Falling Third-Trimester Insulin Requirements in Diabetic Pregnancies and Adverse Pregnancy Outcomes: A Systematic Review and Meta-Analysis †
Abstract
1. Introduction
2. Material and Methods
2.1. Data Sources and Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Data Synthesis
2.5. Quality Assessment
2.6. Grading of Recommendations Assessment, Development and Evaluation
3. Results
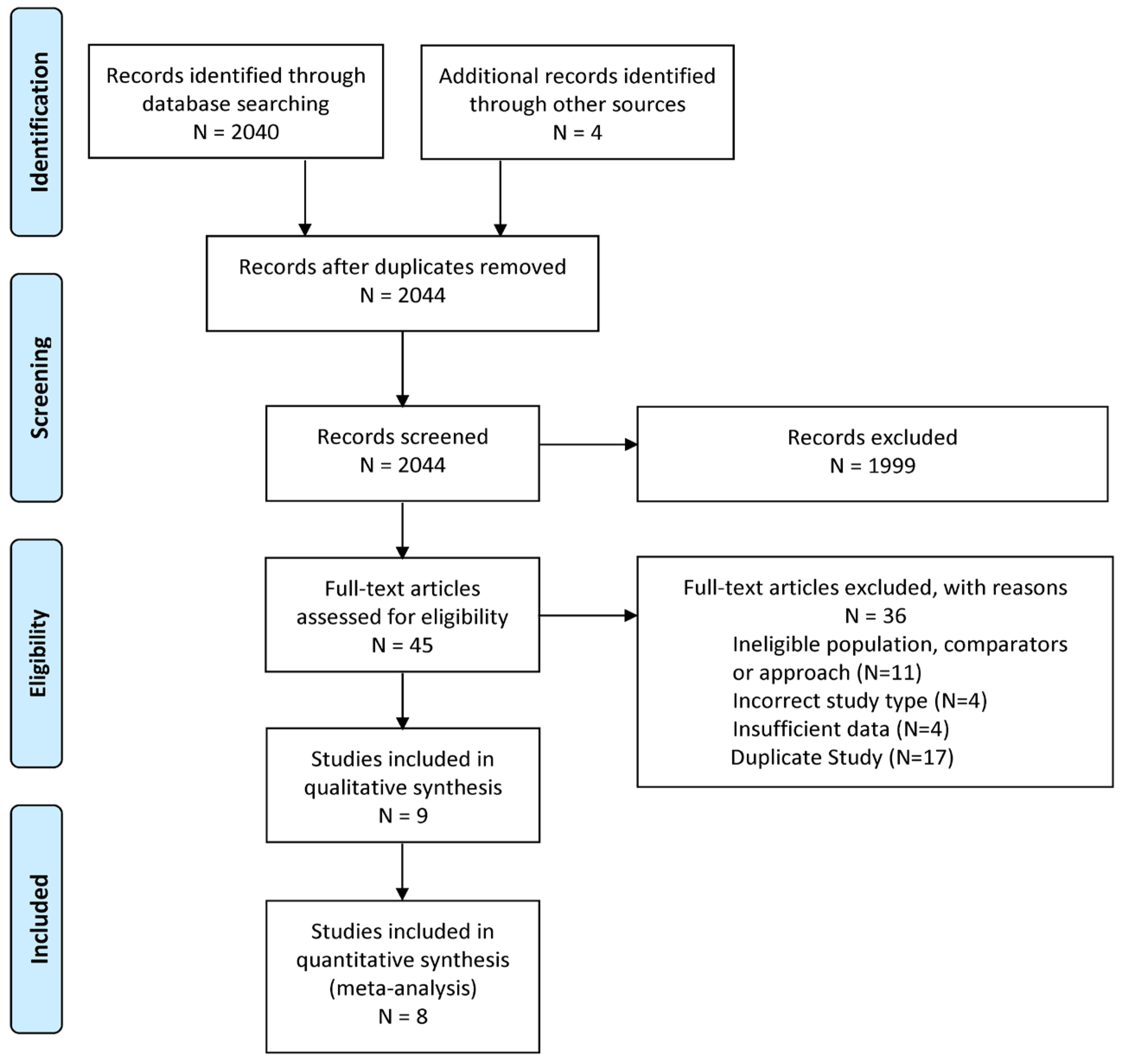
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias of Included Studies
3.4. Synthesis of Results
3.5. GRADE Assessment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chivese, T.; Hoegfeldt, C.A.; Werfalli, M.; Yuen, L.; Sun, H.; Karuranga, S.; Li, N.; Gupta, A.; Immanuel, J.; Divakar, H.; et al. IDF Diabetes Atlas: The prevalence of pre-existing diabetes in pregnancy—A systematic reviewand meta-analysis of studies published during 2010–2020. Diabetes Res. Clin. Pract. 2022, 183, 109049. [Google Scholar] [CrossRef] [PubMed]
- Ngwezi, D.P.; Savu, A.; Yeung, R.O.; Butalia, S.; Kaul, P. Temporal Trends in Type 1, Type 2, and Gestational Diabetes in Pregnancy: Impact of Rural Residence, Ethnicity, and Material Deprivation. Can. J. Diabetes 2023, 47, 672–679.e673. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.K.; Tan, E.L. Alterations in physiology and anatomy during pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2013, 27, 791–802. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes, A. 13. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41, S137–S143. [Google Scholar] [CrossRef]
- Ryan, E.A.; Enns, L. Role of gestational hormones in the induction of insulin resistance. J. Clin. Endocrinol. Metab. 1988, 67, 341–347. [Google Scholar] [CrossRef]
- Rayburn, W.; Piehl, E.; Lewis, E.; Schork, A.; Sereika, S.; Zabrensky, K. Changes in insulin therapy during pregnancy. Am. J. Perinatol. 1985, 2, 271–275. [Google Scholar] [CrossRef]
- Jovanovic, L.; Druzin, M.; Peterson, C.M. Effect of euglycemia on the outcome of pregnancy in insulin-dependent diabetic women as compared with normal control subjects. Am. J. Med. 1981, 71, 921–927. [Google Scholar] [CrossRef]
- Sutherland, H.W.; Stowers, J.M. Carbohydrate Metabolism in Pregnancy and the Newborn; Springer: Berlin/Heidelberg, Germany, 1979. [Google Scholar]
