Neoadjuvant Chemotherapy for Early Breast Cancer: A Study on Response Rate and Toxicity
Abstract
1. Introduction
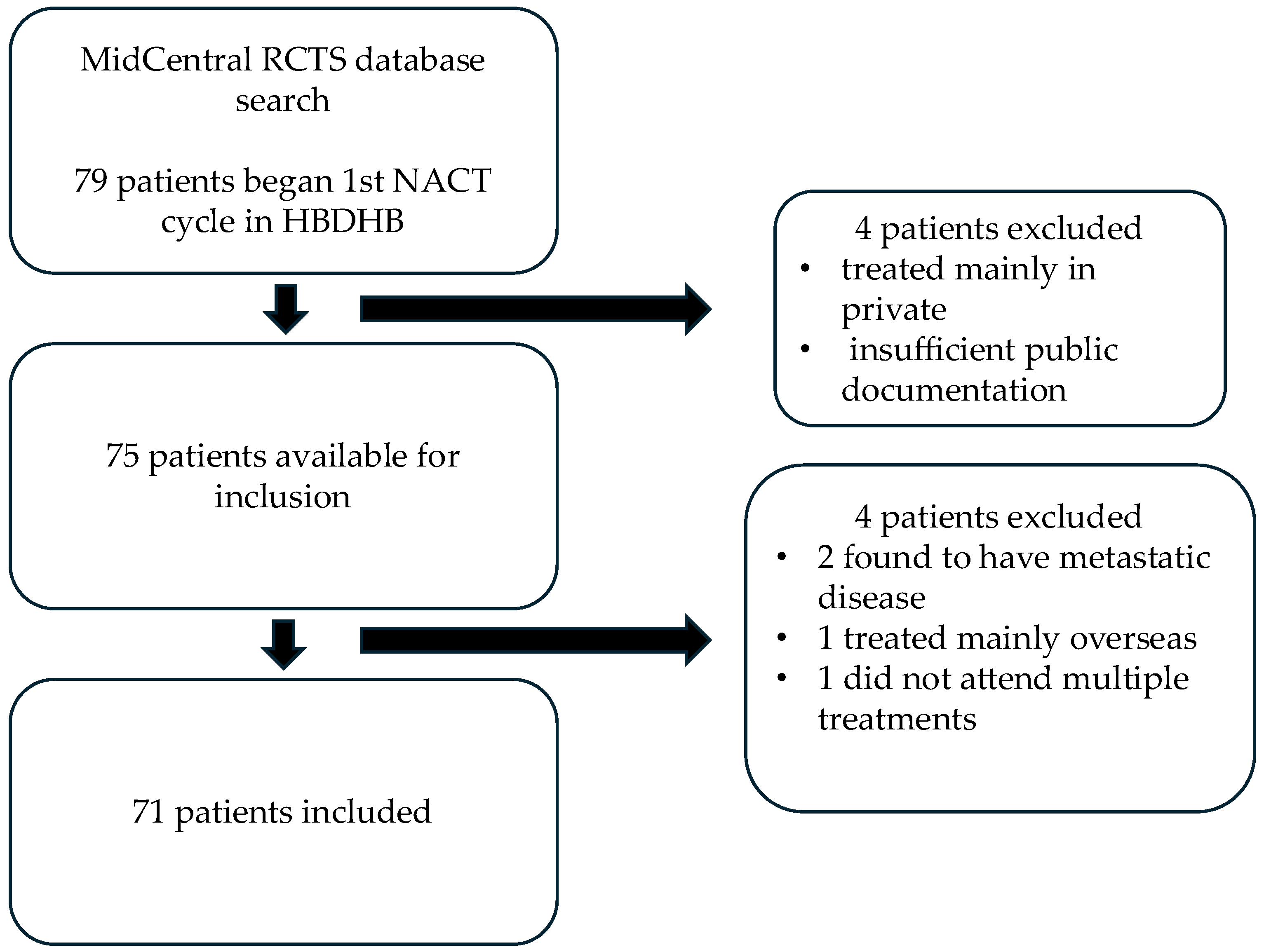
2. Method
2.1. Patient Recruitment
2.2. Data Collection
2.3. Process Clarifications and Definitions
3. Results
3.1. pCR Rates
3.2. Severe NACT Toxicity
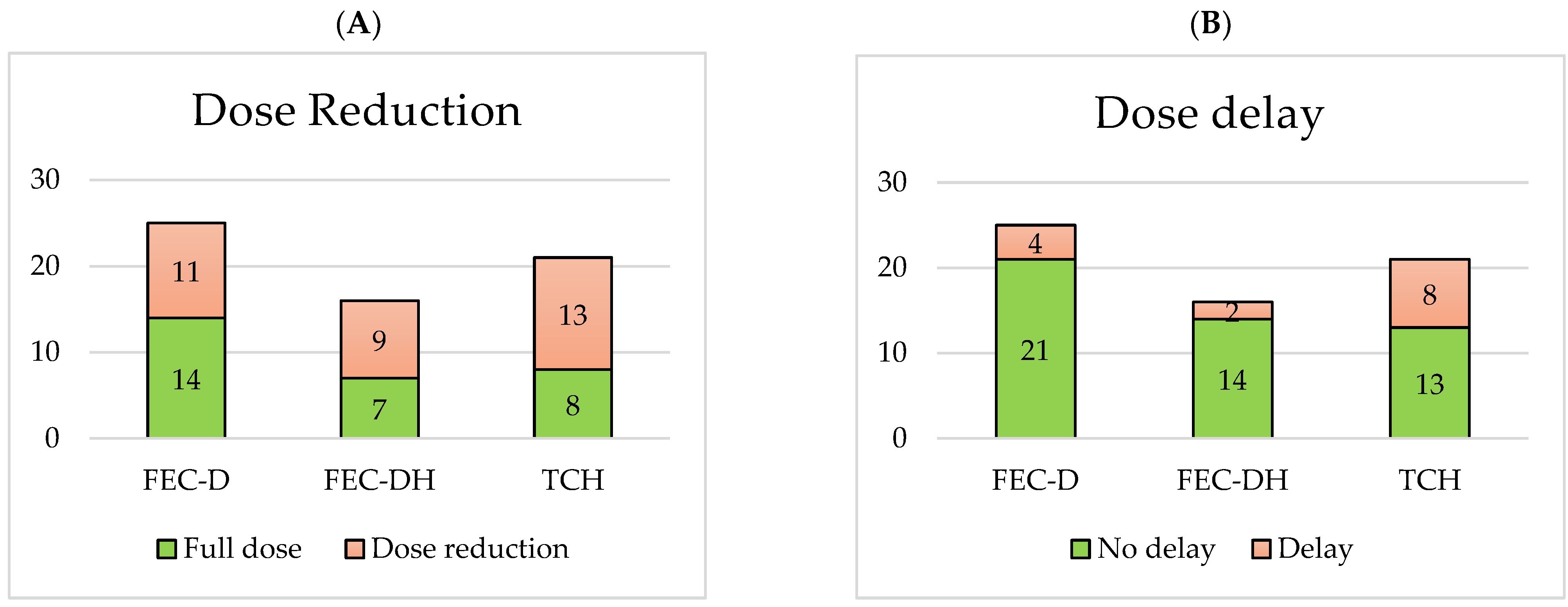
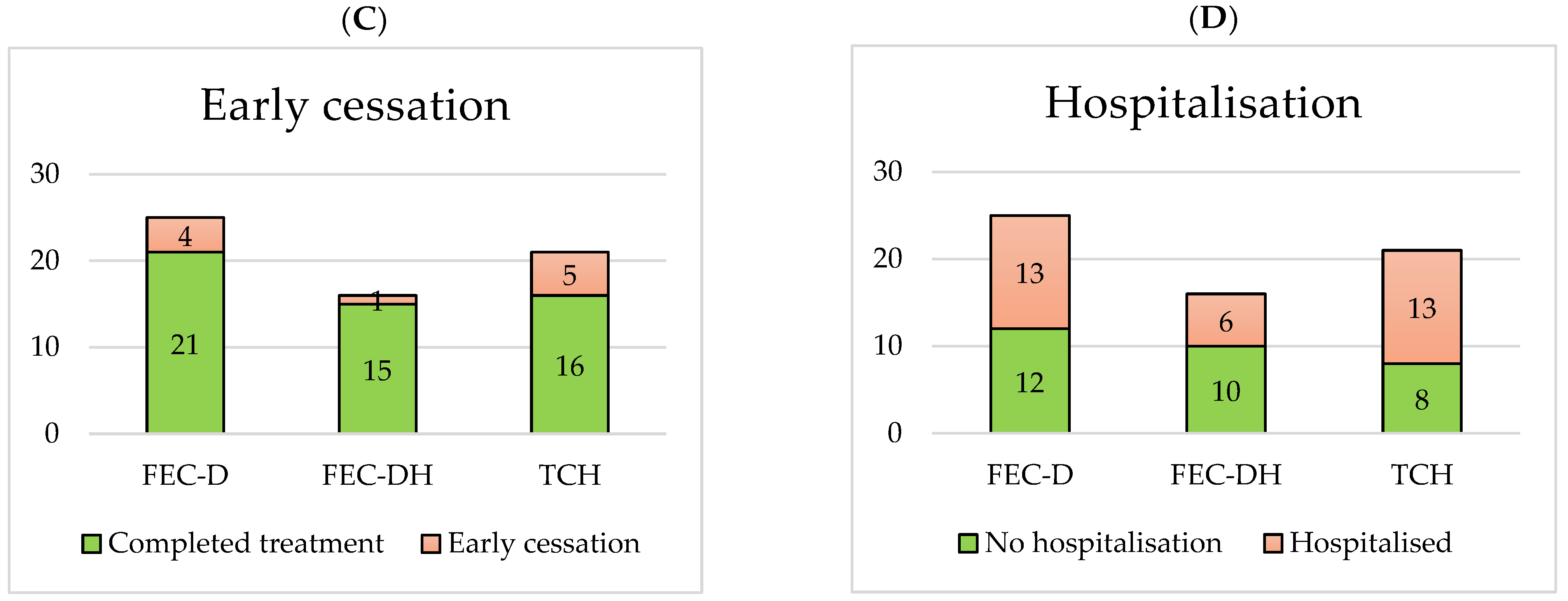
3.3. Treatment-Limiting NACT Toxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korde, L.A.; Sommerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Shelley Hwang, E.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Onc. 2021, 39, 1485–1505. [Google Scholar] [CrossRef]
- Ang, E.; Wewala, N.; Carrol, R.; Forgeson, G.; Anderson, M.; Fernando, J.; Jordan, J.; Isaacs, R. Neoadjuvant chemotherapy in non-metastatic breast cancer: A study on practice trends in a regional cancer treatment service. Intern. Med. J. 2020, 50, 315–321. [Google Scholar] [CrossRef]
- Buzatto, I.P.C.; Ribeiro-Silva, A.; Andrade, J.M.; Carrara, H.H.A.; Silveira, W.A.; Tiezzi, D.G. Neoadjuvant chemotherapy with trastuzumab in HER2-positive breast cancer: Pathologic complete response rate, predictive and prognostic factors. Braz. J. Med. Biol. Res. 2017, 50, e5674. [Google Scholar] [CrossRef]
