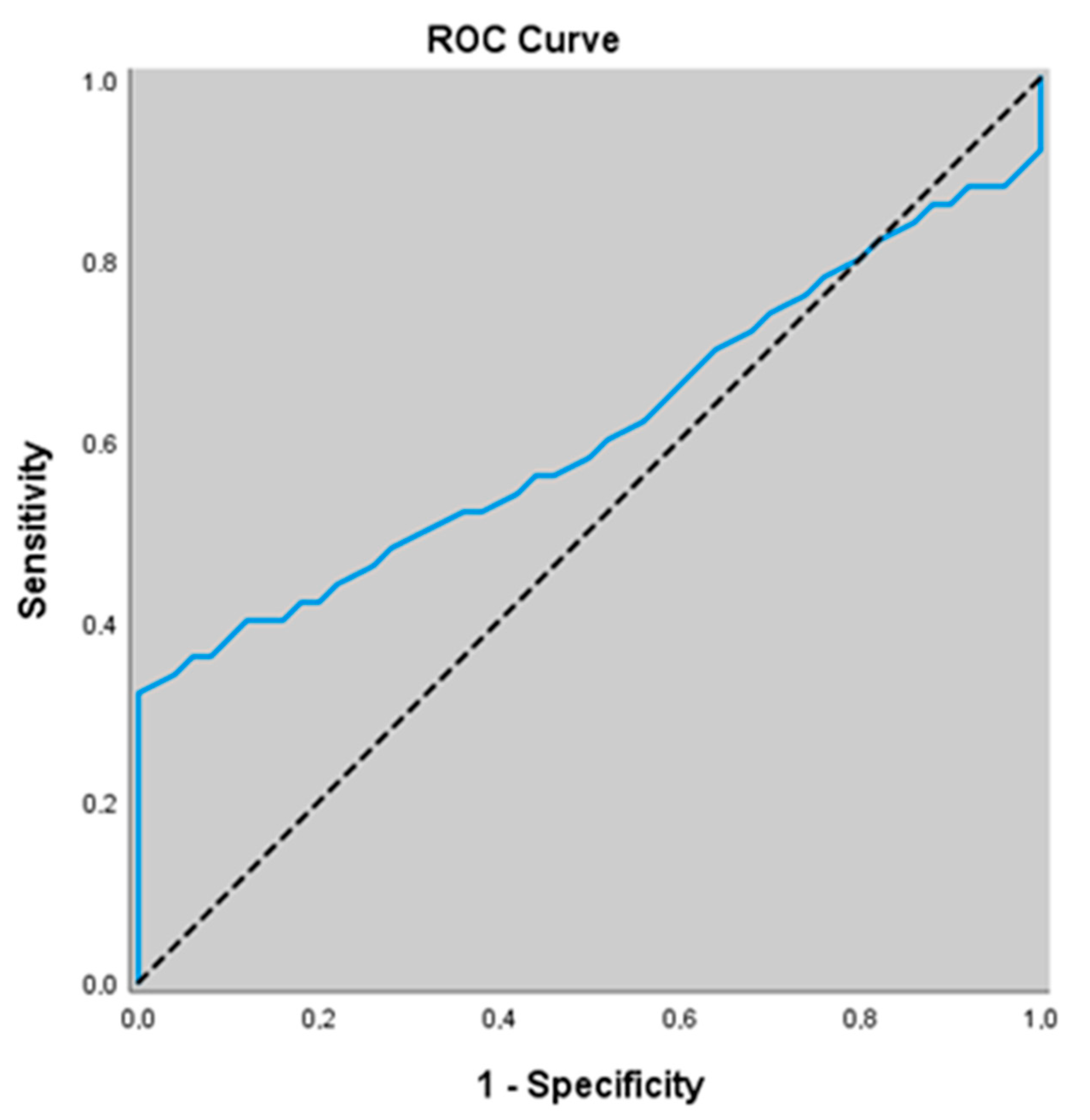
3.7. Diagnostic Value of Non-Related Biomarkers for Predicting Stable COPD
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. gold executive summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, A.I.; Wedzicha, J.A. Definition, Causes, Pathogenesis, and Consequences of Chronic Obstructive Pulmonary Disease Exacerbations. Clin. Chest Med. 2020, 41, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Celli, B.; Fabbri, L.; Criner, G.; Martinez, F.J.; Mannino, D.; Vogelmeier, C.; de Oca, M.M.; Papi, A.; Sin, D.D.; Han, M.K.; et al. Definition and Nomenclature of Chronic Obstructive Pulmonary Disease: Time for Its Revision. Am. J. Respir. Crit. Care Med. 2022, 206, 1317–1325. [Google Scholar] [CrossRef]
- Boers, E.; Barrett, M.; Su, J.G.; Benjafield, A.V.; Sinha, S.; Kaye, L.; Zar, H.J.; Vuong, V.; Tellez, D.; Gondalia, R.; et al. Global Burden of Chronic Obstructive Pulmonary Disease Through 2050. JAMA Netw. Open 2023, 6, e2346598. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). 2025. Available online: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) (accessed on 12 June 2025).