- Padmanabhan, S.; McLean, M.; Cheung, N.W. Falling insulin requirements are associated with adverse obstetric outcomes in women with preexisting diabetes. Diabetes Care 2014, 37, 2685–2692. [Google Scholar] [CrossRef]
- Achong, N.; Callaway, L.; d’Emden, M.; McIntyre, H.D.; Lust, K.; Barrett, H.L. Insulin requirements in late pregnancy in women with type 1 diabetes mellitus: A retrospective review. Diabetes Res. Clin. Pract. 2012, 98, 414–421. [Google Scholar] [CrossRef]
- McManus, R.M.; Ryan, E.A. Insulin requirements in insulin-dependent and insulin-requiring GDM women during final month of pregnancy. Diabetes Care 1992, 15, 1323–1327. [Google Scholar] [CrossRef]
- García-Patterson, A.; Gich, I.; Amini, S.B.; Catalano, P.M.; de Leiva, A.; Corcoy, R. Insulin requirements throughout pregnancy in women with type 1 diabetes mellitus: Three changes of direction. Diabetologia 2009, 53, 446–451. [Google Scholar] [CrossRef]
- Oka, R.; Iura, T.; Miyamoto, S. Large decreases in insulin requirement occurred repeatedly in two pregnancies in a type 1 diabetic woman. Acta Diabetol. 2006, 43, 34–35. [Google Scholar] [CrossRef]
- Steel, J.M.; Johnstone, F.D.; Hume, R.; Mao, J.H. Insulin requirements during pregnancy in women with type I diabetes. Obstet. Gynecol. 1994, 83, 253–258. [Google Scholar] [PubMed]
- Søholm, J.C.; Do, N.C.; Vestgaard, M.; Ásbjörnsdóttir, B.; Nørgaard, S.K.; Pedersen, B.W.; Storgaard, L.; Nielsen, B.B.; Holmager, P.; Ringholm, L.; et al. Falling Insulin Requirement in Pregnant Women With Diabetes Delivering Preterm: Prevalence, Predictors, and Consequences. J. Clin. Endocrinol. Metab. 2022, 107, e2237–e2244. [Google Scholar] [CrossRef] [PubMed]
- Vohr, B. Long-term outcomes of moderately preterm, late preterm, and early term infants. Clin. Perinatol. 2013, 40, 739–751. [Google Scholar] [CrossRef]
- Brooke, B.S.; Schwartz, T.A.; Pawlik, T.M. MOOSE Reporting Guidelines for Meta-analyses of Observational Studies. JAMA Surg. 2021, 156, 787–788. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- D’Souza, R.; Sayyar, P.; Feig, D. Falling Third-Trimester Insulin Requirements in Diabetic Pregnancies and Adverse Pregnancy Outcomes: A Systematic Review. PROSPERO CRD42017064878. 2017. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42017064878 (accessed on 10 July 2025).