- Pusztai, L.; Denkert, C.; O’Shaughnessy, J.; Cortes, J.; Dent, R.; McArthur, H.; Kummel, S.; Bergh, J.; Park, Y.H.; Hui, R.; et al. Event-free survival by residual cancer burden with pembrolizumab in early-stage TNBC: Exploratory analysis from KEYNOTE-522. Ann. Oncol. 2024, 35, 429–436. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: Meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018, 19, 27–39. [Google Scholar] [CrossRef]
- Feng, K.; Jia, Z.; Liu, G.; Xing, Z.; Li, J.; Li, F.; Ren, F.; Wu, J.; Wang, W.; Wang, J.; et al. A review of studies on omitting surgery after neoadjuvant chemotherapy in breast cancer. Am. J. Cancer Res. 2022, 12, 3512–3531. [Google Scholar]
- Muller, C.; Juhasz-Boss, I.; Schmidt, G.; Jungmann, P.; Solomayer, E.F.; Breitbach, G.P.; Juhasz-Boss, S. Factors influencing the time to surgery after neoadjuvant chemotherapy in breast cancer patients. Arch. Gyneocl. Obstet. 2020, 301, 1055–1059. [Google Scholar] [CrossRef]
- Wang, D.; Sun, X.; Sun, W.; Wang, R.; Pan, H.; Zhou, W. Influence of surgical timing post-neoadjuvant chemotherapy on survival outcomes in breast cancer patients: A comprehensive systematic review and meta-analysis. Breast 2025, 81, 104454. [Google Scholar] [CrossRef]