- Wedzicha, J.A.; Seemungal, T.A. COPD exacerbations: Defining their cause and prevention. Lancet 2007, 370, 786–796. [Google Scholar] [CrossRef]
- Atchley, W.T.; Kakkera, T.K. Pulmonary hypertension in chronic obstructive pulmonary disease: Current understanding, knowledge gaps and future directions. Curr. Opin. Pulm. Med. 2024, 30, 150–155. [Google Scholar] [CrossRef]
- Farah, C.; Michel, L.Y.M.; Balligand, J.L. Nitric oxide signalling in cardiovascular health and disease. Nat. Rev. Cardiol. 2018, 15, 292–316. [Google Scholar] [CrossRef]
- Leo, F.; Suvorava, T.; Heuser, S.K.; Li, J.; LoBue, A.; Barbarino, F.; Piragine, E.; Schneckmann, R.; Hutzler, B.; Good, M.E.; et al. Red Blood Cell and Endothelial eNOS Independently Regulate Circulating Nitric Oxide Metabolites and Blood Pressure. Circulation 2021, 144, 870–889. [Google Scholar] [CrossRef]
- Seimetz, M.; Parajuli, N.; Pichl, A.; Veit, F.; Kwapiszewska, G.; Weisel, F.C.; Milger, K.; Egemnazarov, B.; Turowska, A.; Fuchs, B.; et al. Inducible NOS Inhibition Reverses Tobacco-Smoke-Induced Emphysema and Pulmonary Hypertension in Mice. Cell 2011, 147, 293–305. [Google Scholar] [CrossRef] [PubMed]
- di Gangi, I.M.; Pirillo, P.; Carraro, S.; Gucciardi, A.; Naturale, M.; Baraldi, E.; Giordano, G. Online trapping and enrichment ultra performance liquid chromatography–tandem mass spectrometry method for sensitive measurement of “arginine-asymmetric dimethylarginine cycle” biomarkers in human exhaled breath condensate. Anal. Chim. Acta 2012, 754, 67–74. [Google Scholar] [CrossRef]
- Wood, K.C.; Cortese-Krott, M.M.; Kovacic, J.C.; Noguchi, A.; Liu, V.B.; Wang, X.; Raghavachari, N.; Boehm, M.; Kato, G.J.; Kelm, M.; et al. Circulating blood endothelial nitric oxide synthase contributes to the regulation of systemic blood pressure and nitrite homeostasis. Arter. Thromb. Vasc. Biol. 2013, 33, 1861–1871. [Google Scholar] [CrossRef] [PubMed]
- Patel, N. An update on COPD prevention, diagnosis, and management: The 2024 GOLD Report. Nurse Pract. 2024, 49, 29–36. [Google Scholar] [CrossRef]
- Soler-Cataluna, J.J.; Martínez-García, M.Á.; Sánchez, P.R.; Salcedo, E.; Navarro, M.; Ochando, R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005, 60, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Anthonisen, N.R.; Manfreda, J.; Warren, C.P.W.; Hershfield, E.S.; Harding, G.K.M.; Nelson, N.A. Antibiotic Therapy in Exacerbations of Chronic Obstructive Pulmonary Disease. Ann. Intern. Med. 1987, 106, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Stanojevic, S.; Kaminsky, D.A.; Miller, M.R.; Thompson, B.; Aliverti, A.; Barjaktarevic, I.; Cooper, B.G.; Culver, B.; Derom, E.; Hall, G.L.; et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur. Respir. J. 2022, 60, 2101499. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, Y.; Gao, X.; Li, R.; Zhao, L. Correlation between serum nitric oxide synthase levels and readmission due to acute exacerbation within 30 days in patients with acute exacerbations of chronic obstructive pulmonary disease. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2024, 36, 712–716. [Google Scholar]