- Allison Pihelgas, V.J.; Prior, A.; Ospina, M. Decreasing Insulin Requirements During the Third Trimester Amongst Women with Insulin Dependent Gestational and Pregestational Diabetes and Its Association to Maternal-Fetal Outcomes: A Systematic Review. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019142976 (accessed on 10 July 2025).
- DataParty. Available online: https://dataparty.ca/ (accessed on 21 September 2025).
- Hayden, J.A.; van der Windt, D.A.; Cartwright, J.L.; Côté, P.; Bombardier, C. Assessing Bias in Studies of Prognostic Factors. Ann. Intern. Med. 2013, 158, 280–286. [Google Scholar] [CrossRef]
- The Cochrane Collaboration Prognosis Methods Group, Review Tools. 2018. Available online: https://methods.cochrane.org/prognosis/tools (accessed on 21 September 2025).
- Foroutan, F.; Guyatt, G.; Zuk, V.; Vandvik, P.O.; Alba, A.C.; Mustafa, R.; Vernooij, R.; Arevalo-Rodriguez, I.; Munn, Z.; Roshanov, P.; et al. GRADE Guidelines 28: Use of GRADE for the assessment of evidence about prognostic factors: Rating certainty in identification of groups of patients with different absolute risks. J. Clin. Epidemiol. 2020, 121, 62–70. [Google Scholar] [CrossRef]
- Iorio, A.; Spencer, F.A.; Falavigna, M.; Alba, C.; Lang, E.; Burnand, B.; McGinn, T.; Hayden, J.; Williams, K.; Shea, B.; et al. Use of GRADE for assessment of evidence about prognosis: Rating confidence in estimates of event rates in broad categories of patients. BMJ 2015, 350, h870. [Google Scholar] [CrossRef]
- Guyatt, G.; Agoritsas, T.; Brignardello-Petersen, R.; Mustafa, R.A.; Rylance, J.; Foroutan, F.; Prasad, M.; Agarwal, A.; De Beer, H.; Murad, M.H.; et al. Core GRADE 1: Overview of the Core GRADE approach. BMJ 2025, 389, e081903. [Google Scholar] [CrossRef]
- Guyatt, G.; Wang, Y.; Eachempati, P.; Iorio, A.; Murad, M.H.; Hultcrantz, M.; Chu, D.K.; Florez, I.D.; Hemkens, L.G.; Agoritsas, T.; et al. Core GRADE 4: Rating certainty of evidence—Risk of bias, publication bias, and reasons for rating up certainty. BMJ 2025, 389, e083864. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.; Iorio, A.; De Beer, H.; Owen, A.; Agoritsas, T.; Murad, M.H.; Karthikeyan, G.; Cuello, C.; Prasad, M.; Kim, K.; et al. Core GRADE 5: Rating certainty of evidence—Assessing indirectness. BMJ 2025, 389, e083865. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.; Schandelmaier, S.; Brignardello-Petersen, R.; De Beer, H.; Prasad, M.; Murad, M.H.; Eachempati, P.; Chu, D.K.; D’Souza, R.; Iorio, A.; et al. Core GRADE 3: Rating certainty of evidence—Assessing inconsistency. BMJ 2025, 389, e081905. [Google Scholar] [CrossRef]
- Guyatt, G.; Zeng, L.; Brignardello-Petersen, R.; Prasad, M.; De Beer, H.; Murad, M.H.; Iorio, A.; Agarwal, A.; Yao, L.; Agoritsas, T.; et al. Core GRADE 2: Choosing the target of certainty rating and assessing imprecision. BMJ 2025, 389, e081904. [Google Scholar] [CrossRef]
