Clinical Characteristics, Treatment Approaches, and Survival Predictors in Adult Acute Myeloid Leukemia: Interim Results from the Turkish Society of Hematology AML Registry †
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Data Collection and Definitions
2.3. AML Subtypes and Risk Stratification
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
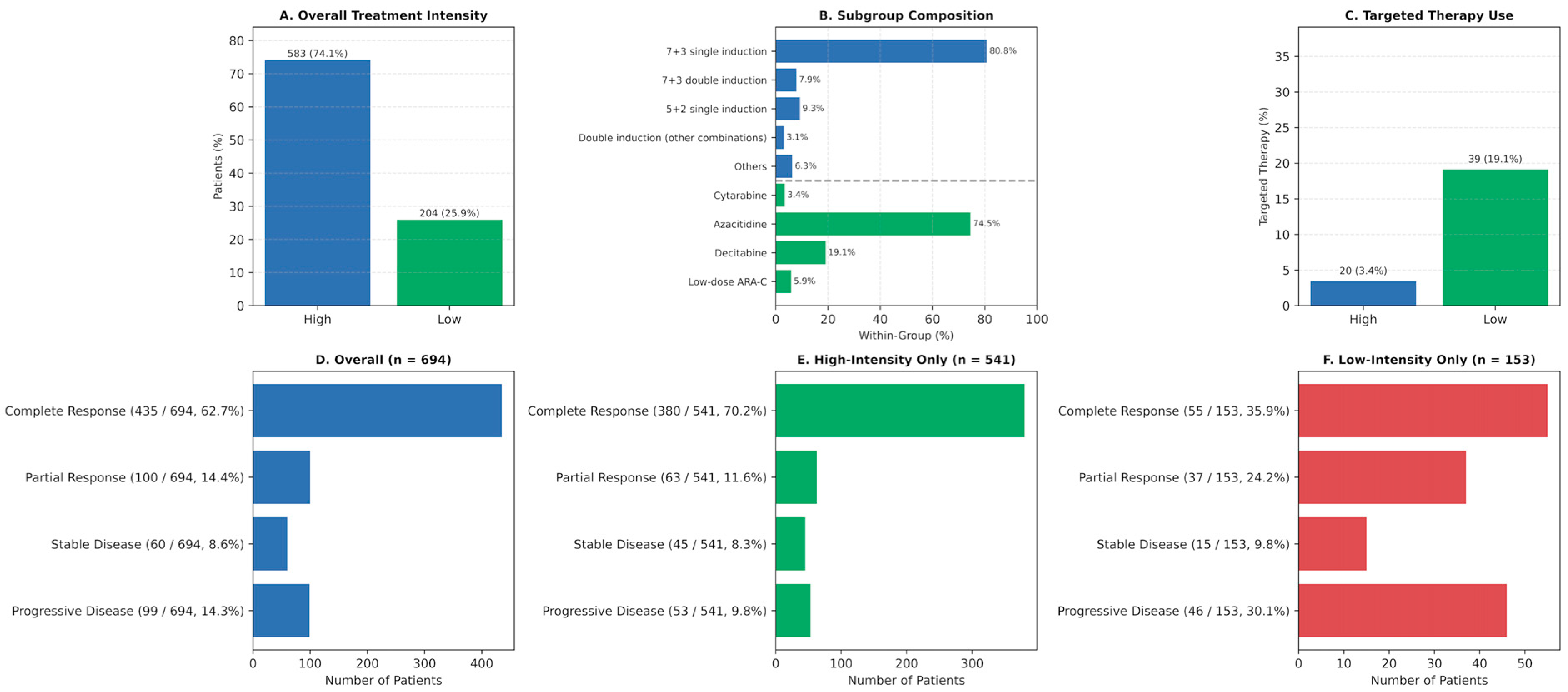
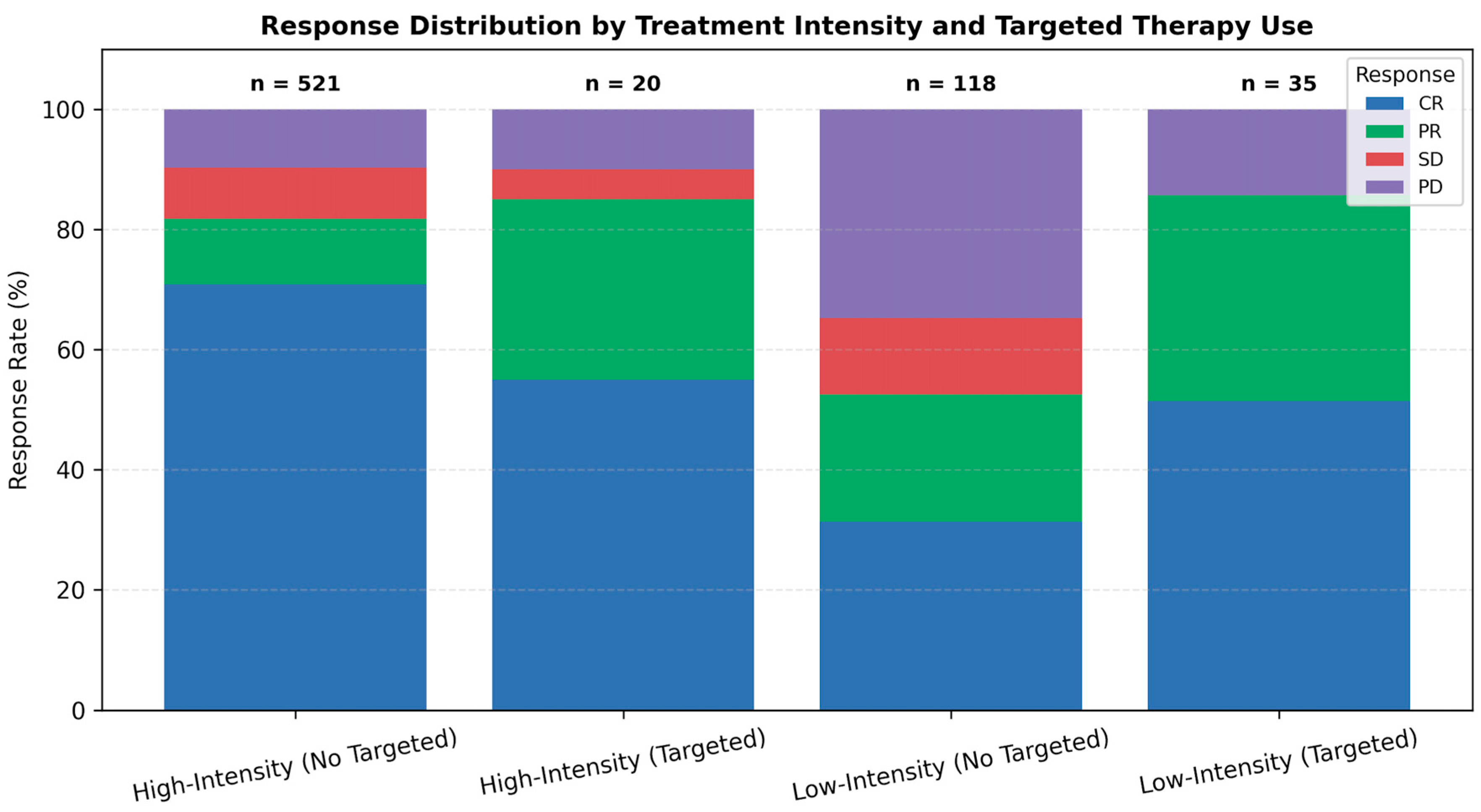
3.2. Treatment Distribution and Targeted Therapy Use
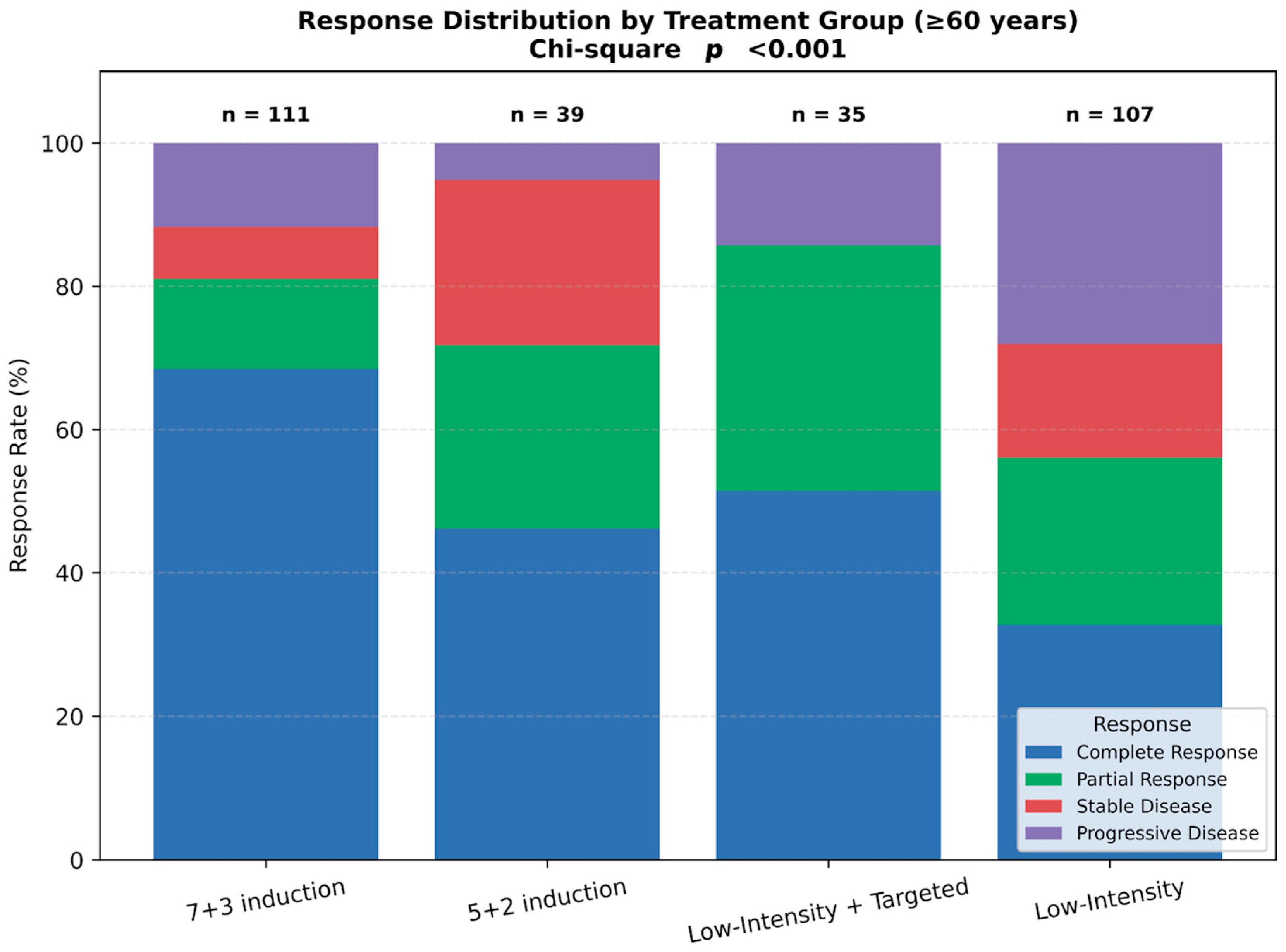
3.3. Response Rates
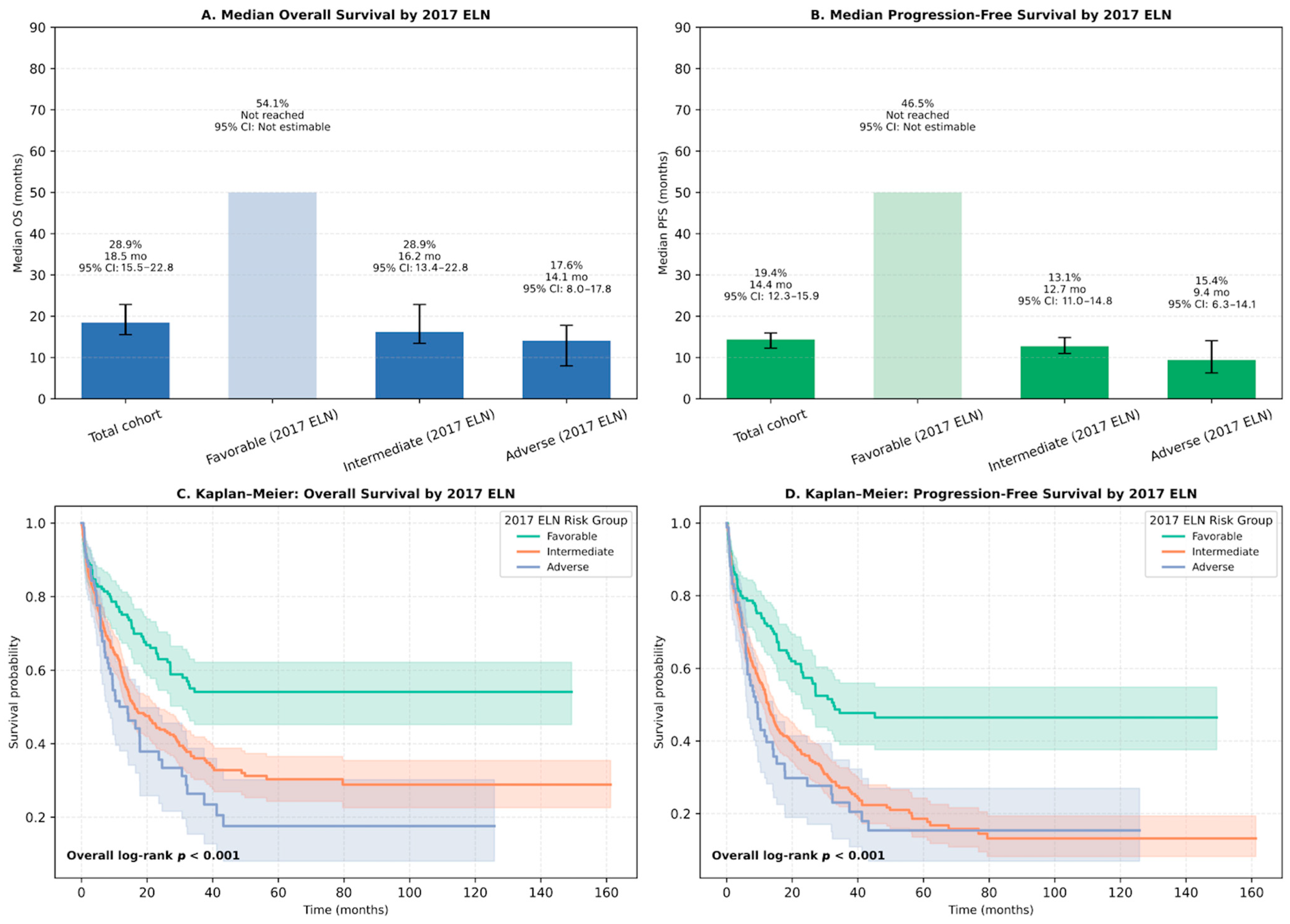
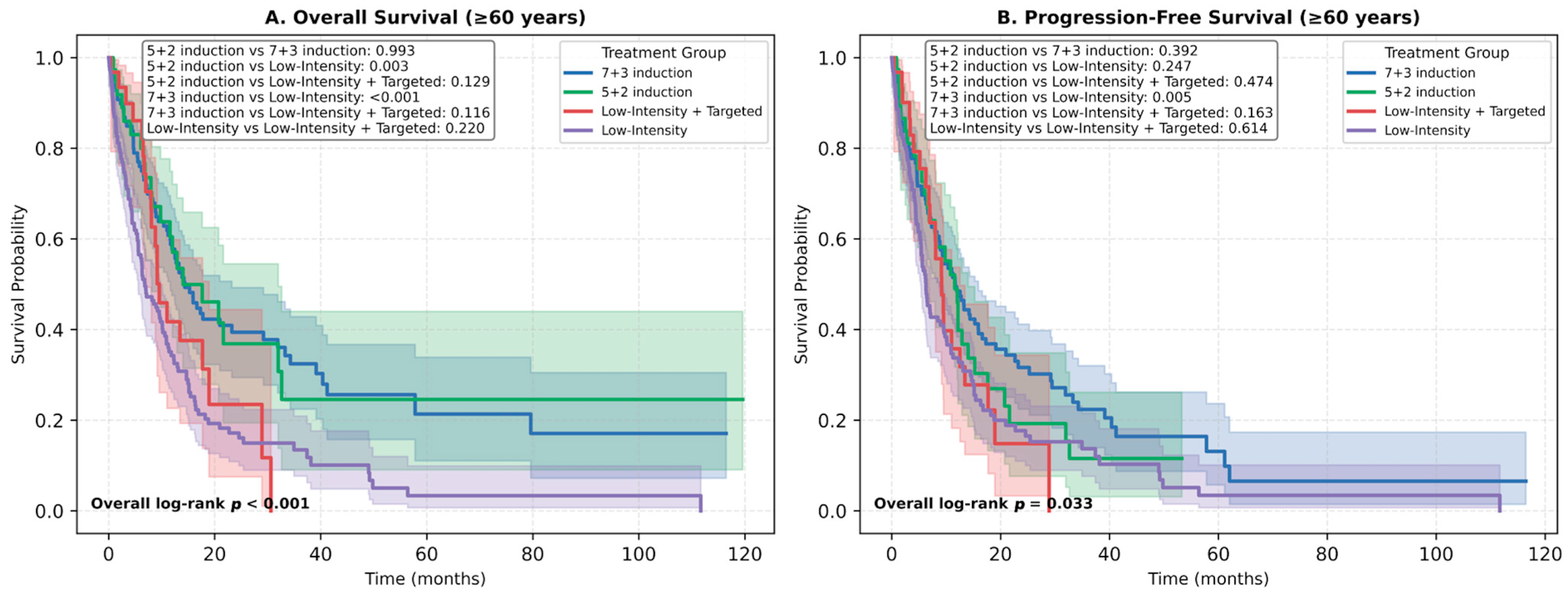