- Duan, W.; Cheng, M. Diagnostic value of serum neuroactive substances in the acute exacerbation of chronic obstructive pulmonary disease complicated with depression. Open Life Sci. 2023, 18, 20220693. [Google Scholar] [CrossRef]
- Csoma, B.; Bikov, A.; Nagy, L.; Tóth, B.; Tábi, T.; Szűcs, G.; Komlósi, Z.I.; Müller, V.; Losonczy, G.; Lázár, Z. Dysregulation of the endothelial nitric oxide pathway is associated with airway inflammation in COPD. Respir. Res. 2019, 20, 156. [Google Scholar] [CrossRef]
- Hannemann, J.; Thorarinnsdottir, E.H.; Amaral, A.F.S.; Schwedhelm, E.; Schmidt-Hutten, L.; Stang, H.; Benediktsdottir, B.; Gunnarsdóttir, I.; Gislason, T.; Böger, R. Biomarkers of the L-Arginine/Dimethylarginine/Nitric Oxide Pathway in People with Chronic Airflow Obstruction and Obstructive Sleep Apnoea. J. Clin. Med. 2023, 12, 5230. [Google Scholar] [CrossRef]
- Telo, S.; Kırkıl, G.; Kuluöztürk, M.; Balin, M.; Deveci, F. Can ADMA play a role in determining pulmonary hypertension related to chronic obstructive pulmonary disease? Clin. Respir. J. 2018, 12, 1433–1438. [Google Scholar] [CrossRef]
- Aydin, M.; Altintas, N.; Mutlu, L.C.; Bilir, B.; Oran, M.; Tulubas, F.; Topçu, B.; Tayfur, I.; Kucukyalcin, V.; Kaplan, G.; et al. Asymmetric dimethylarginine contributes to airway nitric oxide deficiency in patients with COPD. Clin. Respir. J. 2017, 11, 318–327. [Google Scholar] [CrossRef]
- Zinellu, A.; Fois, A.G.; Sotgia, S.; Sotgiu, E.; Zinellu, E.; Bifulco, F.; Mangoni, A.A.; Pirina, P.; Carru, C. Arginines Plasma Concentration and Oxidative Stress in Mild to Moderate COPD. PLoS ONE 2016, 11, e0160237. [Google Scholar] [CrossRef]
- Corradi, M.; Majori, M.; Cacciani, G.C.; Consigli, G.F.; De’Munari, E.; Pesci, A. Increased exhaled nitric oxide in patients with stable chronic obstructive pulmonary disease. Thorax 1999, 54, 572–575. [Google Scholar] [CrossRef]
- Maziak, W.; Loukides, S.; Culpitt, S.; Sullivan, P.; Kharitonov, S.A.; Barnes, P.J. Exhaled nitric oxide in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1998, 157, 998–1002. [Google Scholar] [CrossRef]
- Rutgers, S.R.; van der Mark, T.W.; Coers, W.; Moshage, H.; Timens, W.; Kauffman, H.F.; Koëter, G.H.; Postma, D.S. Markers of nitric oxide metabolism in sputum and exhaled air are not increased in chronic obstructive pulmonary disease. Thorax 1999, 54, 576–580. [Google Scholar] [CrossRef]