- Guyatt, G.; Yao, L.; Murad, M.H.; Hultcrantz, M.; Agoritsas, T.; De Beer, H.; Schandelmaier, S.; Iorio, A.; Zeng, L.; Prasad, M.; et al. Core GRADE 6: Presenting the evidence in summary of findings tables. BMJ 2025, 389, e083866. [Google Scholar] [CrossRef]
- Padmanabhan, S.; Lee, V.; McLean, M.; Athayde, N.; Lanzarone, V.; Peek, M.J.; Quinton, A.; Cheung, N.W. The relationship between falling insulin requirements and serial ultrasound measurements in women with preexisting diabetes: A prospective cohort study. J. Matern.-Fetal Neonatal Med. 2022, 35, 10239–10245. [Google Scholar] [CrossRef]
- Padmanabhan, S.; Lee, V.W.; McLean, M.; Athayde, N.; Lanzarone, V.; Khoshnow, Q.; Peek, M.J.; Cheung, N.W. The Association of Falling Insulin Requirements With Maternal Biomarkers and Placental Dysfunction: A Prospective Study of Women With Preexisting Diabetes in Pregnancy. Diabetes Care 2017, 40, 1323–1330. [Google Scholar] [CrossRef]
- Ram, M.; Feinmesser, L.; Shinar, S.; Maslovitz, S. The importance of declining insulin requirements during pregnancy in patients with pre-gestational gestational diabetes mellitus. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 215, 148–152. [Google Scholar] [CrossRef]
- Wilkinson, B.; McDonnell, M.; Palermo, N.; Lassey, S.; Little, S. Falling insulin requirement in late pregnancy: Association with obstetric and neonatal outcomes. J. Perinatol. 2021, 41, 1043–1049. [Google Scholar] [CrossRef]
- Vainder, M.; Natt, N.; D’Souza, R.D.; Syeda, A.; Mitsakakis, N. Falling third trimester insulin requirements and adverse pregnancy outcomes in individuals with pre-existing diabetes. J. Clin. Med. 2025. revisions submitted. [Google Scholar]
- Søholm, J.C.; Vestgaard, M.; Ásbjörnsdóttir, B.; Do, N.C.; Pedersen, B.W.; Storgaard, L.; Nielsen, B.B.; Ringholm, L.; Damm, P.; Mathiesen, E.R. Potentially modifiable risk factors of preterm delivery in women with type 1 and type 2 diabetes. Diabetologia 2021, 64, 1939–1948. [Google Scholar] [CrossRef]
- Wang, A.; Rana, S.; Karumanchi, S.A. Preeclampsia: The role of angiogenic factors in its pathogenesis. Physiology 2009, 24, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Mustary, M.; Ansariadi; Syam, A.; Riskiyani, S.; Erika, K.A.; Moedjiono, A.I.; Lubis, M. Preeclampsia: Etiology, Pathophysiology, Risk Factors, Impact and Prevention: A Narrative Review. Iran. J. Public Health 2024, 53, 2392–2403. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.C.; Rayol, P.; Gozzani, L.; Reis, L.M.; Zampieri, G.; Dias, C.B.; Woronik, V. The relationship of angiogenic factors to maternal and neonatal manifestations of early-onset and late-onset preeclampsia. Prenat. Diagn. 2014, 34, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Sibai, B.M. Preeclampsia as a cause of preterm and late preterm (near-term) births. Semin. Perinatol. 2006, 30, 16–19. [Google Scholar] [CrossRef]
- Davies, E.L.; Bell, J.S.; Bhattacharya, S. Preeclampsia and preterm delivery: A population-based case-control study. Hypertens. Pregnancy 2016, 35, 510–519. [Google Scholar] [CrossRef]