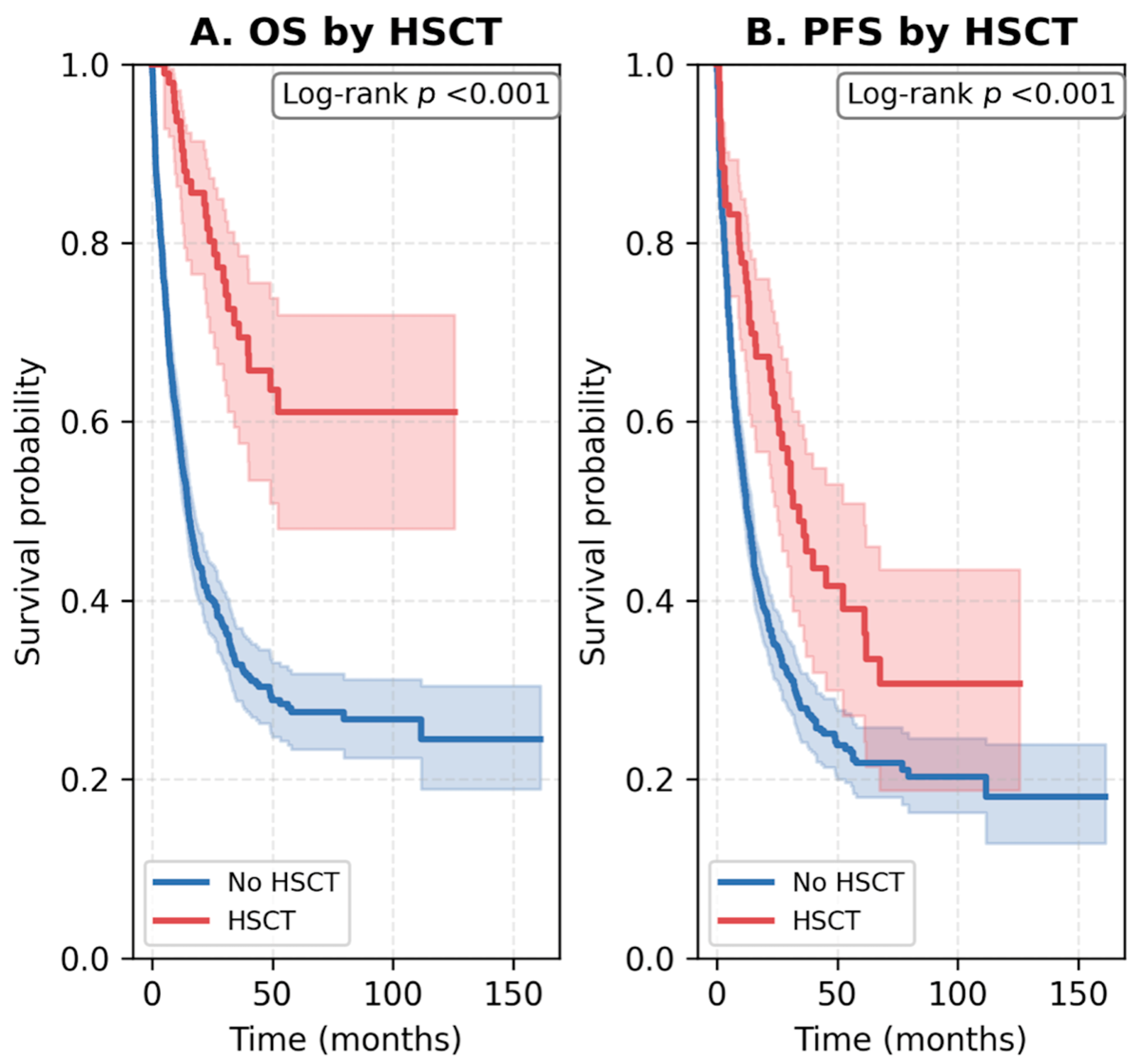
3.4. Survival Outcomes
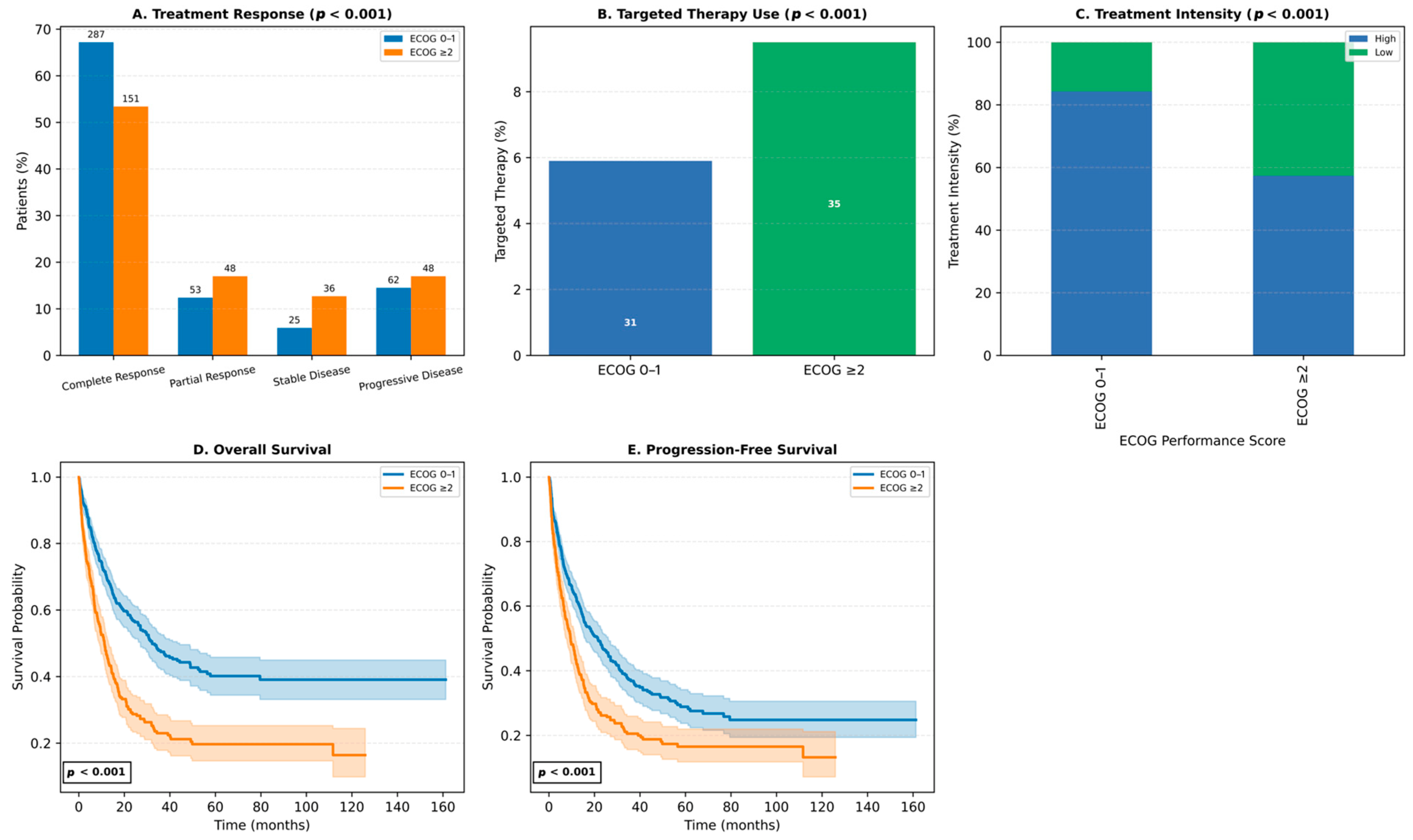
3.5. Impact of ECOG Performance Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DiNardo, C.D.; Erba, H.P.; Freeman, S.D.; Wei, A.H. Acute myeloid leukaemia. Lancet 2023, 401, 2073–2086. [Google Scholar] [CrossRef]
- Shimony, S.; Stahl, M.; Stone, R.M. Acute myeloid leukemia: 2023 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2023, 98, 502–526. [Google Scholar] [CrossRef]
- National Cancer Institute. SEER Cancer Stat Facts: Acute Myeloid Leukemia. Available online: https://seer.cancer.gov/statfacts/html/amyl.html (accessed on 4 August 2024).