- Sikov, W.M.; Polley, M.Y.; Twohy, E.; Perou, C.M.; Singh, B.; Berry, D.A.; Tolaney, S.M.; Somlo, G.; Port, E.; Ma, C.; et al. CALGB (Alliance) 40603: Long-term outcomes (LTOs) after neoadjuvant chemotherapy (NACT) +/− carboplatin (Cb) and bevacizumab (Bev) in triple-negative breast cancer (TNBC). J. Clin. Oncol. 2019, 37, 591. [Google Scholar] [CrossRef]
- Budman, D.R.; Berry, D.A.; Cirrincione, C.T.; Henderson, I.C.; Wood, W.C.; Weiss, R.B.; Reree, C.R.; Muss, H.B.; Green, M.; Norton, L.; et al. Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The Cancer and Leukaemia Group B. J. Natl. Cancer Inst. 1998, 90, 1205–1211. [Google Scholar] [CrossRef]
- Spring, L.M.; Fell, G.; Arfe, A.; Sharma, C.; Greenup, R.; Reynolds, K.L.; Smith, B.L.; Alexander, B.; Moy, B.; Isakoff, S.J.; et al. Pathological complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: A comprehensive meta-analysis. Clin. Cancer Res. 2020, 26, 2838–2848. [Google Scholar] [CrossRef]
- Holanek, M.; Selingerova, I.; Bilek, O.; Kazda, T.; Fabian, P.; Foretova, L.; Zvarikova, M.; Obermannova, R.; Kolouskova, I.; Coufal, O.; et al. Neoadjuvant Chemotherapy of Triple-Negative Breast Cancer: Evaluation of Early Clinical Response, Pathological Complete Response Rates, and Addition of Platinum Salts Benefit Based on Real-World Evidence. Cancers 2021, 13, 1586. [Google Scholar] [CrossRef]
