Antithrombotic Therapy in the Elderly with Cardiovascular Disease: Walking the Tightrope Between Efficacy and Bleeding Risk—A Narrative Review
Abstract
1. Introduction
2. Methodology
3. Definition of “Elderly” Patient
4. Age-Related Changes in the Thrombotic Pathway
5. Increased Bleeding Risk in Elderly
6. Atherosclerotic Cardiovascular Disease
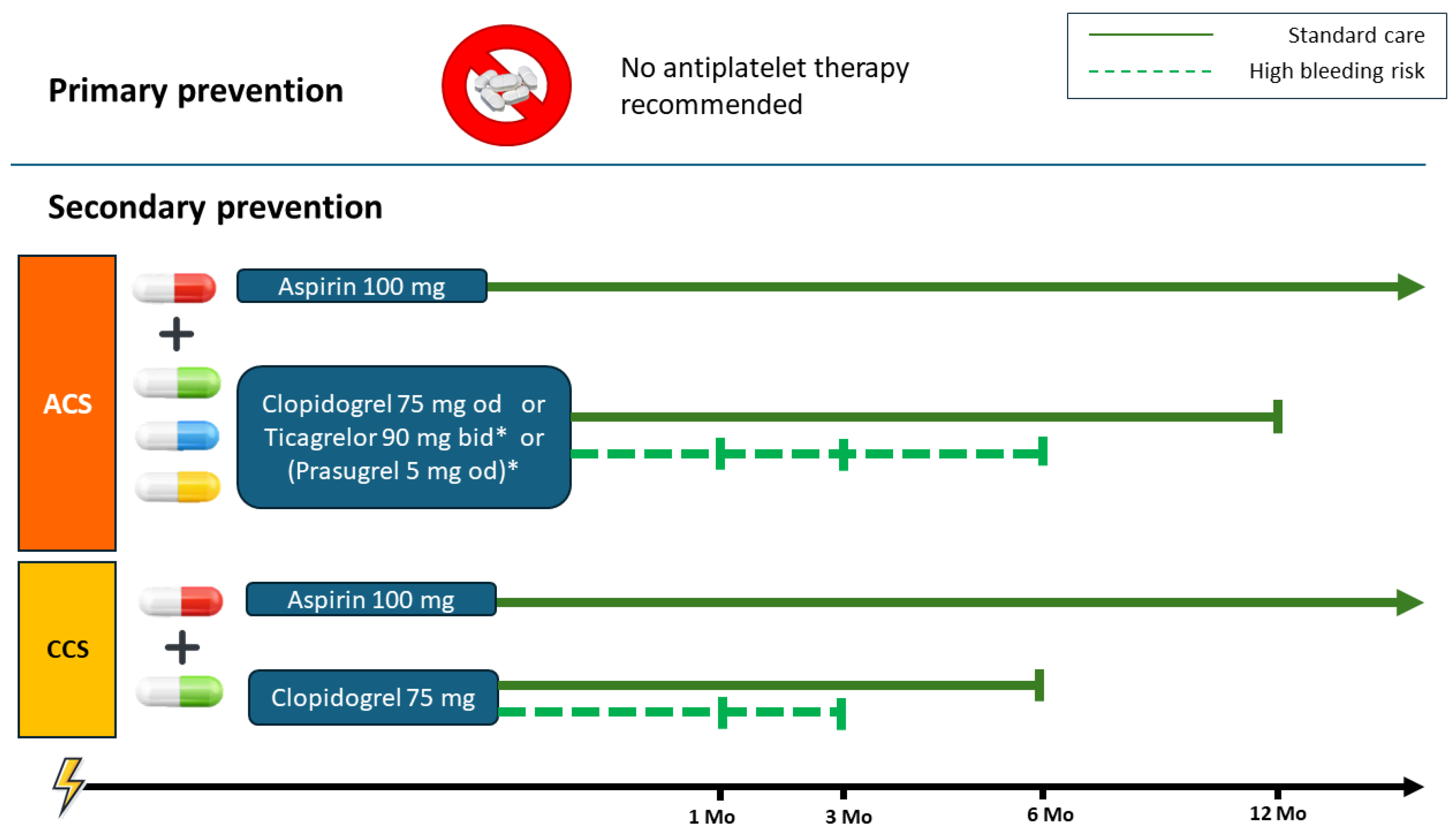
6.1. Primary Prevention
6.2. Secondary Prevention
7. Dual Antiplatelet Therapy in Acute Coronary Syndrome in Elderly Patients
DAPTDuration After an Acute Coronary Syndromes
8. Dual Antiplatelet Therapy in Chronic Coronary Syndrome in Elderly Patients
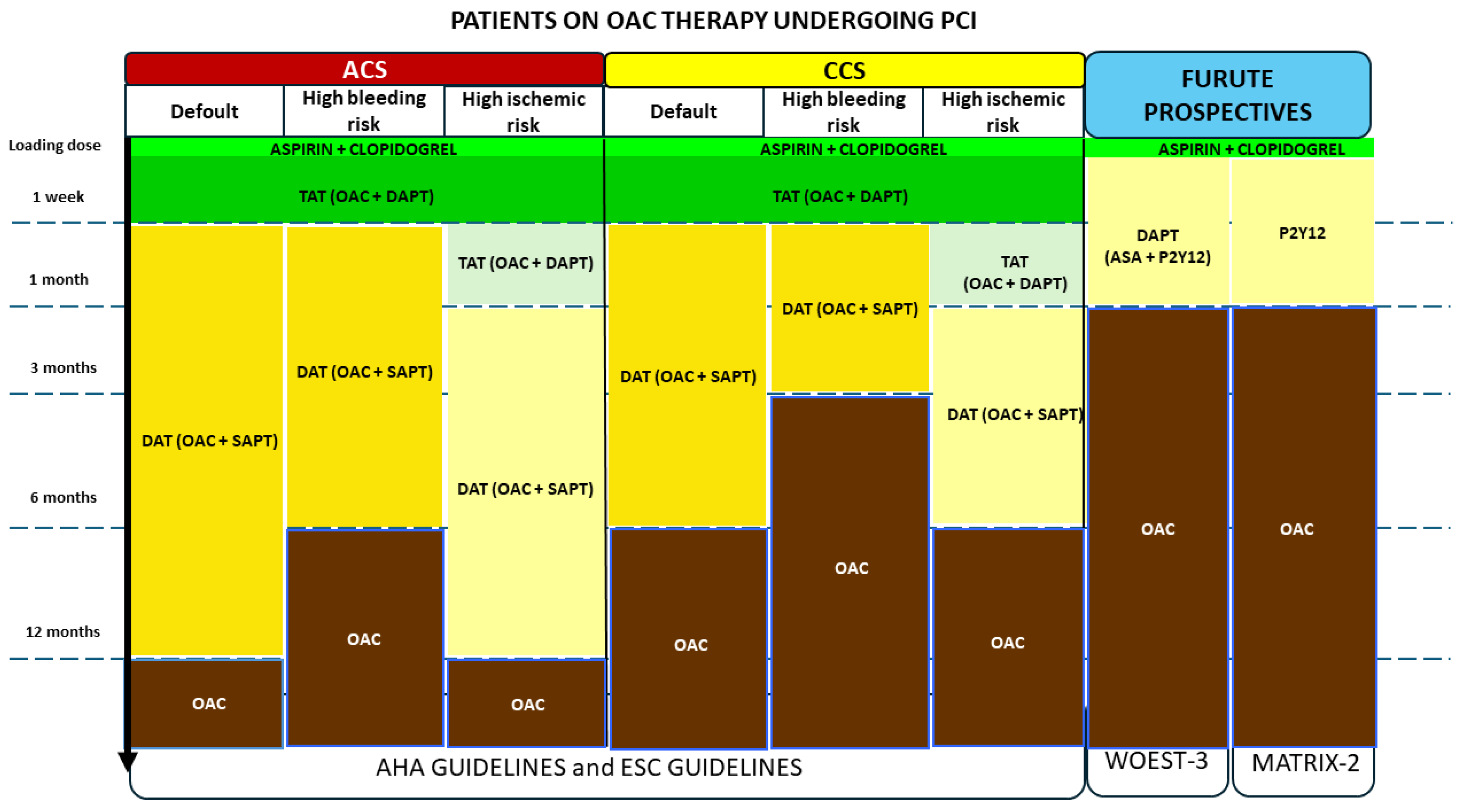
9. Anticoagulant Therapy in Elderly
10. New Anticoagulants Under Development
- -
- Asundexian: is a selective oral Factor XIa inhibitor administered once daily. It has a favorable pharmacokinetic profile, with minimal renal elimination and no significant dependence on hepatic cytochrome P450 metabolism. The Phase II trial, PACIFIC-AF [84], which enrolled patients with atrial fibrillation at increased risk of stroke, demonstrated a comparable antithrombotic effect of Asundexian to Apixaban but was associated with a significantly lower rate of bleeding with Asundexian. However, the following Phase III trials of the OCEANIC program, particularly the OCEANIC-AF [85], showed that treatment with Asundexian at a dose of 50 mg once daily resulted in a higher rate of stroke or systemic embolism compared to Apixaban, which led to the premature discontinuation of the trial. Despite this, Asundexian was associated with fewer major bleeding events over the course of the study.