- Talati, C.; Sweet, K. Recently approved therapies in acute myeloid leukemia: A complex treatment landscape. Leuk. Res. 2018, 73, 58–66. [Google Scholar] [CrossRef]
- Chen, P.; Liu, X.; Zhao, Y.; Hu, Y.; Guo, J.; Wang, H. Global, national, and regional burden of acute myeloid leukemia among 60–89 years-old individuals: Insights from a study covering the period 1990 to 2019. Front. Public Health 2024, 11, 1329529. [Google Scholar] [CrossRef]
- Yi, M.; Li, A.; Zhou, L.; Chu, Q.; Song, Y.; Wu, K. The global burden and attributable risk factor analysis of acute myeloid leukemia in 195 countries and territories from 1990 to 2017: Estimates based on the global burden of disease study 2017. J. Hematol. Oncol. 2020, 13, 72. [Google Scholar] [CrossRef]
- Han, H.J.; Choi, K.; Suh, H.S. Impact of aging on acute myeloid leukemia epidemiology and survival outcomes: A real-world, population-based longitudinal cohort study. PLoS ONE 2024, 19, e0300637. [Google Scholar] [CrossRef]
- Pollyea, D.A.; Altman, J.K.; Assi, R.; Bixby, D.; Fathi, A.T.; Foran, J.M.; Gojo, I.; Hall, A.C.; Jonas, B.A.; Kishtagari, A. Acute myeloid leukemia, version 3.2023, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 503–513. [Google Scholar] [CrossRef]
- Burnett, A.K.; Milligan, D.; Prentice, A.G.; Goldstone, A.H.; McMullin, M.F.; Hills, R.K.; Wheatley, K. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2007, 109, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Bittar, G.; De Oliveira-Gomes, D.; Rivero, G. Advances and Future Goals in Acute Myeloid Leukaemia Therapy. Oncol. Haematol. 2022, 18, 130–138. [Google Scholar] [CrossRef]
- Meillon-Garcia, L.A.; Demichelis-Gómez, R. Access to Therapy for Acute Myeloid Leukemia in the Developing World: Barriers and Solutions. Curr. Oncol. Rep. 2020, 22, 125. [Google Scholar] [CrossRef] [PubMed]
- Bhansali, R.S.; Pratz, K.W.; Lai, C. Recent advances in targeted therapies in acute myeloid leukemia. J. Hematol. Oncol. 2023, 16, 29. [Google Scholar] [CrossRef]
- Bazinet, A.; Kantarjian, H.; Arani, N.; Popat, U.; Bataller, A.; Sasaki, K.; DiNardo, C.D.; Daver, N.; Yilmaz, M.; Abbas, H.A.; et al. Evolving trends and outcomes in older patients with acute myeloid leukemia including allogeneic stem cell transplantation. Am. J. Hematol. 2023, 98, 1383–1393. [Google Scholar] [CrossRef]
- Kantarjian, H.; Borthakur, G.; Daver, N.; DiNardo, C.D.; Issa, G.; Jabbour, E.; Kadia, T.; Sasaki, K.; Short, N.J.; Yilmaz, M.; et al. Current status and research directions in acute myeloid leukemia. Blood Cancer J. 2024, 14, 163. [Google Scholar] [CrossRef]
- O’Donnell, M.R.; Abboud, C.N.; Altman, J.; Appelbaum, F.R.; Arber, D.A.; Attar, E.; Borate, U.; Coutre, S.E.; Damon, L.E.; Goorha, S. Acute myeloid leukemia. J. Natl. Compr. Cancer Netw. 2012, 10, 984–1021. [Google Scholar] [CrossRef]
- Brandwein, J.M.; Saini, L.; Geddes, M.N.; Yusuf, D.; Liu, F.; Schwann, K.; Billawala, A.; Westcott, C.; Kurniawan, J.A.; Cheung, W.Y. Outcomes of patients with relapsed or refractory acute myeloid leukemia: A population-based real-world study. Am. J. Blood Res. 2020, 10, 124. [Google Scholar] [PubMed]
- Dombret, H.; Seymour, J.F.; Butrym, A.; Wierzbowska, A.; Selleslag, D.; Jang, J.H.; Kumar, R.; Cavenagh, J.; Schuh, A.C.; Candoni, A. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood J. Am. Soc. Hematol. 2015, 126, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.M.; Thomas, X.G.; Dmoszynska, A.; Wierzbowska, A.; Mazur, G.; Mayer, J.; Gau, J.-P.; Chou, W.-C.; Buckstein, R.; Cermak, J. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J. Clin. Oncol. 2012, 30, 2670–2677. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood J. Am. Soc. Hematol. 2017, 129, 424–447. [Google Scholar] [CrossRef]
- Liesveld, J. Management of AML: Who do we really cure? Leuk. Res. 2012, 36, 1475–1480. [Google Scholar] [CrossRef]
- Ciftciler, R.; Demiroglu, H.; Haznedaroglu, I.C.; Sayınalp, N.; Aksu, S.; Ozcebe, O.; Goker, H.; Aydın, M.S.; Buyukasık, Y. Impact of time between induction chemotherapy and complete remission on survival outcomes in patients with acute myeloid leukemia. Clin. Lymphoma Myeloma Leuk. 2019, 19, 729–734. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Huang, H.-H.; Chen, H.-M.; Hsiao, F.-Y.; Ko, B.-S. Real-world outcomes of patients with acute myeloid leukemia in Taiwan: A nationwide population-based study, 2011–2015. Clin. Lymphoma Myeloma Leuk. 2021, 21, e649–e657. [Google Scholar] [CrossRef] [PubMed]
- Shallis, R.M.; Boddu, P.C.; Bewersdorf, J.P.; Zeidan, A.M. The golden age for patients in their golden years: The progressive upheaval of age and the treatment of newly-diagnosed acute myeloid leukemia. Blood Rev. 2020, 40, 100639. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo Segura, S.; Schlenk, R.F. Update on current treatments for adult acute myeloid leukemia. Haematologica 2023, 108, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Heuser, M.; Fernandez, C.; Hauch, O.; Klibanov, O.M.; Chaudhary, T.; Rives, V. Therapies for acute myeloid leukemia in patients ineligible for standard induction chemotherapy: A systematic review. Future Oncol. 2023, 19, 789–810. [Google Scholar] [CrossRef] [PubMed]
- Juliusson, G.; Lazarevic, V.; Hörstedt, A.-S.; Hagberg, O.; Höglund, M.; Swedish Acute Leukemia Registry Group. Acute myeloid leukemia in the real world: Why population-based registries are needed. Blood 2012, 119, 3890–3899. [Google Scholar] [CrossRef]