- ben Anes, A.; Fetoui, H.; Bchir, S.; ben Nasr, H.; Chahdoura, H.; Chabchoub, E.; Yacoub, S.; Garrouch, A.; Benzarti, M.; Tabka, Z.; et al. Increased oxidative stress and altered levels of nitric oxide and peroxynitrite in tunisian patients with chronic obstructive pulmonary disease: Correlation with disease severity and airflow obstruction. Biol. Trace Element Res. 2014, 161, 20–31. [Google Scholar] [CrossRef]
- Çalikoglu, M.; Tamer, L.; ÇaIkoglu, I.; AtI, S.; Uluba, B.; Ercan, B. Oxidative stress and products of nitric oxide metabolism in chronic obstructive pulmonary disease and in healthy smokers. Turk. Resp. J. 2002, 3, 24–27. [Google Scholar]
- Dinh-Xuan, A.T.; Higenbottam, T.W.; Clelland, C.A.; Pepke-Zaba, J.; Cremona, G.; Butt, A.Y.; Large, S.R.; Wells, F.C.; Wallwork, J. Impairment of endothelium-dependent pulmonary-artery relaxation in chronic obstructive lung disease. N. Engl. J. Med. 1991, 324, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Assreuy, J.; Cunha, F.; Liew, F.; Moncada, S. Feedback inhibition of nitric oxide synthase activity by nitric oxide. Br. J. Pharmacol. 1993, 108, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Eisele, H.-J.; Markart, P.; Schulz, R. Obstructive Sleep Apnea, Oxidative Stress, and Cardiovascular Disease: Evidence from Human Studies. Oxidative Med. Cell. Longev. 2015, 2015, 608438. [Google Scholar] [CrossRef]
- Zhang, D.; Luo, J.; Qiao, Y.; Xiao, Y.; Huang, R.; Zhong, X. Measurement of exhaled nitric oxide concentration in patients with obstructive sleep apnea: A meta-analysis. Medicine 2017, 96, e6429. [Google Scholar] [CrossRef] [PubMed]
| 1 Control (n = 50) | 2 Stable COPD (n = 50) | 3 Acute Exacerbation (n = 50) | p | 1−2p | 1−3p | 2−3p | ||
|---|---|---|---|---|---|---|---|---|
| Age (Year) | Mean ± SD | 55.00 ± 6.93 | 53.30 ± 6.93 | 53.16 ± 7.30 | a0.351 | aa0.690 | aa0.583 | aa1.000 |
| Median (Min–Max) | 56 (42–67) | 52 (39–68) | 54 (35–68) | |||||
| Gender (F/M) | Female | 26 (52.0) | 25 (50.0) | 25 (50.0) | b1.000 | |||
| Male | 24 (48.0) | 25 (50.0) | 25 (50.0) | |||||
| BMI | Mean ± SD | 23.99 ± 1.75 | 28.27 ± 3.53 | 29.37 ± 4.87 | a0.001** | aa0.001** | aa0.001** | aa0.403 |
| Median (Min–Max) | 24.7 (19.6–26.3) | 28.1 (22.3–35.7) | 28.5 (21.1–40.6) | |||||
| Systolic blood pressure | Mean ± SD | 126.88 ± 19.08 | 125.56 ± 22.23 | 129.50 ± 6.53 | a0.357 | aa1.000 | aa1.000 | aa0.770 |
| Median (Min–Max) | 126.3 (81.5–165.4) | 126.3 (66.2–162.3) | 129.5 (120–140) | |||||
| Diastolic blood pressure | Mean ± SD | 77.62 ± 11.09 | 75.77 ± 9.35 | 94.40 ± 9.99 | a0.001** | aa1.000 | aa0.001** | aa0.001** |
| Median (Min–Max) | 78.8 (52.8–104.4) | 74.9 (58.8–93) | 96 (75–110) | |||||
| FEV1 (% predicted) | Mean ± SD | 85.33 ± 2.13 | 52.61 ± 12.50 | 46.00 ± 13.22 | a0.001** | aa0.001** | aa0.001** | aa0.031* |
