Optical Coherence Tomography Angiography Is Associated with Disease Activity Expressed by NEDA-3 Status in Patients with Relapsing Multiple Sclerosis
Abstract
1. Introduction
2. Materials and Methods
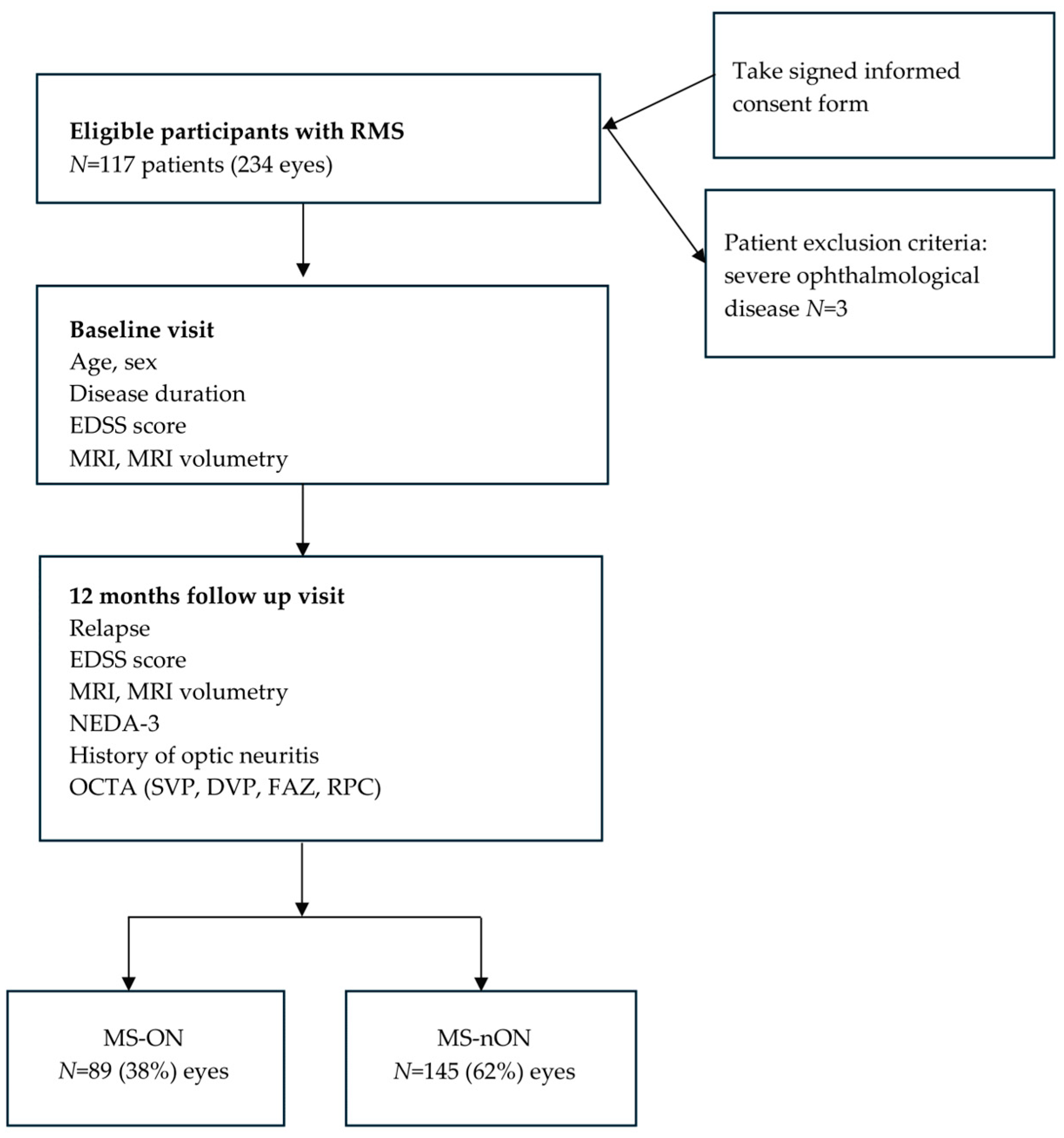
2.1. Study Design
2.2. Study Population
2.3. Study Procedures
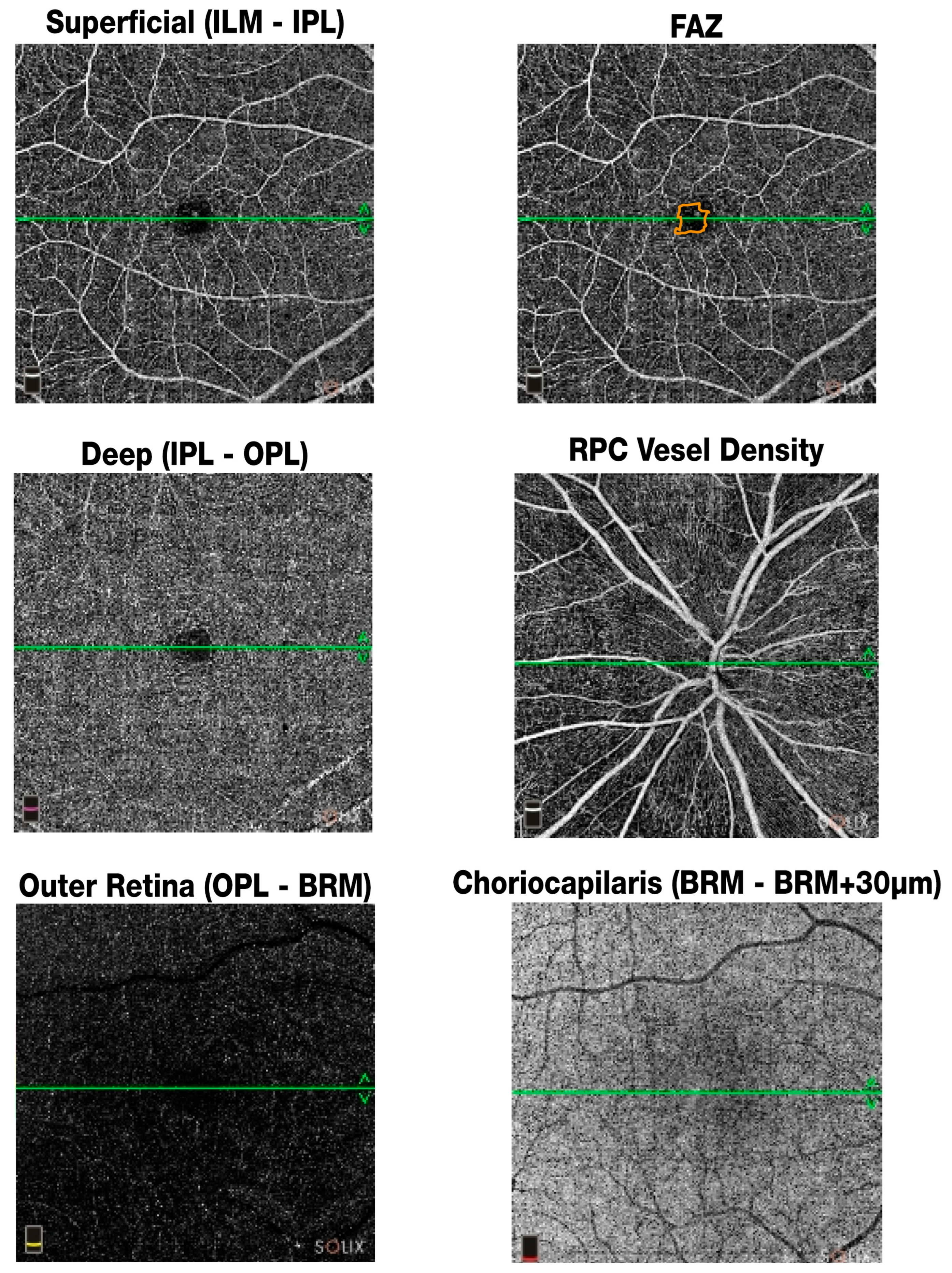
2.3.1. Optical Coherence Tomography Angiography (OCTA)
- SVP (Superficial Vascular Plexus): vessel density in the superficial retinal layer extending from the internal limiting membrane to the inner plexiform layer (in %).
- DVP (Deep Vascular Plexus): vessel density in the deep retinal layer spanning the inner nuclear layer to the outer plexiform layer (in %).
- FAZ area: area of the foveal avascular zone measured in mm2.
- FAZ perimeter: length of the boundary surrounding the FAZ in mm.
- FAZ FD (Foveal Density): represents the vessel density within a 300-μm radius around the foveal avascular zone (FAZ), as automatically generated by the SOLIX software (version Solix 2019 V1.0.0.382) (FD-300) (in %).
- RPC (Radial Peripapillary Capillary) density: vessel density in the peripapillary region surrounding the optic nerve head (in %).
2.3.2. Brain MRI Protocol and Analysis
2.3.3. NEDA-3 Assessment
2.4. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Retinal Vascular Findings
3.3. Correlations
- SVP vessel density negatively correlated with age (r = −0.16, p < 0.01), time since last relapse (r = −0.25, p < 0.001), and disease duration (r = −0.19, p < 0.001).