| Year, Author (Country) | Study Period | Pregnancies | Type of Diabetes | Case Definition | Control Definition | Gestational Age | Pre/Early Pregnancy BMI (kg/m2) | ||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Case | Control | ||||||
| 2025, Vainder [36] (Canada) | 2000–2016 | 54 | 296 | T1 and T2 | ≥15% FIR, where PFID = (PTID-TTID)/ PTID × 100 | <15% FIR | 28 weeks to childbirth | 28.32 (7.85) * | 29.48 (7.21) * |
| 2022, Padmanabhan [32] (Australia) | June 2013–May 2016 | 30 | 112 | T1, T2 and GDM | ≥15% FIR, where PFID = (PTID − TTID)/PTID × 100 | No FIR | 20 weeks to childbirth | 27.7 (25.1–32.9) ** | 30.2 (26.2–38.1) ** |
| 2022, Soholm [15] (Denmark) | Unspecified 5-year period | 27 | 74 | T1 and T2 | ≥20% from PTID based on 20-year clinical data | No FIR | Pregnancy | 24.3 (22–28) ** | 28.2 (23–33) ** |
| 2021, Wilkinson [35] (United States) | Unspecified 2-year period | 21 | 136 | T1, T2 and GDM | Falling insulin requirement (undefined) | No FIR | Third trimester | 30.9 (24.0–37.0) ** | 31.0 (25.2–36.5) ** |
| 2017, Padmanabhan [33] (Australia) | June 2013–October 2015 | 32 | 126 | T1, T2 and GDM | ≥15% FIR where PFID = (PTID − TTID)/PTID × 100 | <15% FIR | 20 weeks to childbirth | 27.7 (24.2–33.3) ** | 30.45 (36.3–38.1) ** |
| 2017, Ram [34] (Israel) | January 2010–December 2014 | 101 | 203 | T1, T2 and GDM | <30% FIR, where PFID = (PTID − TTID)/PTID × 100 | No FIR | Two-week period | 26.1 (2.8) ** | 26.4 (2.5) ** |
| 2014, Padmanabhan [9] (Australia) | January 2010–January 2013 | 35 | 104 | T1, T2 and GDM | ≥15% PFID, where PFID = (PTID − TTID)/PTID × 100 | <15% FIR | 20 weeks to childbirth | 29.8 (25.0–34.0) ** | 29.7 (26.0–35.6) ** |
| 2012, Achong [10] (Australia) | 2006–2010 | 5 | 49 | T1 | ≥15% fall in weight-adjusted basal insulin | <15% FIR | 30 weeks to childbirth | 23.9 (4.3) * | 25.7 (3.8) * |
| 1992, McManus [11] (Canada) | Unspecified 5-year period | 20 | 12 | T1 | any FIR | Any rise in insulin requirements | 36 weeks to childbirth | 25 (1) *** | 25 (1) *** |
| Outcome | No. of Studies | Events/Pregnancies | Odds Ratios [95% CI] | I2 | |
|---|---|---|---|---|---|
| FIR | No FIR | ||||
| Preterm birth | 4 | 21/126 (16.7%) | 68/575 (11.8%) | 2.0 [0.51–7.85] | 26% |
| Preeclampsia | 4 | 32/189 (16.9%) | 62/569 (10.9%) | 3.0 [1.4–6.38] | 27% |
| Stillbirth | 3 | 1/121 (0.8%) | 5/526 (1.0%) | 1.5 [0.27–8.4] | 0 |
| Neonatal respiratory distress | 4 | 40/93 (43.0%) | 110/415 (26.5%) | 2.03 [1.27–3.26] | 0 |
| Composite of clinical outcomes reflecting placental dysfunction | 4 | 41/189 (21.7%) | 80/569 (14.1%) | 2.32 [1.07–5.03] | 41% |
| Small-for-gestational-age | 7 | 23/278 (8.3%) | 73/1026 (7.1%) | 1.29 [0.77–2.15] | 0 |
| Low Apgar score at 5 min | 5 | 7/189 (3.7%) | 20/566 (3.5%) | 1.68 [0.68–4.14] | 0 |