| Median (Min–Max) | 85.2 (81.6–89.3) | 50 (32.6–78.9) | 47.9 (20.5–69.8) | |||||
| FEV1/FVC | Mean ± SD | 83.30 ± 4.19 | 65.94 ± 6.46 | 56.58 ± 8.70 | a0.001** | aa0.001** | aa0.001** | aa0.001** |
| Median (Min–Max) | 83 (76–90) | 69 (42–70) | 57.5 (38–70) | |||||
| PaO2 (mmHg) | Mean ± SD | 87.26 ± 7.51 | 61.43 ± 7.73 | 57.43 ± 8.47 | a0.001** | aa0.001** | aa0.001** | aa0.038* |
| Median (Min–Max) | 87.5 (75–100) | 61.5 (43.7–73.5) | 58 (42.9–71.2) | |||||
| SaO2 (%) | Mean ± SD | 94.75 ± 1.64 | 80.05 ± 6.15 | 77.70 ± 6.35 | a0.001** | aa0.001** | aa0.001** | aa0.074 |
| Median (Min–Max) | 94.6 (91.9–97.6) | 80 (68.2–91) | 77.6 (65.4–91.3) | |||||
| pH | Mean ± SD | 7.40 ± 0.03 | 7.35 ± 0.03 | 7.30 ± 0.03 | a0.001** | aa0.001** | aa0.001** | aa0.001** |
| Median (Min–Max) | 7.4 (7.4–7.4) | 7.4 (7.3–7.4) | 7.3 (7.3–7.3) | |||||
| pCO2 | Mean ± SD | 40.06 ± 2.82 | 52.18 ± 4.44 | 63.79 ± 5.91 | a0.001** | aa0.001** | aa0.001** | aa0.001** |
| Median (Min–Max) | 40 (35.5–44.8) | 51.9 (45.5–59.7) | 63.1 (55.2–73) |
| 1 Control (n = 50) | 2 Stable COPD (n = 50) | 3 Acute Exacerbation (n = 50) | p | 1−2p | 1−3p | ||
|---|---|---|---|---|---|---|---|
| FBG | Mean ± SD | 86.74 ± 6.92 | 88.6 ± 11.39 | 95.52 ± 9.21 | a0.001** | aa0.961 | aa0.001** |
| Median (Min–Max) | 86.5 (75–99) | 87.7 (69.1–122.5) | 95 (80–110) | ||||
| Insulin (μU/mL) | Mean ± SD | 9.96 ± 3.02 | 10.08 ± 3.26 | 9.72 ± 3.02 | a0.840 | aa1.000 | aa1.000 |
| Median (Min–Max) | 10 (5–15) | 10 (5–15) | 9.5 (5–15) | ||||
| HOMA-IR | Mean ± SD | 2.13 ± 0.67 | 2.22 ± 0.86 | 2.29 ± 0.76 | a0.582 | aa1.000 | aa0.901 |
| Median (Min–Max) | 2.1 (1–3.4) | 2.1 (1–4.5) | 2.1 (1.1–4) | ||||
| CRP (mg/L) | Mean ± SD | 1.20 ± 0.34 | 1.97 ± 1.37 | 1.05 ± 2.70 | c0.001** | cc0.330 | cc0.001** |
| Median (Min–Max) | 1.2 (0.6–1.8) | 1.9 (0.1–5.2) | 0.5 (0.1–19.3) | ||||
| ESR (mm/h) | Mean ± SD | 7.34 ± 2.21 | 22.18 ± 9.69 | 40.58 ± 16.91 | a0.001** | aa0.001** | aa0.001** |
| Median (Min–Max) | 7.2 (3–13) | 21.8 (5–42.1) | 40.2 (11.4–78.4) | ||||
| Triglyceride (mg/dL) | Mean ± SD | 104.14 ± 15.62 | 105.4 ± 14.44 | 118.34 ± 21.42 | a0.001** | aa0.908 | aa0.001** |
| Median (Min–Max) | 104 (78–130) | 105.5 (79–130) | 115.5 (75–175) | ||||
| LDL (mg/dL) | Mean ± SD | 99.69 ± 11.06 | 103.66 ± 12.85 | 139.41 ± 24.84 | a0.001** | aa0.228 | aa0.001** |
| Median (Min–Max) | 99.9 (75–118.8) | 103.3 (75.6–133.2) | 137.4 (96–184.6) | ||||
| Total cholesterol (mg/dL) | Mean ± SD | 173.00 ± 10.82 | 172.70 ± 10.5 | 200.82 ± 24.61 | a0.001** | aa1.000 | aa0.001** |
| Median (Min–Max) | 172.5 (155–198) | 172.5 (155–197) | 199.5 (162–250) | ||||
| HDL (mg/dL) | Mean ± SD | 52.48 ± 7.25 | 47.96 ± 5.42 | 37.74 ± 4.77 | a0.001** | aa0.002** | aa0.001** |
| Median (Min–Max) | 52.5 (40–65) | 48 (39–59) | 38 (30–47) | ||||