- SVP positively correlated with new FLAIR lesion volume (r = 0.26, p < 0.001), whole brain (WB) volume (r = 0.19, p < 0.05), and gray matter (GM) volume (r = 0.19, p < 0.05).
- DVP vessel density negatively correlated with time since last relapse (r = −0.18, p < 0.05), and positively with new FLAIR lesion volume (r = 0.23, p < 0.05), WB volume (r = 0.16, p < 0.05), and GM volume (r = 0.17, p < 0.05).
- FAZ area showed a positive correlation with EDSS score (r = 0.14, p < 0.05) and T1 lesion volume (r = 0.19, p < 0.05).
- FAZ FD was negatively correlated with age (r = −0.21, p < 0.001), time since last relapse (r = −0.21, p < 0.001), and disease duration (r = −0.14, p < 0.05).
- RPC vessel density negatively correlated with time since last relapse (r = −0.19, p < 0.001), disease duration (r = −0.22, p < 0.001), and FLAIR lesion volume (r = −0.22, p < 0.001). It was positively associated with WB volume (r = 0.27, p < 0.001) and GM volume (r = 0.16, p < 0.05) (Table 2).
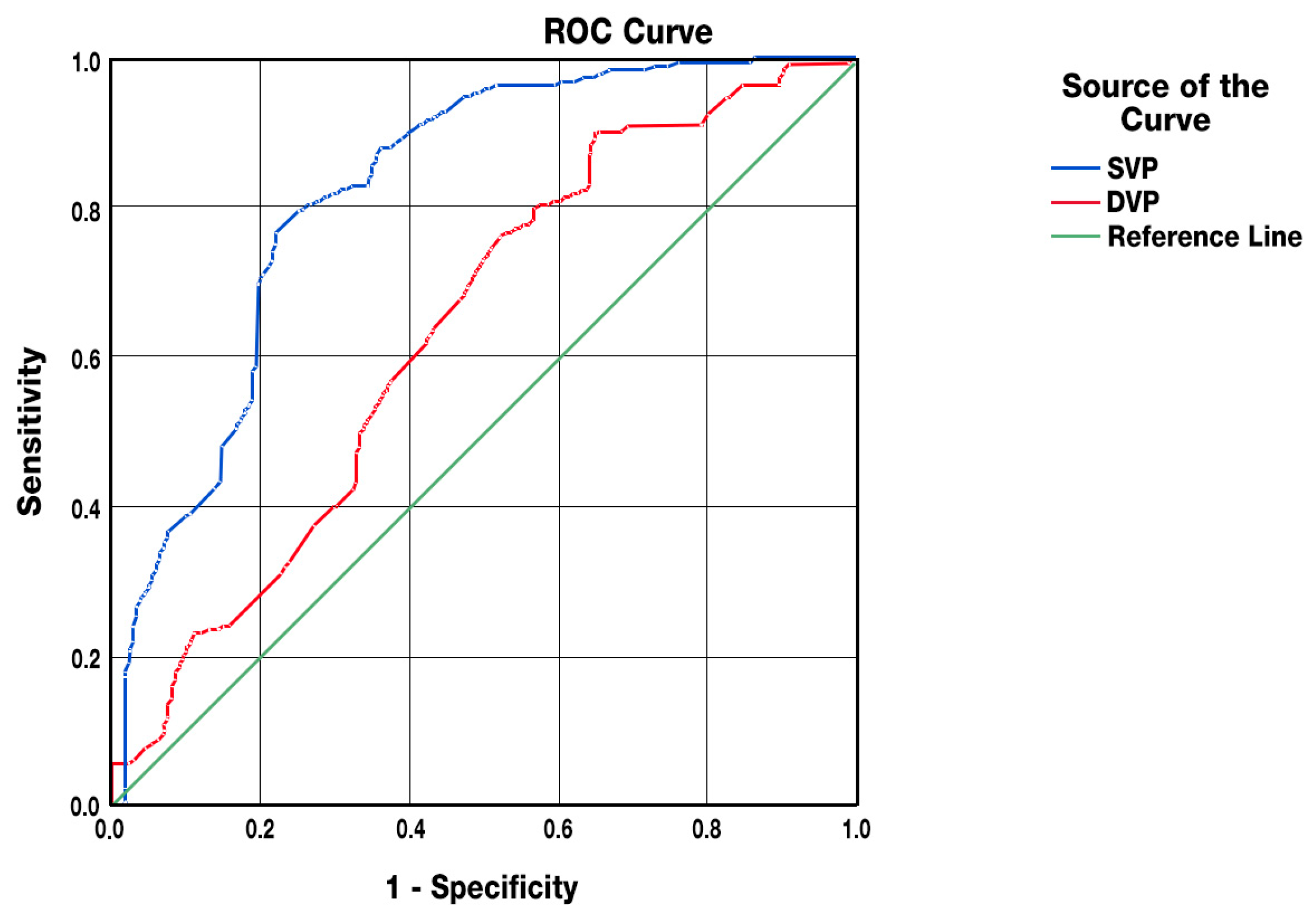
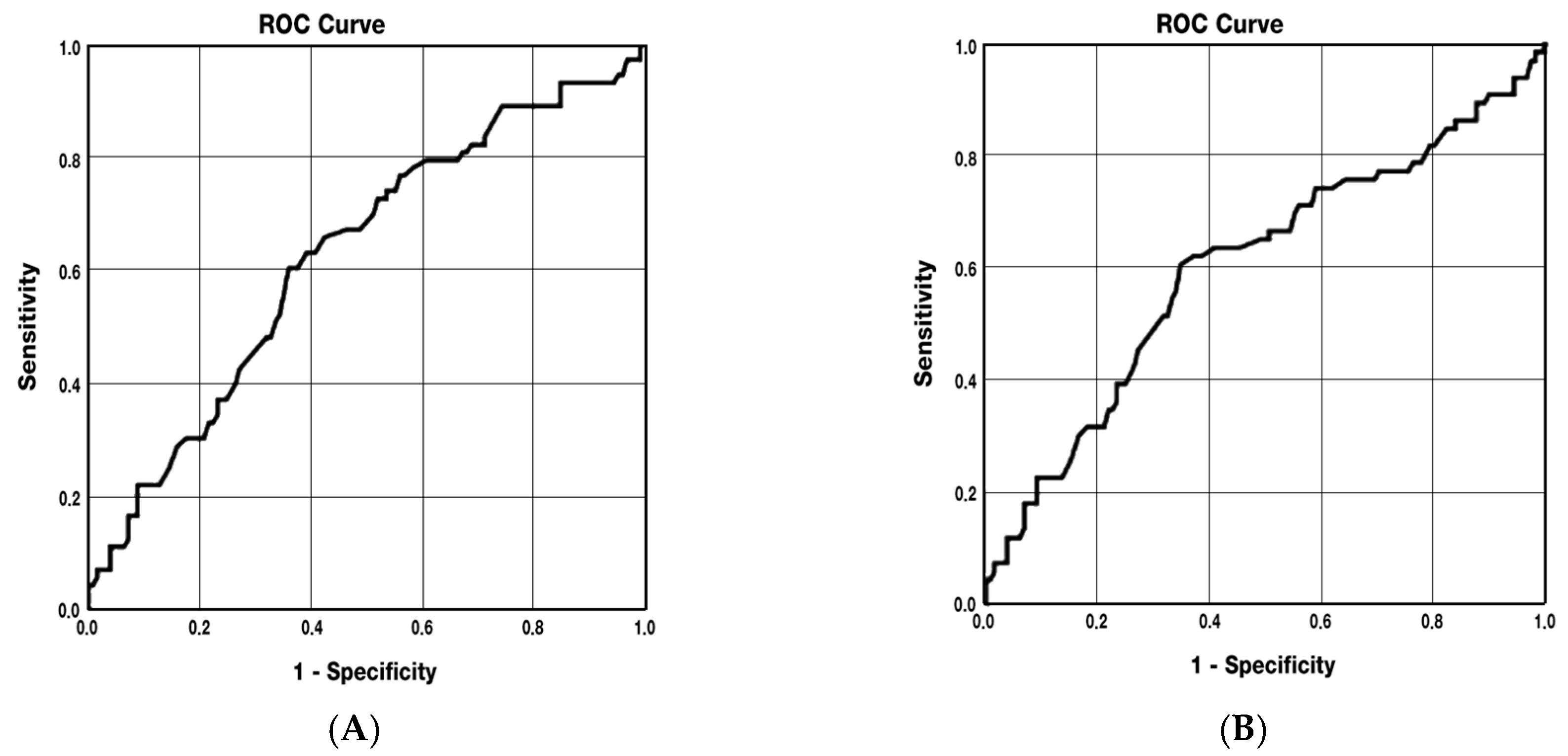
3.4. Predictive Analyses
4. Discussion
4.1. Main Findings and Comparison with Previous Studies
4.2. Vascular Changes in Patients with Optic Neuritis
4.3. Associations Between Vascular Parameters and Neurodegeneration
4.4. Retinal Vascular Parameters as Markers of Disease Activity
4.5. The Significance of Foveal Avascular Zone (FAZ)
4.6. Retinal Vascular Changes as a Reflection of Cerebral Neurodegeneration
4.7. Potential Pathophysiological Mechanisms and Implications
4.8. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| CI | Confidence Interval |
| DMT | Disease Modifying Therapy |
| DVP | Deep vascular plexus |
| EDSS | Expanded Disability Status Scale |
| FAZ | Foveal avascular zone |
| FAZ FD | Foveal avascular zone floor density |
| FLAIR | Fluid-attenuated inversion recovery |
| FUV | follow-up visit |
| Gd | gadolinium |
| GM | Gray Matter |
| HCs | Healthy Controls |
| IQR | Interquartile Range |
| MRI | Magnetic Resonance Imaging |
| NEDA | No Evidence of Disease Activity |
| OCT | Optical coherence tomography |
| OCTA | Optical coherence tomography angiography |
| ON | Optic neuritis |
| RMS | Relapsing Multiple Sclerosis |
| PwMS | Patients with Multiple sclerosis |
| ROC | Receiver operating characteristics |
| RPC | Radial peripapillary capillary |
| SD | Standard deviation |
| SVP | Superficial vascular plexus |
| VD | Vessel density |
| WB | Whole Brain |
References
- Thompson, A.J.; Baranzini, S.E.; Geurts, J.; Hemmer, B.; Ciccarelli, O. Multiple sclerosis. Lancet 2018, 391, 1622–1636. [Google Scholar] [CrossRef]