- Fayanju, O.M.; Ren, Y.; Thomas, S.M.; Greenup, R.A.; Plichta, J.K.; Rosenberger, L.H.; Tamirisa, N.; Force, J.; Boughey, J.C.; Hyslop, T.; et al. The Clinical Significance of Breast-only and Node-only Pathologic Complete Response (pCR) After Neoadjuvant Chemotherapy (NACT): A Review of 20,000 Breast Cancer Patients in the National Cancer Data Base (NCDB). Ann. Surg. 2018, 268, 591–601. [Google Scholar] [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Park, Y.H.; Ahn, J.S.; Im, Y.H.; Nam, S.J.; Cho, S.Y.; Cho, E.Y. Evaluation of Pathologic Complete Response in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy: Experience in a Single Institution over a 10-Year Period. J. Pathol. Transl. Med. 2016, 51, 69–78. [Google Scholar] [CrossRef] [PubMed]
- von Minckwitz, G.; Huang, C.S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Masuda, N.; Lee, S.J.; Ohtani, S.; Im, Y.H.; Lee, E.S.; Yokota, I.; Kuroi, K.; Im, S.A.; Park, B.W.; Kim, S.B.; et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef]
- UpToDate: Choice of Neoadjuvant Chemotherapy for Triple-Negative Breast Cancer. Available online: https://www.uptodate.com/contents/choice-of-neoadjuvant-chemotherapy-for-triple-negative-breast-cancer (accessed on 21 June 2025).