- -
- Milvexian: is another oral, highly selective Factor XIa inhibitor, taken once or twice daily. Like Asundexian, Milvexian is not renally cleared to a significant extent, and its metabolism does not rely heavily on hepatic enzymes. Initial Phase I studies established Milvexian’s safety, tolerability, and favorable pharmacokinetic profile [90]. The following Phase II study, AXIOMATIC-SSP trial, examined the safety and the efficacy of Milvexian versus placebo in patients with acute ischemic stroke or high-risk, showing no statistically significant reduction in the primary composite outcome of symptomatic ischemic stroke and covert brain infarction at 90 days [87]. The AXIOMATIC-TKR trial evaluated thromboprophylaxis after total knee replacement, showing that Milvexian significantly reduces the incidence of VTE in a dose-dependent manner, with bleeding rates similar to placebo and lower than those seen with enoxaparin [88]. The LIBREXIA Phase III program is ongoing with the aim of comparing Milvexian with standard care in the context of stroke prevention in nonvalvular AF (LIBREXIA-AF. NCT05757869), acute ischemic stroke, or high-risk TIA (LIBREXIA-STROKE. NCT05702034) and ACS (LIBREXIA-ACS. NCT05754957).
- -
- Abelacimab: stands out among the Factor XIa inhibitors due to its monoclonal antibody structure, which allows it to bind to the inactive form of Factor XI and block its activation. It is administered parenterally (either intravenously or subcutaneously) and provides long-lasting anticoagulation with a monthly injection. In a first-in-human Phase 1 study, single subcutaneous administration of Abelacimab was demonstrated to be safe and well-tolerated [91]. The safety profile of Abelacimab was evaluated in a Phase II trial comparing intravenous doses to the standard of care (Enoxaparin) for the prevention of VTE [90]. In the Phase III AZALEA-TIMI 71 trial, which compared Abelacimab to Rivaroxaban in patients with atrial fibrillation, treatment with Abelacimab resulted in markedly lower levels of free factor XI and fewer bleeding events than treatment with Rivaroxaban [89].
11. Atherosclerotic Cardiovascular Disease and Concomitant Indication to Anticoagulant Therapy
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forman, D.E.; Maurer, M.S.; Boyd, C.; Brindis, R.; Salive, M.E.; Horne, F.M.; Bell, S.P.; Fulmer, T.; Reuben, D.B.; Zieman, S.; et al. Multimorbidity in Older Adults with Cardiovascular Disease. J. Am. Coll. Cardiol. 2018, 71, 2149–2161. [Google Scholar] [CrossRef]
- Bencivenga, L.; Komici, K.; Nocella, P.; Grieco, F.V.; Spezzano, A.; Puzone, B.; Cannavo, A.; Cittadini, A.; Corbi, G.; Ferrara, N.; et al. Atrial fibrillation in the elderly: A risk factor beyond stroke. Ageing Res. Rev. 2020, 61, 101092. [Google Scholar] [CrossRef]
- Technical Advisory Group for Measurement, Monitoring and Evaluation of the United Nation’s Decade of Healthy Ageing. Available online: https://www.who.int/groups/technical-advisory-group-for-measurement-monitoring-and-evaluation-of-the-un-decade-of-healthy-ageing/about-us (accessed on 15 October 2025).