- Kleerekooper, I.; Petzold, A.; Trip, S.A. Anterior visual system imaging to investigate energy failure in multiple sclerosis. Brain 2020, 143, 1999–2008. [Google Scholar] [CrossRef]
- Yang, R.; Dunn, J.F. Multiple sclerosis disease progression: Contributions from a hypoxia–inflammation cycle. Mult. Scler. J. 2018, 25, 1715–1718. [Google Scholar] [CrossRef]
- D’Haeseleer, M.; Hostenbach, S.; Peeters, I.; El Sankari, S.; Nagels, G.; De Keyser, J. Cerebral hypoperfusion: A new pathophysiologic concept in multiple sclerosis? J. Cereb. Blood Flow. Metab. 2015, 35, 1406–1410. [Google Scholar] [CrossRef]
- Juurlink, B.H.J. The evidence for hypoperfusion as a factor in multiple sclerosis lesion development. Mult. Scler. Int. 2013, 2013, 598093. [Google Scholar] [CrossRef]
- Varga, A.W.; Johnson, G.; Babb, J.S.; Herbert, J.; Grossman, R.I.; Inglese, M. White matter hemodynamic abnormalities precede subcortical gray matter changes in multiple sclerosis. J. Neurol. Sci. 2009, 282, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Curcio, C.A. Evaluation of segmentation of the superficial and deep vascular layers of the retina by optical coherence tomography angiography instruments in normal eyes. JAMA Ophthalmol. 2017, 135, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Delgado, S.; Tan, J.; Liu, C.; Rammohan, K.W.; DeBuc, D.C.; Wilkins, A.; Green, A.J.; Sriram, S. Impaired retinal microcirculation in multiple sclerosis. Mult. Scler. 2016, 22, 1812–1820. [Google Scholar] [CrossRef] [PubMed]
- Modrzejewska, M.; Karczewicz, D.; Wilk, G. Assessment of blood flow velocity in eyeball arteries in multiple sclerosis patients with past retrobulbar optic neuritis in color Doppler ultrasonography. Klin. Ocz. 2007, 109, 183–186. [Google Scholar]
- Murphy, O.C.; Kwakyi, O.; Iftikhar, M.; Zafar, S.; Lambe, J.; Pellegrini, N.; O’Neill, C.; Farooqi, N.; Sriram, S.; Sadiq, S. Alterations in the retinal vasculature occur in multiple sclerosis and exhibit novel correlations with disability and visual function measures. Mult. Scler. J. 2019, 26, 815–828. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, C.; Zhou, L.; Huang, S.; Shen, M.; He, Z. The detection of retina microvascular density in subclinical aquaporin-4 antibody seropositive neuromyelitis optica spectrum disorders. Front. Neurol. 2020, 11, 35. [Google Scholar]
- Yilmaz, H.; Ersoy, A.; Icel, E. Assessments of vessel density and foveal avascular zone metrics in multiple sclerosis: An optical coherence tomography angiography study. Eye 2020, 34, 771–778. [Google Scholar] [CrossRef]