| № of Studies | Certainty Assessment | Effect | Certainty | Importance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | № of Events | № of Individuals | Absolute Event Rate (95% CI) | |||
| Preterm Birth | |||||||||||
| 4 | Non-randomized studies | Serious a | Not serious | Not serious | Very Serious b | None | 21 | 126 | 93 per 1000 (−54 to 395) | ⨁◯◯◯ Very low a,b | Critical |
| Preeclampsia | |||||||||||
| 4 | Non-randomized studies | Not serious | Not Serious | Not serious | Not Serious | None | 32 | 189 | 159 per 1000 (38 to 329) | ⨁⨁⨁⨁ High | Critical |
| Stillbirth | |||||||||||
| 3 | Non-randomized studies | Not serious | Not serious | Not serious | very serious c | None | 1 | 121 | 5 per 1000 (−7 to 65) | ⨁⨁◯◯ Low c | Critical |
| Neonatal respiratory distress | |||||||||||
| 4 | Non-randomized studies | Not serious | Not serious | Not serious | Serious d | None | 40 | 93 | 158 per 1000 (49 to 275) | ⨁⨁⨁◯ Moderate d | Important |
| Composite of clinical outcomes reflecting placental dysfunction * | |||||||||||
| 4 | Non-randomized studies | Not serious | Serious e | Not serious | Serious d | None | 41 | 189 | 135 per 1000 (8 to 311) | ⨁⨁◯◯ Low d,e | Important |
| Small-for-gestational-age | |||||||||||
| 7 | Non-randomised studies | Not serious | Serious f | Not serious | Serious d | None | 23 | 278 | 18 per 1000 (−15 to 69) | ⨁⨁◯◯ Low d.f | Important |
| Low Apgar score at 5 min | |||||||||||
| 5 | Non-randomized studies | Not serious | Serious f | Not serious | Serious g | None | 7 | 189 | 23 per 1000 (−11 to 96) | ⨁⨁◯◯ Low f,g | Important |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Souza, R.; Ashraf, R.; Sayfi, S.; Prior, A.; Pihelgas, A.; Sanni, O.; Sayyar, P.; Alavifard, S.; Ospina, M.B.; Jain, V. Falling Third-Trimester Insulin Requirements in Diabetic Pregnancies and Adverse Pregnancy Outcomes: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 7357. https://doi.org/10.3390/jcm14207357
D’Souza R, Ashraf R, Sayfi S, Prior A, Pihelgas A, Sanni O, Sayyar P, Alavifard S, Ospina MB, Jain V. Falling Third-Trimester Insulin Requirements in Diabetic Pregnancies and Adverse Pregnancy Outcomes: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(20):7357. https://doi.org/10.3390/jcm14207357
Chicago/Turabian StyleD’Souza, Rohan, Rizwana Ashraf, Shahab Sayfi, Alexandra Prior, Allison Pihelgas, Omolara Sanni, Parastoo Sayyar, Sepand Alavifard, Maria B. Ospina, and Venu Jain. 2025. "Falling Third-Trimester Insulin Requirements in Diabetic Pregnancies and Adverse Pregnancy Outcomes: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 20: 7357. https://doi.org/10.3390/jcm14207357
APA StyleD’Souza, R., Ashraf, R., Sayfi, S., Prior, A., Pihelgas, A., Sanni, O., Sayyar, P., Alavifard, S., Ospina, M. B., & Jain, V. (2025). Falling Third-Trimester Insulin Requirements in Diabetic Pregnancies and Adverse Pregnancy Outcomes: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(20), 7357. https://doi.org/10.3390/jcm14207357