- Blum, J.L.; Flynn, P.J.; Yothers, G.; Asmar, L.; Geyer, C.E., Jr.; Jacobs, S.A.; Robert, N.J.; Hopkins, J.O.; O’Shaughnessy, J.A.; Dang, C.T.; et al. Anthracyclines in Early Breast Cancer: The ABC Trials—USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology). J. Clin. Oncol. 2017, 35, 2647–2655. [Google Scholar] [CrossRef]
- Loibl, S.; Weber, K.E.; Timms, K.M.; Elkin, E.P.; Hahnen, E.; Fasching, P.A.; Lederer, B.; Denkert, C.; Schneeweiss, A.; Braun, S.; et al. Survival analysis of carboplatin added to an anthracycline/taxane-based neoadjuvant chemotherapy and HRD score as predictor of response-final results from GeparSixto. Ann. Oncol. 2018, 29, 2341–2347. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Dent, R.; McArthur, H.; Pusztai, L.; Kummel, S.; Denkert, C.; Park, Y.H.; Hui, R.; Hardbek, N.; et al. Overall Survival with Pembrolizumab in Early-Stage Triple-Negative Breast Cancer. N. Engl. J. Med. 2024, 391, 1981–1991. [Google Scholar] [CrossRef]
- UpToDate: Neoadjuvant Therapy for Patients with HER2-Positive Breast Cancer. Available online: https://www.uptodate.com/contents/neoadjuvant-therapy-for-patients-with-her2-positive-breast-cancer (accessed on 21 June 2025).
- Untch, M.; Rezai, M.; Loibl, S.; Fasching, P.A.; Huober, J.; Tesch, H.; Bauerfeind, I.; Hilfrich, L.; Eidtmann, H.; Gerber, B.; et al. Neoadjuvant Treatment With Trastuzumab in HER2-Positive Breast Cancer: Results From the GeparQuattro Study. J. Clin. Oncol. 2010, 28, 2024–2032. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Anthracycline-containing and taxane-containing chemotherapy for early-stage operable breast cancer: A patient-level meta-analysis of 100,000 women from 86 randomised trials. Lancet 2023, 401, 1277–1292. [Google Scholar] [CrossRef] [PubMed]
- van Ramshorst, M.S.; van der Voort, A.; van Werkhoven, E.D.; Mandjes, I.A.; Kemper, I.; Dezentje, V.O.; Oving, I.M.; Honkoop, A.H.; Tick, L.W.; van de Wouw, A.J.; et al. Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1630–1640. [Google Scholar] [CrossRef] [PubMed]
- Volkova, M.; Russell III, R. Anthracycline Cardiotoxicity: Prevalence, Pathogenesis and Treatment. Curr. Cardiol. Rev. 2011, 7, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Copeland-Halperin, R.S.; Liu, J.E.; Yu, A.F. Cardiotoxicity of HER2-targeted therapies. Curr. Opin. Cardiol. 2019, 34, 451–458. [Google Scholar] [CrossRef]
- Kerr, A.J.; Dodwell, D.; McGale, P.; Holt, F.; Duane, F.; Mannu, G.; Darby, S.C.; Taylor, C.W. Adjuvant and neoadjuvant breast cancer treatments: A systematic review of their effects on mortality. Cancer Treat. Rev. 2022, 105, 203275. [Google Scholar] [CrossRef]
- Lee, J.W.; Oh, H.; You, J.Y.; Lee, E.S.; Lee, J.H.; Song, S.E.; Lee, K.L.; Jung, S.P.; An, J.S.; Cho, K.R.; et al. Therapy-related myeloid neoplasm in early breast cancer patients treated with adjuvant chemotherapy. Eur. J. Cancer 2023, 191, 112952. [Google Scholar] [CrossRef]
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Available online: https://dctd.cancer.gov/research/ctep-trials/trial-development (accessed on 11 July 2025).
- ECOG-ACRIN Cancer Research Group. ECOG Performance Status Scale. Available online: https://ecog-acrin.org/re-sources/ecog-performance-status/ (accessed on 2 October 2025).