- Feucht, N.; Maier, M.; Lepennetier, G.; Pettenkofer, M.; Wetzlmair, C.; Daltrozzo, T.; Watzke, S.; Rist, M.; Haschke, M.; Stögbauer, F. Optical coherence tomography angiography indicates associations of the retinal vascular network and disease activity in multiple sclerosis. Mult. Scler. J. 2018, 25, 224–234. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Tewarie, P.; Balk, L.; Costello, F.; Cassels, J.; Petzold, A.; Balcer, L.J.; Schippling, S.; Murueta-Goyena, A.; Enzinger, C.; Bilder, R.; et al. The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS ONE 2012, 7, e34823. [Google Scholar] [CrossRef] [PubMed]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Petzold, A.; Fraser, C.L.; Abegg, M.; Alroughani, R.; Alshowaeir, D.; Alvarenga, R.; Arnold, D.L.; Balcer, L.J.; Bennett, J.L.; Boland, R.; et al. Diagnosis and classification of optic neuritis. Lancet Neurol. 2022, 21, 1120–1134. [Google Scholar] [CrossRef]
- Steenwijk, M.D.; Amiri, H.; Schoonheim, M.M.; Daams, M.; Wattjes, M.P.; Pouwels, P.J.W.; Tewarie, P.K.; Vrenken, H.; Barkhof, F.; Polman, C.H.; et al. Agreement of MSmetrix with established methods for measuring cross-sectional and longitudinal brain atrophy. Neuroimage Clin. 2017, 15, 843–853. [Google Scholar] [CrossRef]
- De Stefano, N.; Stromillo, M.L.; Giorgio, A.; Rossi, E.; Carone, D.; La Cava, G.; Battaglini, M.; Pitteri, M.; Petruzzo, M.; Federico, A.; et al. Establishing pathological cut-offs of brain atrophy rates in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2016, 87, 93–99. [Google Scholar] [CrossRef]
- Smeets, D.; Ribbens, A.; Sima, D.M.; Heersema, D.; D’hooghe, M.; Suneel, N.; Van Hecke, W.; D’Hooge, R.; Van Damme, P.; Vandenberghe, R. Reliable measurements of brain atrophy in individual patients with multiple sclerosis. Brain Behav. 2016, 6, e00518. [Google Scholar] [CrossRef]
- Giovannoni, G.; Tomic, D.; Bright, J.R.; Phillips, J.; Finkelsztejn, A.; Lechner-Scott, J.; Rice, C.M.; Van der Mei, I. “No evident disease activity”: The use of combined assessments in the management of patients with multiple sclerosis. Mult. Scler. 2017, 23, 1179–1187. [Google Scholar] [CrossRef]
- European Medicines Agency Guideline on Clinical Investigation of Medicinal Products for the Treatment of Multiple Sclerosis. CHMP/771815/2011 Rev.2, 26 March 2015. Available online: https://www.tga.gov.au/resources/resource/international-scientific-guidelines/guideline-clinical-investigation-medical-products-treatment-multiple-sclerosis (accessed on 6 September 2025).
- Koustenis, A., Jr.; Harris, A.; Gross, J.; Januleviciene, I.; Shah, A.; Siesky, B. Optical coherence tomography angiography: An overview of the technology and applications for clinical research. Br. J. Ophthalmol. 2017, 101, 16–20. [Google Scholar] [CrossRef]