- Østgård, L.S.; Nørgaard, J.M.; Raaschou-Jensen, K.K.; Pedersen, R.S.; Rønnov-Jessen, D.; Pedersen, P.T.; Dufva, I.H.; Marcher, C.W.; Nielsen, O.J.; Severinsen, M.T.; et al. The Danish National Acute Leukemia Registry. Clin. Epidemiol. 2016, 8, 553–560. [Google Scholar] [CrossRef]
- Campos, C.; Orathes Ponte Silva, A.M.; Feistauer, F.; Teixeira Da Silva, L.; Favano, T.; Salvino, M.A. 10-Year Real-World Data on Acute Myeloid Leukemia. J. Bone Marrow Transplant. Cell. Ther. 2024, 5, 245. [Google Scholar] [CrossRef]
- Burd, A.; Levine, R.L.; Ruppert, A.S.; Mims, A.S.; Borate, U.; Stein, E.M.; Patel, P.A.; Baer, M.R.; Stock, W.; Deininger, M.W. Precision medicine treatment in older AML: Results of Beat AML Master Trial. Blood 2019, 134, 175. [Google Scholar] [CrossRef]
- Sahasrabudhe, K.; Huang, Y.; Rebechi, M.; Elder, P.; Mims, A.; Wall, S. Survival, response rates, and post-transplant outcomes in patients with acute myeloid leukemia aged 60–75 treated with high intensity chemotherapy vs. lower intensity targeted therapy. Front. Oncol. 2022, 12, 1017194. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; Kadia, T.M.; DiNardo, C.D.; Welch, M.A.; Ravandi, F. Acute myeloid leukemia: Treatment and research outlook for 2021 and the MD Anderson approach. Cancer 2021, 127, 1186–1207. [Google Scholar] [CrossRef]
- Bhatt, V.R. Advances and unanswered questions in management of acute myeloid leukemia in older adults: A glimpse into the future. J. Geriatr. Oncol. 2021, 12, 980–984. [Google Scholar] [CrossRef]
- Göker, H.; Çınar, O.E.; Demiroğlu, H.; Malkan, Ü.Y.; Aladağ Karakulak, E.; Büyükaşık, Y. Venetoclax and Azacitidine Treatment in Relapsed Acute Myeloid Leukemia after Hematopoietic Stem Cell Transplantation: A Cohort Study in the Real-World Setting of a Tertiary Center. Turk. J. Hematol. 2023, 40, 213–215. [Google Scholar] [CrossRef]
- Gemici, A.; Ozkalemkas, F.; Dogu, M.H.; Tekinalp, A.; Alacacioglu, I.; Guney, T.; Ince, I.; Geduk, A.; Cagliyan, G.A.; Maral, S.; et al. A Real-life Turkish Experience of Venetoclax Treatment in High-risk Myelodysplastic Syndrome and Acute Myeloid Leukemia. Clin. Lymphoma Myeloma Leuk. 2021, 21, e686–e692. [Google Scholar] [CrossRef]
- American Cancer Society. Acute Myeloid Leukemia (AML) Subtypes and Prognostic Factors. Available online: https://www.cancer.org/cancer/types/acute-myeloid-leukemia/detection-diagnosis-staging/how-classified.html (accessed on 4 August 2024).
- Bataller Torralba, A.; Garrido, A.; Guijarro, F.; Oñate, G.; Díaz-Beyá, M.; Arnan, M.; Salamero, O. European LeukemiaNet 2017 risk stratification for acute myeloid leukemia: Validation in a risk-adapted protocol. Blood Adv. 2022, 6, 1193–1206. [Google Scholar] [CrossRef]
- Marshalek, J.P.; Epistola, R.; Tomassetti, S. Real-world treatment outcomes from a retrospective cohort of patients with acute myeloid leukemia from an urban safety net hospital. J. Oncol. Pharm. Pract. 2025, 31, 182–189. [Google Scholar] [CrossRef]
- Perl, A.E.; Martinelli, G.; Cortes, J.E.; Neubauer, A.; Berman, E.; Paolini, S.; Montesinos, P.; Baer, M.R.; Larson, R.A.; Ustun, C.; et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N. Engl. J. Med. 2019, 381, 1728–1740. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.S.; Montesinos, P.; Minden, M.D.; Lee, J.-H.; Heuser, M.; Naoe, T.; Chou, W.-C.; Laribi, K.; Esteve, J.; Altman, J.K.; et al. Phase 3 trial of gilteritinib plus azacitidine vs azacitidine for newly diagnosed FLT3mut+ AML ineligible for intensive chemotherapy. Blood 2022, 140, 1845–1857. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Schuh, A.C.; Stein, E.M.; Montesinos, P.; Wei, A.H.; de Botton, S.; Zeidan, A.M.; Fathi, A.T.; Kantarjian, H.M.; Bennett, J.M. Enasidenib plus azacitidine versus azacitidine alone in patients with newly diagnosed, mutant-IDH2 acute myeloid leukaemia (AG221-AML-005): A single-arm, phase 1b and randomised, phase 2 trial. Lancet Oncol. 2021, 22, 1597–1608. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.S.; Montesinos, P.; Minden, M.D.; Lee, J.H.; Heuser, M.; Naoe, T.; Chou, W.C.; Laribi, K.; Esteve, J.; Altman, J.K.; et al. Phase 3, Open-Label, Randomized Study of Gilteritinib and Azacitidine Vs Azacitidine for Newly Diagnosed-Mutated Acute Myeloid Leukemia in Patients Ineligible for Intensive Induction Chemotherapy. Blood 2021, 138, 700. [Google Scholar] [CrossRef]
- Matthews, A.H.; Perl, A.E.; Luger, S.M.; Gill, S.I.; Lai, C.; Porter, D.L.; Skuli, S.; Bruno, X.J.; Carroll, M.P.; Freyer, C.W. Real-world effectiveness of intensive chemotherapy with 7&3 versus venetoclax and hypomethylating agent in acute myeloid leukemia. Am. J. Hematol. 2023, 98, 1254–1264. [Google Scholar] [PubMed]
- Gore, S.D.; Fenaux, P.; Santini, V.; Bennett, J.M.; Silverman, L.R.; Seymour, J.F.; Hellström-Lindberg, E.; Swern, A.S.; Beach, C.L.; List, A.F. A multivariate analysis of the relationship between response and survival among patients with higher-risk myelodysplastic syndromes treated within azacitidine or conventional care regimens in the randomized AZA-001 trial. Haematologica 2013, 98, 1067. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Sapkota, S.; Parker, S.; Defor, T.; Warlick, E.; Ustun, C.; Eckfeldt, C.; Rashidi, A.; Kurtzweil, A.; Weisdorf, D. A real-world study of clofarabine and cytarabine combination therapy for patients with acute myeloid leukemia. Leuk. Lymphoma 2018, 59, 2352–2359. [Google Scholar] [CrossRef] [PubMed]
- Shallis, R.M.; Wang, R.; Davidoff, A.; Ma, X.; Zeidan, A.M. Epidemiology of acute myeloid leukemia: Recent progress and enduring challenges. Blood Rev. 2019, 36, 70–87. [Google Scholar] [CrossRef]