- Esserman, L.J.; Berry, A.D.; DeMichele, A.; Carey, L.; Davis, S.E.; Buxton, M.; Hudis, C.; Gray, J.W.; Perou, C.; Yau, C.; et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: Results from the I-SPY 1 TRIAL--CALGB 150007/150012, ACRIN 6657. J. Clin. Oncol. 2012, 30, 3242–3249. [Google Scholar] [CrossRef]
- Swain, S.M.; Schneeweiss, A.; Gianni, L.; Goa, J.J.; Stein, A.; Waldron-Lynch, M.; Heeson, S.; Beattie, M.S.; Yoo, B.; Cortes, J.; et al. Incidence and management of diarrhea in patients with HER2-positive breast cancer treated with pertuzumab. Ann. Oncol. 2017, 28, 761–768. [Google Scholar] [CrossRef]
- Crown, J.; Eustace, A.J.; Collins, D.M.; Keane, M.; Coate, L.; Kennedy, J.; O’Reilly, S.; Kelly, C.; O’Connor, M.; Martin, M.; et al. TCHL—A phase II neo-adjuvant study assessing TCH (docetaxel, carboplatin and trastuzumab) and TCHL (docetaxel, carboplatin, trastuzumab and lapatinib) in HER-2 positive breast cancer patients: A 5-year follow-up with serum biomarker analysis. Acta Oncol. 2025, 64, 751–760. [Google Scholar] [CrossRef]
- Bayraktar, S.; Gonzalez-Angulo, A.M.; Lei, X.; Buzdar, A.U.; Valero, V.; Melhem-Bertrandt, A.; Kuerer, H.M.; Hortobagyi, G.N.; Sahin, A.A.; Meric-Bernstam, F. Efficacy of neoadjuvant therapy with trastuzumab concurrent with anthracycline- and nonanthracycline-based regimens for HER2-positive breast cancer. Cancer 2012, 118, 2385–2393. [Google Scholar] [CrossRef]
- Ginzac, A.; Molnar, I.; Durando, X.; De La Motte Rouge, T.; Petit, T.; D’hondt, V.; Campone, M.; Bonichon-Lamichhane, N.; Bouvet, L.V.; Levy, C.; et al. Neoadjuvant anthracycline-based (5-FEC) or anthracycline-free (docetaxel/carboplatin) chemotherapy plus trastuzumab and pertuzmab in HER2 + BC patients according to their TOP2A: A multicentre, open-label, non-randomized phase II trial. Breast Cancer Res. Treat. 2024, 205, 267–279. [Google Scholar] [CrossRef]
- Swain, S.M.; Baselga, J.; Kim, S.B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef]
- Mulrooney, D.A.; Yeazel, M.W.; Kawashima, T.; Mertens, A.C.; Mitby, P.; Stovall, M.; Donaldson, S.S.; Green, D.M.; Sklar, C.A.; Robison, L.L.; et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 2009, 339, b4606. [Google Scholar] [CrossRef]
| Molecular Subtype | TNBC | HER2+ (Overall) | HER2+/HR- | HER2+/HR+ | HR+/HER2− |
|---|---|---|---|---|---|
| pCR rate | 8/24 (33%) | 19/45 (42%) | 8/13 (62%) | 11/32 (34%) | 0/3 (0%) |
| Molecular Subtype | pCR Rate * | Regimen ^ | pCR Rate * | Treatment Base | pCR Rate * |
|---|---|---|---|---|---|
| TNBC | 8/24 (33.3%) | AC + P | 0/1 | Non-FEC-D | 0/2 (0%) |
| C/P/Pem + AC | 0/1 | ||||
| FEC-D | 1/6 | FEC-D | 8/25 (32%) | ||
| FEC-DC | 5/13 | ||||
| FEC-DC + Pem | 1/2 | ||||
| FEC-DC + Azet | 1/1 | ||||
| HR+/HER2− | 0/3 (0%) | FEC-D | 0/3 | ||
| HER2+ | 19/45 (42.2%) | AC + P/H | 0/1 | P/H | 5/8 (62.5%) |
| P/H | 4/6 | ||||
| P/H + Pert | 1/1 | ||||
| FEC-DH | 5/14 | FEC-DH | 7/16 (43.8%) | ||
| FEC-DH + Pert | 2/2 | ||||
| TCH | 5/18 | TCH | 7/21 (33.3%) | ||
| TCH + Pert | 2/3 |