| Albumin (g/dL) | Mean ± SD | 42.78 ± 4.79 | 43.26 ± 4.08 | 39.06 ± 4.67 | a0.001** | aa1.000 | aa0.001** |
| Median (Min–Max) | 42.5 (35–50) | 43.5 (35–50) | 40.4 (28.1–52) | ||||
| Total protein (g/dL) | Mean ± SD | 71.36 ± 6.06 | 70.24 ± 5.1 | 69.12 ± 7.07 | a0.191 | aa1.000 | aa0.207 |
| Median (Min–Max) | 70.5 (61–80) | 70.5 (60–80) | 68.5 (51.7–81) | ||||
| ADMA (umol/L) | Mean ± SD | 1.17 ± 0.03 | 1.27 ± 0.03 | 1.61 ± 0.031 | d0.001** | dd0.085 | dd0.001** |
| Median (Min–Max) | 1.2 (1–1.4) | 1.2 (0.9–2) | 1.5 (1.3–2.1) | ||||
| NO (μmol/L) | Mean ± SD | 18.52 ± 0.33 | 13.75 ± 0.3 | 11.07 ± 0.32 | d0.001** | dd0.001** | dd0.001** |
| Median (Min–Max) | 18.4 (15–21.9) | 13.6 (10.1–18) | 11.1 (7.4–14) | ||||
| eNOS (IU/mL) | Mean ± SD | 47.27 ± 0.62 | 43.97 ± 0.56 | 42.19 ± 0.58 | d0.001** | dd0.001** | dd0.001** |
| Median (Min–Max) | 47.6 (40.2–55) | 44.2 (35.3–49.9) | 42 (35.1–51.3) |
| ADMA (umol/L) | NO (μmol/L) | eNOS (IU/mL) | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Age (Year) | −0.005 | 0.959 | 0.152 | 0.132 | 0.077 | 0.446 |
| •BMI | 0.265 | 0.001 ** | −0.430 | <0.001 *** | −0.330 | <0.001 *** |
| Duration of COPD | −0.011 | 0.910 | −0.062 | 0.543 | −0.084 | 0.407 |
| Systolic blood pressure | 0.030 | 0.765 | −0.103 | 0.310 | −0.067 | 0.511 |
| Diastolic blood pressure | 0.394 | <0.001 *** | −0.313 | 0.002 ** | −0.097 | 0.340 |
| FEV1 (% predicted) | −0.481 | <0.001 *** | 0.286 | 0.004** | 0.372 | <0.001 *** |
| FEV1/FVC | −0.293 | 0.003 ** | 0.120 | 0.236 | 0.086 | 0.397 |
| PaO2 (mmHg) | −0.209 | 0.038 * | 0.067 | 0.511 | 0.098 | 0.337 |
| SaO2 (%) | −0.272 | 0.006 ** | 0.345 | <0.001 *** | 0.067 | 0.512 |
| FBG | 0.133 | 0.189 | −0.312 | 0.002 ** | −0.107 | 0.292 |
| Insulin (μU/mL) | 0.026 | 0.798 | −0.024 | 0.813 | 0.072 | 0.477 |
| HOMA-IR | 0.057 | 0.579 | −0.130 | 0.199 | 0.030 | 0.766 |
| CRP(mg/L) | −0.106 | 0.294 | 0.130 | 0.198 | −0.002 | 0.982 |
| ESR (mm/h) | 0.344 | <0.001 *** | −0.282 | 0.005 ** | −0.080 | 0.430 |
| Triglyceride (mg/dL) | 0.255 | 0.011 * | −0.177 | 0.080 | −0.192 | 0.057 |
| LDL (mg/dL) | 0.512 | <0.001 *** | −0.442 | <0.001 *** | −0.210 | 0.037 * |
| Total cholesterol (mg/dL) | 0.484 | <0.001 *** | −0.392 | <0.001 *** | −0.194 | 0.054 |
| HDL (mg/dL) | −0.452 | <0.001 *** | 0.450 | <0.001 *** | 0.249 | 0.013 * |
| Albumin (g/dL) | −0.274 | 0.006 ** | 0.205 | 0.042 * | 0.087 | 0.394 |
| Total protein (g/dL) | −0.071 | 0.488 | 0.032 | 0.754 | 0.015 | 0.879 |
| pH | −0.419 | <0.001 *** | 0.366 | <0.001 *** | 0.070 | 0.488 |
| PCO2 | 0.393 | <0.001 *** | −0.418 | <0.001 *** | −0.211 | 0.036 * |
| ADMA (μmol/L) | NO (μmol/L) | eNOS (IU/mL) | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | Median (Min–Max) | Mean ± SD | Median (Min–Max) | Mean ± SD | Median (Min–Max) | |