- Song, X.; Peng, Y.; Wang, X.; Chen, Y.; Jin, L.; Yang, T.; Qian, M.; Ni, W.; Tong, X.; Lan, J. Incidence, survival, and risk factors for adults with acute myeloid leukemia not otherwise specified and acute myeloid leukemia with recurrent genetic abnormalities: Analysis of the surveillance, epidemiology, and end results (SEER) database, 2001–2013. Acta Haematol. 2018, 139, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Ravandi, F.; Kadia, T.M.; DiNardo, C.D.; Short, N.J.; Borthakur, G.; Jabbour, E.; Kantarjian, H.M. De novo acute myeloid leukemia: A population-based study of outcome in the United States based on the Surveillance, Epidemiology, and End Results (SEER) database, 1980 to 2017. Cancer 2021, 127, 2049–2061. [Google Scholar] [CrossRef]
- Pinar, I.E.; Celik, S.; Polat, M.G.; Karatas, A.F.; Dogan, A.; Iltar, U.; Seval, G.C.; Malkan, U.Y.; Ince, I.; Yenihayat, E.M. Idarubicin Versus Daunorubicin Versus Mitoxantrone for Induction Chemotherapy in Acute Myeloid Leukemia: Patient Registration Study of Turkish Society of Hematology-Acute Myeloid Leukemia Working Group. Blood 2023, 142, 5838. [Google Scholar] [CrossRef]
- Urlu, S.M.; Cengiz Seval, G.; Erdogan Yücel, E.; Mehtap, O.; Yenihayat, E.M.; Polat, M.G.; Malkan, U.Y.; Ozbalci, D.; Yigit Kaya, S.; Durusoy, S.S.; et al. Acute Myeloid Leukemia in Elderly, Unfit Patients: Analysis of Turkish AML Prospective Registry Database, on Behalf of Acute Leukemia Working Group of Turkish Society of Hematology. Blood 2023, 142, 5931. [Google Scholar] [CrossRef]
- Dinardo, C.D.; Stein, E.M.; De Botton, S.; Roboz, G.J.; Altman, J.K.; Mims, A.S.; Swords, R.; Collins, R.H.; Mannis, G.N.; Pollyea, D.A.; et al. Durable Remissions with Ivosidenib in IDH1-Mutated Relapsed or Refractory AML. N. Engl. J. Med. 2018, 378, 2386–2398. [Google Scholar] [CrossRef]
- Montesinos, P.; Recher, C.; Vives, S.; Zarzycka, E.; Wang, J.; Bertani, G.; Heuser, M.; Calado, R.T.; Schuh, A.C.; Yeh, S.-P.; et al. Ivosidenib and Azacitidine in IDH1-Mutated Acute Myeloid Leukemia. N. Engl. J. Med. 2022, 386, 1519–1531. [Google Scholar] [CrossRef]
- Van Weelderen, R.E.; Klein, K.; Natawidjaja, M.D.; De Vries, R.; Kaspers, G.J. Outcome of pediatric acute myeloid leukemia (AML) in low- and middle-income countries: A systematic review of the literature. Expert. Rev. Anticancer. Ther. 2021, 21, 765–780. [Google Scholar] [CrossRef]
- Guolo, F.; Fianchi, L.; Minetto, P.; Clavio, M.; Gottardi, M.; Galimberti, S.; Rizzuto, G.; Rondoni, M.; Bertani, G.; Dargenio, M. CPX-351 treatment in secondary acute myeloblastic leukemia is effective and improves the feasibility of allogeneic stem cell transplantation: Results of the Italian compassionate use program. Blood Cancer J. 2020, 10, 96. [Google Scholar] [CrossRef]
- Minetto, P.; Guolo, F.; Fianchi, L.; Clavio, M.; Gottardi, M.; Endri, M.; Galimberti, S.; Rizzuto, G.; Sciumé, M.; Rondoni, M. CPX-351 Induction in Secondary Acute Myeloblastic Leukemia: Extended Follow up from the Italian Compassionate Use Program. Blood 2021, 138, 1262. [Google Scholar] [CrossRef]
- Lemoli, R.M.; Montesinos, P.; Jain, A. Real-world experience with CPX-351 in high-risk acute myeloid leukemia. Crit. Rev. Oncol./Hematol. 2023, 185, 103984. [Google Scholar] [CrossRef] [PubMed]
- Lancet, J.E.; Uy, G.L.; Newell, L.F.; Lin, T.L.; Ritchie, E.K.; Stuart, R.K.; Strickland, S.A.; Hogge, D.; Solomon, S.R.; Bixby, D.L. CPX-351 versus 7+ 3 cytarabine and daunorubicin chemotherapy in older adults with newly diagnosed high-risk or secondary acute myeloid leukaemia: 5-year results of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol. 2021, 8, e481–e491. [Google Scholar] [CrossRef] [PubMed]
- Matthews, A.H.; Perl, A.E.; Luger, S.M.; Loren, A.W.; Gill, S.I.; Porter, D.L.; Babushok, D.V.; Maillard, I.P.; Carroll, M.P.; Frey, N.V. Real-world effectiveness of CPX-351 vs venetoclax and azacitidine in acute myeloid leukemia. Blood Adv. 2022, 6, 3997–4005. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, A.; Ebadi, M.; Colditz, G.A.; DiPersio, J.F. Outcomes of Allogeneic Stem Cell Transplantation in Elderly Patients with Acute Myeloid Leukemia: A Systematic Review and Meta-analysis. Biol. Blood Marrow Transplant. 2016, 22, 651–657. [Google Scholar] [CrossRef]
- Tokaz, M.C.; Baldomero, H.; Cowan, A.J.; Saber, W.; Greinix, H.; Koh, M.B.C.; Kröger, N.; Mohty, M.; Galeano, S.; Okamoto, S.; et al. An Analysis of the Worldwide Utilization of Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia. Transplant. Cell. Ther. 2023, 29, e271–e279. [Google Scholar] [CrossRef]
- Begna, K.H.; Kittur, J.; Gangat, N.; Alkhateeb, H.; Patnaik, M.S.; Al-Kali, A.; Elliott, M.A.; Hogan, W.J.; Litzow, M.R.; Pardanani, A.; et al. European LeukemiaNet-defined primary refractory acute myeloid leukemia: The value of allogeneic hematopoietic stem cell transplant and overall response. Blood Cancer J. 2022, 12, 7. [Google Scholar] [CrossRef]
- Masetti, R.; Muratore, E.; Gori, D.; Prete, A.; Locatelli, F. Allogeneic hematopoietic stem cell transplantation for pediatric acute myeloid leukemia in first complete remission: A meta-analysis. Ann. Hematol. 2022, 101, 2497–2506. [Google Scholar] [CrossRef]
- Servais, S.; Beguin, Y.; Baron, F. Current Status and Perspectives of Allogeneic Hematopoietic Stem Cell Transplantation in Elderly Patients with Acute Myeloid Leukemia. Stem Cells Transl. Med. 2022, 11, 461–477. [Google Scholar] [CrossRef]