| Treatment Base | FEC-D | FEC-DH | TCH |
|---|---|---|---|
| Total patients | 25 | 16 | 21 |
| ECOG Performance status | |||
| 0 | 17 (68%) | 8 (50%) | 12 (57%) |
| 1 | 6 (24%) | 1 (6%) | 8 (38%) |
| 2 | 0 (0%) | 1 (6%) | 1 (5%) |
| Not documented | 2 (8%) | 6 (38%) | 0 (0%) |
| Treatment Base | FEC-D | FEC-DH | TCH |
|---|---|---|---|
| Total patients | 25 | 16 | 21 |
| Patients with severe toxicity | 16 | 12 | 14 |
| Severe toxicity percentage | 64% (16/25) | 75% (12/16) | 67% (14/21) |
| Toxicity | Number of patients (%) | ||
| Diarrhoea | 4 (16%) | 1 (6%) | 8 (38%) |
| Neutropaenia (afebrile) | 3 (12%) | 8 (50%) | 1 (5%) |
| Anaemia | 4 (16%) | 1 (6%) | 4 (19%) |
| Febrile neutropaenia | 4 (16%) | 1 (6%) | 2 (10%) |
| Lung infection | 1 (4%) | 2 (13%) | 2 (10%) |
| Mucositis | 2 (8%) | 0 (0%) | 3 (14%) |
| Fever | 2 (8%) | 3 (19%) | 0 (0%) |
| Vomiting | 1 (4%) | 1 (6%) | 1 (5%) |
| Anorexia | 1 (4%) | 1 (6%) | 1 (5%) |
| Aches * | 0 (0%) | 3 (19%) | 0 (0%) |
| Colitis | 0 (0%) | 0 (0%) | 2 (10%) |
| Device-related infection | 2 (8%) | 0 (0%) | 0 (0%) |
| Hyperglycaemia | 1 (4%) | 0 (0%) | 1 (5%) |
| Dyspnoea | 1 (4%) | 0 (0%) | 1 (5%) |
| Treatment Base | FEC-D | FEC-DH | TCH |
|---|---|---|---|
| Total patients | 25 | 16 | 21 |
| Patients with treatment-limiting toxicity | 12 | 13 | 16 |
| Treatment-limiting toxicity percentage | 48% (12/25) | 81% (13/16) | 76% (16/21) |
| Toxicity | Number of patients (%) | ||
| Diarrhoea | 4 (16%) | 3 (19%) | 5 (24%) |
| Fatigue | 5 (20%) | 2 (13%) | 4 (19%) |
| Neuropathy | 1 (4%) | 2 (13%) | 4 (19%) |
| Nausea | 2 (8%) | 1 (6%) | 3 (14%) |
| Anorexia | 2 (8%) | 1 (6%) | 2 (10%) |
| Febrile neutropaenia | 4 (16%) | 0 (0%) | 2 (10%) |
| Cardiotoxicity (decline in LVEF) | 0 (0%) | 2 (13%) | 2 (10%) |
| Lung infection | 1 (4%) | 0 (0%) | 1 (5%) |
| Anaemia | 2 (8%) | 0 (0%) | 1 (5%) |
| Aches * | 0 (0%) | 4 (25%) | 0 (0%) |
| Vomiting | 1 (4%) | 1 (6%) | 1 (5%) |
| Neutropaenia (afebrile) | 2 (8%) | 1 (6%) | 0 (0%) |
| Mucositis | 1 (4%) | 0 (0%) | 1 (5%) |
| Colitis | 0 (0%) | 0 (0%) | 2 (10%) |
| DKA | 1 (4%) | 0 (0%) | 1 (5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galloway, M.; Barlow, P.; Jordan, J.; Lo, E. Neoadjuvant Chemotherapy for Early Breast Cancer: A Study on Response Rate and Toxicity. J. Clin. Med. 2025, 14, 7362. https://doi.org/10.3390/jcm14207362
Galloway M, Barlow P, Jordan J, Lo E. Neoadjuvant Chemotherapy for Early Breast Cancer: A Study on Response Rate and Toxicity. Journal of Clinical Medicine. 2025; 14(20):7362. https://doi.org/10.3390/jcm14207362
Chicago/Turabian StyleGalloway, Matt, Paula Barlow, Jody Jordan, and Edward Lo. 2025. "Neoadjuvant Chemotherapy for Early Breast Cancer: A Study on Response Rate and Toxicity" Journal of Clinical Medicine 14, no. 20: 7362. https://doi.org/10.3390/jcm14207362
APA StyleGalloway, M., Barlow, P., Jordan, J., & Lo, E. (2025). Neoadjuvant Chemotherapy for Early Breast Cancer: A Study on Response Rate and Toxicity. Journal of Clinical Medicine, 14(20), 7362. https://doi.org/10.3390/jcm14207362