- Mohammadi, S.; Gouravani, M.; Salehi, M.A.; Sadeghi, M.; Khajeh, E.; Farokhi, F.; Yektaei, M.; Sadeghi, M.; Teshnehlab, M.; Akbari, A.; et al. Optical coherence tomography measurements in multiple sclerosis: A systematic review and meta-analysis. J. Neuroinflammation 2023, 20, 85. [Google Scholar] [CrossRef]
- Wang, X.; Jia, Y.; Spain, R.; Liu, L.; Wang, J.; Zhang, M.; Liu, H.; Wang, J.; Zeng, H.; Ko, T.; et al. Optical coherence tomography angiography of optic nerve head and parafovea in multiple sclerosis. Br. J. Ophthalmol. 2014, 98, 1368–1373. [Google Scholar] [CrossRef]
- Spain, R.I.; Liu, L.; Zhang, X.; Jia, Y.; Tan, O.; Bourdette, D.; Huang, D. Optical coherence tomography angiography enhances the detection of optic nerve damage in multiple sclerosis. Br. J. Ophthalmol. 2017, 102, 532–537. [Google Scholar] [CrossRef]
- Lanzillo, R.; Cennamo, G.; Criscuolo, C.; Carotenuto, A.; Pio, L.; De Stefano, N.; Esposito, F.; Ruggieri, S. Optical coherence tomography angiography retinal vascular network assessment in multiple sclerosis. Mult. Scler. 2018, 24, 1706–1714. [Google Scholar] [CrossRef]
- Ulusoy, M.O.; Horasanli, B.; Işik-Ulusoy, S. Optical coherence tomography angiography findings of multiple sclerosis with or without optic neuritis. Neurol. Res. 2020, 42, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Romahn, E.F.; Wiltgen, T.; Bussas, M.; Kumpfel, T.; Gärtner, J.; Kappos, L.; Nef, T.; Enzinger, C.; Mayer, M. Association of retinal vessel pathology and brain atrophy in relapsing-remitting multiple sclerosis. Front. Immunol. 2023, 14, 1284986. [Google Scholar] [CrossRef] [PubMed]
- Lanzillo, R.; Cennamo, G.; Moccia, M.; Carotenuto, A.; Luongo, L.; Rizzo, A.; Carotenuto, A.; Esposito, R. Retinal vascular density in multiple sclerosis: A 1-year follow-up. Eur. J. Neurol. 2019, 26, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Aly, L.; Noll, C.; Wicklein, R.; Schlaeger, F.; Bittner, S.; Sämann, P.G.; Leist, T.; Ueberham, U.; Wuerfel, J.; Paul, F. Dynamics of retinal vessel loss after acute optic neuritis in patients with relapsing multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm 2022, 9, e1159. [Google Scholar] [CrossRef]
- Noll, C.; Hiltensperger, M.; Aly, L.; Wicklein, R.; Maisam Afzali, A.; Mardin, C.; Gasperi, C.; Berthele, A.; Hemmer, B.; Korn, T.; et al. Association of the retinal vasculature, intrathecal immunity, and disability in multiple sclerosis. Front. Immunol. 2022, 13, 997043. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Kaneko, H.; Ito, Y.; Kataoka, K.; Iwase, T.; Yasuma, T.; Matsuura, T.; Tsunekawa, T.; Shimizu, H.; Suzumura, A.; et al. Novel Classification of Early-stage Systemic Hypertensive Changes in Human Retina Based on OCTA Measurement of Choriocapillaris. Sci. Rep. 2018, 8, 15163. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.W.; Tan, G.S.W.; Agrawal, R.; Yanagi, Y.; Sie, N.M.; Wong, C.W.; Yeo, I.; Lee, S.Y.; Cheung, C.M.G.; Wong, T.Y. Optical Coherence Tomographic Angiography in Type 2 Diabetes and Diabetic Retinopathy. JAMA Ophthalmol. 2017, 135, 306–312. [Google Scholar] [CrossRef]
- Saidha, S.; Sotirchos, E.S.; Oh, J.; Gelfand, J.M.; Bakshi, R.; Klistorner, A.; Munschauer, F.E.; Syc, S.B.; Stosic, M.; Liu, T.; et al. Relationships between retinal axonal and neuronal measures and global CNS pathology in multiple sclerosis. JAMA Neurol. 2013, 70, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Suwan, Y.; Aghsaei Fard, M.; Geyman, L.S.; Alnawaiseh, M.; Borrás, A.; Liu, Z.; Kuehn, M.; Chen, C.; Liu, J.; Zhang, X.; et al. Association of myopia with peripapillary perfused capillary density in patients with glaucoma: An optical coherence tomography angiography study. JAMA Ophthalmol. 2018, 136, 507–513. [Google Scholar] [CrossRef] [PubMed]
| HCs | RMS Total | MS-ON | MS-nON | MS-ON vs. MS-nON p Value | MS-ON vs. HCs p Value | MS-nON vs. HCs p Value | |
|---|---|---|---|---|---|---|---|
| Participants, n Female, n (%) | 37 23 (62) | 117 74 (63) | |||||
| Eyes | 74 | 234 | 89 (38%) | 145 (62%) | |||
| Demographic characteristics | |||||||
| Age at FUV, mean (SD), years | 38.9 (12.9) | 38 (10.4) | 36.9 (10.4) | 38.5 (10.4) | NS | NS | NS |
| Clinical characteristics | |||||||
| Disease duration at FUV, median (IQR), years | – | 8 (4–13) | 11 (5.5–14) | 6.5 (3–12) | 0.001 | – | – |
| Time from the last relapse to FUV, years, median IQR | – | 1.5 (0.5–3.5) | 2 (0.5–4) | 1.2 (3–12) | NS | – | – |
| EDSS score at FUV, median (IQR) | – | 2.5 (2–3.5) | 2.5 (2–3.5) | 2.5 (2–3.5) | NS | – | – |
| OCTA measures | |||||||
| SVP, median (IQR), % | 49.3 (47.8–50.1) | 49.3 (48.3–50.2) | 48.9 (47.9–50.1) | 49.5 (48.5–50.4) | 0.024 | 0.049 | NS |
| DVP, median (IQR), % | 52.7 (52.7–54.8) | 54.2 (53.2–54.7) | 54.1 (53.9–54.5) | 54.2 (53.2–54.8) | NS | NS | NS |
| FAZ area, median (IQR), mm2 | 0.24 (0.19–0.29) | 0.23 (0.17–0.3) | 0.26 (0.19–0.3) | 0.23 (0.15–0.29) | NS | NS | NS |
| FAZ FD, median (IQR), % | 51.2 (49–54) | 51.6 (49.1–53.7) | 51.9 (48.9–54.2) | 51.6 (49.1–53.6) | NS | NS | NS |
| RPC VD, median (IQR), % | 49.4 (47.9–51) | 48 (45.9–49.7 | 46.1 (42.9–48.5) | 48.7 (47.2–50) | 0.001 | 0.001 | 0.011 |
| MRI measures at FUV | |||||||
| FLAIR lesions volume (mL), median (IQR) | – | 4.2 (2.3–8.9) | 3.3 (2.4–11) | 4.3 (2.3–8) | NS | – | – |