| Gender (F/M) | ||||||
| Female | 1.38 ± 0.031 | 1.3 (0.9–2.1) | 14.12 ± 0.38 | 13.7 (7.4–21.9) | 44.13 ± 0.49 | 43.8 (35.1–55) |
| Male | 1.32 ± 0.031 | 1.3 (0.9–2) | 14.77 ± 0.39 | 14 (8.1–21.5) | 44.84 ± 0.49 | 44 (37.4–54.9) |
| dp | 0.166 | 0.235 | 0.316 | |||
| Frequency of exacerbations | ||||||
| 0 | 1.57 ± 0.05 | 1.5 (1.3–2) | 11.19 ± 0.36 | 11 (8.4–13.9) | 41.82 ± 0.64 | 41.1 (37.4–51.3) |
| 1 | 1.72 ± 0.07 | 1.9 (1.3–2) | 10.81 ± 0.57 | 10.1 (8.1–13.7) | 41.55 ± 1.05 | 41.3 (37.1–46.7) |
| ≥2 | 1.62 ± 0.08 | 1.4 (1.3–2.1) | 11.16 ± 0.61 | 12 (7.4–14) | 43.05 ± 1.10 | 44.1 (35.1–48) |
| dp | 0.206 | 0.852 | 0.567 | |||
| Variables | AUC (95% CI) | Cut-Off | p Value | Sensitivity (%95 CI) | Specificity (%95 CI) | PPV (%95 CI) | NPV (%95 CI) |
|---|---|---|---|---|---|---|---|
| ADMA (umol/L) | 0.983 (0.934–0.998) | ≥1.36 | <0.001 | 86.0 (73.3–94.2) | 96.0 (86.3–99.5) | 95.6 (84.6–98.8) | 87.3 (77.5–93.2) |
| eNOS (IU/mL) | 0.823 (0.734–0.892) | ≤44.52 | <0.001 | 82.0 (68.6–91.4) | 70.0 (55.4–82.1) | 73.2 (63.7–81.0) | 79.5 (67.7–87.8) |
| Variables | AUC (95% CI) | Cut-Off | p Value | Sensitivity (%95 CI) | Specificity (%95 CI) | PPV (%95 CI) | NPV (%95 CI) |
|---|---|---|---|---|---|---|---|
| ADMA (umol/L) | 0.609 (0.506–0.705) | ≥1.36 | 0.062 | 34.0 (21.2–48.8) | 96.0 (86.3–99.5) | 89.5 (67.4–97.2) | 59.3 (54.2–64.1) |
| NO (μmol/L) | 0.921 (0.850–0.966) | ≤14.91 | <0.001 | 68.0 (53.3–80.5) | 100.0 (92.9–100.0) | 100 (100.0–100.0) | 75.8 (67.6–82.4) |
| eNOS (IU/mL) | 0.713 (0.614–0.799) | ≤48.8 | <0.001 | 94.0 (83.5–98.7) | 42.0 (28.2–56.8) | 61.8 (55.9–67.5) | 87.5 (69.0–95.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Jundi, O.; Fenercioglu, A.K.; Uysal, P.; Dumur, S.; Cucu, O.; Uzun, H. Diagnostic Value of NO-Related Biomarkers (ADMA, NO, eNOS) in Stable COPD and Acute Exacerbation of COPD. J. Clin. Med. 2025, 14, 7386. https://doi.org/10.3390/jcm14207386
El Jundi O, Fenercioglu AK, Uysal P, Dumur S, Cucu O, Uzun H. Diagnostic Value of NO-Related Biomarkers (ADMA, NO, eNOS) in Stable COPD and Acute Exacerbation of COPD. Journal of Clinical Medicine. 2025; 14(20):7386. https://doi.org/10.3390/jcm14207386
Chicago/Turabian StyleEl Jundi, Osman, Aysen Kutan Fenercioglu, Pelin Uysal, Seyma Dumur, Oguzhan Cucu, and Hafize Uzun. 2025. "Diagnostic Value of NO-Related Biomarkers (ADMA, NO, eNOS) in Stable COPD and Acute Exacerbation of COPD" Journal of Clinical Medicine 14, no. 20: 7386. https://doi.org/10.3390/jcm14207386
APA StyleEl Jundi, O., Fenercioglu, A. K., Uysal, P., Dumur, S., Cucu, O., & Uzun, H. (2025). Diagnostic Value of NO-Related Biomarkers (ADMA, NO, eNOS) in Stable COPD and Acute Exacerbation of COPD. Journal of Clinical Medicine, 14(20), 7386. https://doi.org/10.3390/jcm14207386