- Lee, S.B.; Oh, J.H.; Park, J.H.; Choi, S.P.; Wee, J.H. Differences in youngest-old, middle-old, and oldest-old patients who visit the emergency department. Clin. Exp. Emerg. Med. 2018, 5, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Damluji, A.A.; Nanna, M.G.; Rymer, J.; Kochar, A.; Lowenstern, A.; Baron, S.J.; Narins, C.R.; Alkhouli, M. Chronological vs Biological Age in Interventional Cardiology: A Com-prehensive Approach to Care for Older Adults: JACC Family Series. JACC Cardiovasc. Interv. 2024, 17, 961–978. [Google Scholar] [CrossRef]
- Dillinger, J.-G.; Laine, M.; Bouajila, S.; Paganelli, F.; Henry, P.; Bonello, L. Antithrombotic strategies in elderly patients with acute coronary syndrome. Arch. Cardiovasc. Dis. 2021, 114, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Balduini, C.L.; Noris, P. Platelet count and aging. Haematologica 2014, 99, 953–955. [Google Scholar] [CrossRef]
- Jones, C.I. Platelet function and ageing. Mamm. Genome 2016, 27, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Mutschlechner, D.; Tscharre, M.; Wadowski, P.P.; Lee, S.; Pultar, J.; Weikert, C.; Panzer, S.; Gremmel, T. Elderly patients are hyperresponsive to potent P2Y12 inhibitors. Res. Pract. Thromb. Haemost. 2025, 9, 102704. [Google Scholar] [CrossRef]
- Gleerup, G.; Winther, K. The effect of ageing on platelet function and fibrinolytic activity. Angiology 1995, 46, 715–718. [Google Scholar] [CrossRef]
- Qian, H.; Luo, N.; Chi, Y. Aging-shifted prostaglandin profile in endothelium as a factor in cardiovascular disorders. J. AgingRes. 2012, 2012, 121390. [Google Scholar] [CrossRef]
- Mazuryk, O.; Gurgul, I.; Oszajca, M.; Polaczek, J.; Kieca, K.; Bieszczad-Żak, E.; Martyka, T.; Stochel, G. Nitric Oxide Signaling and Sensing in Age-Related Diseases. Antioxidants 2024, 13, 1213. [Google Scholar] [CrossRef]
- Rocha, B.S. The Nitrate-Nitrite-Nitric Oxide Pathway on Healthy Ageing: A Review of Pre-clinical and Clinical Data on the Impact of Dietary Nitrate in the Elderly. Front. Aging 2021, 2, 778467. [Google Scholar] [CrossRef]
- Tschan, S.L.; Bolliger, D. Coagulation and Aging: Implications for the Anesthesiologist. Curr. Anesthesiol. 2021, 11, 387–395. [Google Scholar] [CrossRef]
- Capranzano, P.; Angiolillo, D.J. Antithrombotic Management of Elderly Patients With Coronary Artery Disease. JACC Cardiovasc. Interv. 2021, 14, 723–738. [Google Scholar] [CrossRef]
- Ahmed, B.; Rahman, A.A.; Lee, S.; Malhotra, R. The Implications of Aging on Vascular Health. Int. J. Mol. Sci. 2024, 25, 11188. [Google Scholar] [CrossRef]
- Ruiz, A.; Di Cristina, S. Absorption to Excretion: The Aging Body’s Take on Drugs—A Review of Pharmacokinetic Changes and their Impact on Medication Management. Curr. Pharmacol. 2025, 11, 42. [Google Scholar] [CrossRef]
- Wynne, H. Drug metabolism and ageing. J. Br. Menopause Soc. 2005, 11, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.J.; Lee, J.S.; Jang, S.; Lee, S.; Jeon, S.; Lee, S.; Kim, J.H.; Lee, K.H. Polypharmacy and Elevated Risk of Severe Adverse Events in Older Adults Based on the Korea Institute of Drug Safety and Risk Management-Korea Adverse Event Reporting System Database. J. Korean Med. Sci. 2024, 39, e205. [Google Scholar] [CrossRef] [PubMed]