| T1 hypointense lesions volume (mL), median (IQR) | – | 2.2 (1.1–4.6) | 2.2 (1.1–4.6) | 1.9 (1–5.6) | NS | – | – |
| WB volume (mL), median (IQR) | – | 1544 (1507–1588) | 1544 (1492–1582) | 1545 (1511–1588) | NS | – | – |
| WB volume normative percentile, median (IQR) | – | 11.9 (3.8–46) | 8 (2.3–46.8) | 17 (4.4–46) | NS | – | – |
| GM volume (mL), median (IQR) | – | 919 (886–944) | 912 (885–953) | 923 (886–943) | NS | – | – |
| GM volume normative percentile, median (IQR) | – | 16 (1.6–44) | 9.8 (1–42) | 17 (2–52) | NS | – | – |
| OCTA Measures | |||||
|---|---|---|---|---|---|
| Variables | SVP (%) | DVP (%) | FAZ Area (mm2) | FAZ FD (%) | RPC VD (%) |
| Age (years) | −0.16 * | −0.12 | 0.11 | −0.21 ** | 0.09 |
| Last relapse period (years) | −0.25 ** | −0.18 ** | −0.04 | −0.21 ** | −0.19 ** |
| Disease duration (years) | −0.19 ** | −0.07 | 0.05 | −0.14 * | −0.22 ** |
| EDSS | 0.04 | −0.08 | 0.14 * | −0.05 | 0.01 |
| FLAIR lesions volume (mL) | −0.07 | 0.05 | −0.01 | −0.10 | −0.22 ** |
| FLAIR new lesions volume (mL) | 0.26 ** | 0.23 * | 0.04 | 0.06 | −0.07 |
| T1-hypointense lesions volume (mL) | 0.10 | 0.08 | 0.19 * | −0.06 | 0.11 |
| WB volume (mL) | 0.19 * | 0.16 * | −0.05 | −0.03 | 0.27 ** |
| GM volume (mL) | 0.19 * | 0.17 * | −0.12 | −0.05 | 0.16 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szilasi, J.; Vitková, M.; Gdovinová, Z.; Fedičová, M.; Mikula, P.; Frigová, L.; Szilasiová, J. Optical Coherence Tomography Angiography Is Associated with Disease Activity Expressed by NEDA-3 Status in Patients with Relapsing Multiple Sclerosis. J. Clin. Med. 2025, 14, 7370. https://doi.org/10.3390/jcm14207370
Szilasi J, Vitková M, Gdovinová Z, Fedičová M, Mikula P, Frigová L, Szilasiová J. Optical Coherence Tomography Angiography Is Associated with Disease Activity Expressed by NEDA-3 Status in Patients with Relapsing Multiple Sclerosis. Journal of Clinical Medicine. 2025; 14(20):7370. https://doi.org/10.3390/jcm14207370
Chicago/Turabian StyleSzilasi, Jozef, Marianna Vitková, Zuzana Gdovinová, Miriam Fedičová, Pavol Mikula, Lýdia Frigová, and Jarmila Szilasiová. 2025. "Optical Coherence Tomography Angiography Is Associated with Disease Activity Expressed by NEDA-3 Status in Patients with Relapsing Multiple Sclerosis" Journal of Clinical Medicine 14, no. 20: 7370. https://doi.org/10.3390/jcm14207370
APA StyleSzilasi, J., Vitková, M., Gdovinová, Z., Fedičová, M., Mikula, P., Frigová, L., & Szilasiová, J. (2025). Optical Coherence Tomography Angiography Is Associated with Disease Activity Expressed by NEDA-3 Status in Patients with Relapsing Multiple Sclerosis. Journal of Clinical Medicine, 14(20), 7370. https://doi.org/10.3390/jcm14207370