- Backhaus, D.; Brauer, D.; Pointner, R.; Bischof, L.; Vucinic, V.; Franke, G.-N.; Niederwieser, D.; Platzbecker, U.; Jentzsch, M.; Schwind, S. A high hematopoietic cell transplantation comorbidity Index (HCT-CI) does not impair outcomes after non-myeloablative allogeneic stem cell transplantation in acute myeloid leukemia patients 60 years or older. Bone Marrow Transplant. 2023, 58, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-H.; Lian, X.-Y.; Yao, D.-M.; He, P.-F.; Ma, J.-C.; Xu, Z.-J.; Guo, H.; Zhang, W.; Lin, J.; Qian, J. Reduced intensity conditioning of allogeneic hematopoietic stem cell transplantation for myelodysplastic syndrome and acute myeloid leukemia in patients older than 50 years of age: A systematic review and meta-analysis. J. Cancer Res. Clin. Oncol. 2017, 143, 1853–1864. [Google Scholar] [CrossRef] [PubMed]
- Mohty, R.; El Hamed, R.; Brissot, E.; Bazarbachi, A.; Mohty, M. New drugs before, during, and after hematopoietic stem cell transplantation for patients with acute myeloid leukemia. Haematologica 2023, 108, 321–341. [Google Scholar] [CrossRef] [PubMed]
- Perl, A.E.; Larson, R.A.; Podoltsev, N.A.; Strickland, S.; Wang, E.S.; Schiller, G.J.; Martinelli, G.; Neubauer, A.; Sierra, J.; Montesinos, P. Follow-up of patients with FLT3-mutated R/R AML in the phase 3 ADMIRAL trial. J. Clin. Oncol. 2021, 39, 7013. [Google Scholar] [CrossRef]
- Pratz, K.W.; DiNardo, C.D.; Arellano, M.L.; Letai, A.G.; Thirman, M.; Pullarkat, V.A.; Roboz, G.J.; Becker, P.S.; Hong, W.-J.; Jiang, Q. Outcomes after stem cell transplant in older patients with acute myeloid leukemia treated with venetoclax-based therapies. Blood 2019, 134, 264. [Google Scholar] [CrossRef]
- Kennedy, V.E.; Hui, G.; Gaut, D.; Mittal, V.; Oliai, C.; Muffly, L.S.; Logan, A.C.; Mannis, G.N. Hypomethylating agents in combination with venetoclax as a bridge to allogeneic transplant in acute myeloid leukemia. Blood 2020, 136, 32–33. [Google Scholar] [CrossRef]
- Gupta, V.; Tallman, M.S.; Weisdorf, D.J. Allogeneic hematopoietic cell transplantation for adults with acute myeloid leukemia: Myths, controversies, and unknowns. Blood J. Am. Soc. Hematol. 2011, 117, 2307–2318. [Google Scholar] [CrossRef]
- Maakaron, J.E.; Zhang, M.-J.; Chen, K.; Abhyankar, S.; Bhatt, V.R.; Chhabra, S.; El Jurdi, N.; Farag, S.S.; He, F.; Juckett, M. Age is no barrier for adults undergoing HCT for AML in CR1: Contemporary CIBMTR analysis. Bone Marrow Transplant. 2022, 57, 911–917. [Google Scholar] [CrossRef]
- Pinar, I.E.; Celik, S.; Polat, M.G.; Karatas, A.F.; Dogan, A.; Iltar, U.; Cengiz Seval, G.; Malkan, U.Y.; Ince, I.; Yenihayat, E.M.; et al. Comprehensive analysis of FLT3-mutated patients with acute myeloid leukemia with updated 2022 European LeukemiaNet recommendations: Insights from the Turkish AML registry project. BMC Cancer 2025, 25, 1546. [Google Scholar] [CrossRef]
| Age (Year), Median (Min–Max) | 58 (18–91) |
| Gender, n (%) | |
| Female | 402 (45.1) |
| Male | 489 (54.9) |
| WHO subtype, % | |
| AML with recurrent genetic abnormalities | 17.6 |
| AML with myelodysplasia-related changes | 15.4 |
| Therapy-related myeloid neoplasms | 0.8 |
| AML not otherwise specified | 55.8 |
| Myeloid sarcoma | 0.7 |
| Acute leukemias of ambiguous lineage | 9.7 |
| ELN 2017 risk stratification, n (%) | |
| Favorable | 169 (24.7) |
| Intermediate | 416 (60.8) |
| Adverse | 99 (14.5) |
| Missing | 207 |
| Extramedullary involvement, n (%) | |
| Yes | 38 (4.4) |
| No | 834 (95.6) |
| Missing | 19 |
| ECOG at diagnosis | |
| 0–1 | 524 (63.1) |
| 2–4 | 306 (36.9) |
| Missing | 61 |
| FLT3-ITD mutation status | |
| Mutated | 62 (15.7) |
| Unmutated | 332 (84.3) |
| Missing | 497 |
| NPM1 mutation status | |
| Mutated | 55 (20.1) |
| Unmutated | 219 (79.9) |
| Missing | 617 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakus, V.; Pinar, I.E.; Iltar, U.; Yenihayat, E.M.; Polat, M.G.; Celik, S.; Malkan, U.Y.; Cengiz Seval, G.; Dogan, A.; Akdeniz, A.; et al. Clinical Characteristics, Treatment Approaches, and Survival Predictors in Adult Acute Myeloid Leukemia: Interim Results from the Turkish Society of Hematology AML Registry. J. Clin. Med. 2025, 14, 7367. https://doi.org/10.3390/jcm14207367
Karakus V, Pinar IE, Iltar U, Yenihayat EM, Polat MG, Celik S, Malkan UY, Cengiz Seval G, Dogan A, Akdeniz A, et al. Clinical Characteristics, Treatment Approaches, and Survival Predictors in Adult Acute Myeloid Leukemia: Interim Results from the Turkish Society of Hematology AML Registry. Journal of Clinical Medicine. 2025; 14(20):7367. https://doi.org/10.3390/jcm14207367
Chicago/Turabian StyleKarakus, Volkan, Ibrahim Ethem Pinar, Utku Iltar, Emel Merve Yenihayat, Merve Gokcen Polat, Serhat Celik, Umit Yavuz Malkan, Guldane Cengiz Seval, Ali Dogan, Aydan Akdeniz, and et al. 2025. "Clinical Characteristics, Treatment Approaches, and Survival Predictors in Adult Acute Myeloid Leukemia: Interim Results from the Turkish Society of Hematology AML Registry" Journal of Clinical Medicine 14, no. 20: 7367. https://doi.org/10.3390/jcm14207367
APA StyleKarakus, V., Pinar, I. E., Iltar, U., Yenihayat, E. M., Polat, M. G., Celik, S., Malkan, U. Y., Cengiz Seval, G., Dogan, A., Akdeniz, A., Ozbalci, D., Ince, I., Erdem, R., Mehtap, O., Kirkizlar, H. O., Kacmaz, M., Deveci, B., Aykas, F., Korkmaz, G., ... Alacacioglu, I., on behalf of the Turkish Society of Hematology-Acute Leukemias Working Group. (2025). Clinical Characteristics, Treatment Approaches, and Survival Predictors in Adult Acute Myeloid Leukemia: Interim Results from the Turkish Society of Hematology AML Registry. Journal of Clinical Medicine, 14(20), 7367. https://doi.org/10.3390/jcm14207367