- Penner, L.S.; Gavan, S.P.; Ashcroft, D.M.; Peek, N.; Elliott, R.A. Does coprescribing nonsteroidal anti-inflammatory drugs and oral anticoagulants increase the risk of major bleeding, stroke and systemic embolism? Br. J. Clin. Pharmacol. 2022, 88, 4789–4811. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.V. Epidemiology of anemia in older adults. Semin. Hematol. 2008, 45, 210–217. [Google Scholar] [CrossRef]
- Valles, J.; Santos, M.T.; Aznar, J.; Marcus, A.J.; Martinez-Sales, V.; Portoles, M.; Broekman, M.J.; Safier, L.B. Erythrocytes metabolically enhance collagen-induced platelet responsiveness via increased thromboxane production, adenosine diphosphate release, and recruitment. Blood 1991, 78, 154–162. [Google Scholar]
- Aronow, W.S.; Fleg, J.L.; Pepine, C.J.; Artinian, N.T.; Bakris, G.; Brown, A.S.; Ferdinand, K.C.; Ann Forciea, M.; Frishman, W.H.; Jaigobin, C.; et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J. Am. Soc. Hypertens. 2011, 5, 259–352. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, F.; Kleinert, R.; Roob, G.; Kleinert, G.; Kapeller, P.; Schmidt, R.; Hartung, H.P. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral haemorrhage: Evidence of microangiopathy-related microbleeds. AJNR Am. J. Neuroradiol. 1999, 20, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, S.; Brau, F.; Galluzzo, V.; Santagada, D.A.; Loreti, C.; Biscotti, L.; Laudisio, A.; Zuccalà, G.; Bernabei, R. Falls among Older Adults: Screening, Identification, Rehabilitation, and Management. Appl. Sci. 2022, 12, 7934. [Google Scholar] [CrossRef]
- Andreotti, F.; Rocca, B.; Husted, S.; Ajjan, R.A.; ten Berg, J.; Cattaneo, M.; Collet, J.P.; De Caterina, R.; Fox, K.A.; Halvorsen, S.; et al. Antithrombotic therapy in the elderly: Expert position paper of the European Society of Cardiology Working Group on Thrombosis. Eur. Heart J. 2015, 36, 3238–3249. [Google Scholar] [CrossRef] [PubMed]
- Gaziano, J.M.; Brotons, C.; Coppolecchia, R.; Cricelli, C.; Darius, H.; Gorelick, P.B.; Howard, G.; Pearson, T.A.; Rothwell, P.M.; Ruilope, L.M.; et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): A randomised, double-blind, placebo-controlled trial. Lancet 2018, 392, 10361046. [Google Scholar]
- ASCEND Study Collaborative Group; Bowman, L.; Mafham, M.; Wallendszus, K.; Stevens, W.; Buck, G.; Barton, J.; Murphy, K.; Aung, T.; Haynes, R.; et al. Effects of Aspirin for Primary Prevention in Persons with Diabetes Mellitus. N. Engl. J. Med. 2018, 379, 15291539. [Google Scholar]
- Soodi, D.; VanWormer, J.J.; Rezkalla, S.H. Aspirin in Primary Prevention of Cardiovascular Events. Clin. Med. Res. 2020, 18, 89–94. [Google Scholar] [CrossRef]
- McNeil, J.J.; Wolfe, R.; Woods, R.L.; Tonkin, A.M.; Donnan, G.A.; Nelson, M.R.; Reid, C.M.; Lockery, J.E.; Kirpach, B.; Storey, E.; et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N. Engl. J. Med. 2018, 379, 1509–1518. [Google Scholar] [CrossRef]
- McNeil, J.J.; Woods, R.L.; Nelson, M.R.; Reid, C.M.; Kirpach, B.; Wolfe, R.; Storey, E.; Shah, R.C.; Lockery, J.E.; Tonkin, A.M.; et al. Effect of aspirin on disability-free survival in the healthy elderly. N. Engl. J. Med. 2018, 379, 1499–1508. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll.Cardiol. 2019, 74, e177–e232. [Google Scholar] [CrossRef]
- Laudani, C.; Giacoppo, D.; Greco, A.; Ortega-Paz, L.; El Khoury, G.; Capodanno, D.; Angiolillo, D.J. Antiplatelet Monotherapies for Long-Term Secondary Prevention Following Percutaneous Coronary Intervention. J. Clin. Med. 2025, 14, 5536. [Google Scholar] [CrossRef] [PubMed]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes: Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Wei, P.; Wang, X.; Fu, Q.; Cao, B. Progress in the clinical effects and adverse reactions of ticagrelor. Thromb. J. 2024, 22, 8. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, W.; Ye, J.; Lin, Y.; Yu, B.; Zhou, L.; Zhou, Y.; Dong, H. Effect of GP IIb/IIIa inhibitor duration on the clinical prognosis of primary percutaneous coronary intervention in ST-segment elevation myocardial infarction with no-/slow-reflow phenomenon. Biomed. Pharmacother. 2021, 143, 112196. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, A.E.; Nanna, M.G.; Wang, T.Y.; Bhatt, D.L.; Angiolillo, D.J.; Mehran, R.; Banerjee, S.; Cantrell, S.; Jones, W.S.; Rymer, J.A.; et al. Bridging antiplatelet therapy after percutaneous coronary intervention: JACC review topic of the week. J. Am. Coll. Cardiol. 2021, 78, 1550–1563. [Google Scholar] [CrossRef]
- Harrington, R.A.; Stone, G.W.; McNulty, S.; White, H.D.; Lincoff, A.M.; Gibson, C.M.; Pollack, C.V., Jr.; Montalescot, G.; Mahaffey, K.W.; Kleiman, N.S.; et al. Platelet inhibition with cangrelor in patients undergoing PCI. N. Engl. J. Med. 2009, 361, 2318–2329. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Stone, G.W.; Mahaffey, K.W.; Gibson, C.M.; Steg, P.G.; Hamm, C.W.; Price, M.J.; Leonardi, S.; Gallup, D.; Bramucci, E.; et al. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N. Engl. J. Med. 2013, 368, 1303–1313. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Lincoff, A.M.; Gibson, C.M.; Stone, G.W.; McNulty, S.; Montalescot, G.; Kleiman, N.S.; Goodman, S.G.; White, H.D.; Mahaffey, K.W.; et al. Intravenous platelet blockade with cangrelor during PCI. N. Engl. J. Med. 2009, 361, 2330–2341. [Google Scholar] [CrossRef]
- Steg, P.G.; Bhatt, D.L.; Hamm, C.W.; Stone, G.W.; Gibson, C.M.; Mahaffey, K.W.; Leonardi, S.; Liu, T.; Skerjanec, S.; Day, J.R.; et al. Effect of cangrelor on periprocedural outcomes in percutaneous coronary interventions: A pooled analysis of patient-level data. Lancet 2013, 382, 1981–1992. [Google Scholar] [CrossRef]
- Capranzano, P.; Calabro, P.; Musumeci, G.; Mario, C.D.; Chirillo, F.; Rolfo, C.; Menozzi, A.; Menichelli, M.; Maffeo, D.; Talanas, G.; et al. Use of Cangrelor in Older Patients: Findings from the itAlianpRospective Study on CANGrELOr Study. Am. J. Cardiol. 2025, 240, 31–37. [Google Scholar] [CrossRef]
- Yusuf, S.; Zhao, F.; Mehta, S.R.; Chrolavicius, S.; Tognoni, G.; Fox, K.K.; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N. Engl. J. Med. 2001, 345, 494–502. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.J.; Ardissino, D.; De Servi, S.; Murphy, S.A.; et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef] [PubMed]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef]
- Wrishko, R.E.; Ernest, C.S., 2nd; Small, D.S.; Li, Y.G.; Weerakkody, G.J.; Riesmeyer, J.R.; Macias, W.L.; Rohatagi, S.; Salazar, D.E.; Antman, E.M.; et al. Population pharmacokinetic analyses to evaluate the influence of intrinsic and extrinsic factors on exposure of prasugrel active metabolite in TRITON-TIMI 38. J. Clin. Pharmacol. 2009, 49, 984–998. [Google Scholar] [CrossRef]
- Erlinge, D.; Gurbel, P.A.; James, S.; Lindahl, T.L.; Svensson, P.; Ten Berg, J.M.; Foley, D.P.; Wagner, H.; Brown, P.B.; Luo, J.; et al. Prasugrel 5 mg in the very elderly attenuates platelet inhibition but maintains noninferiority to prasugrel 10 mg in nonelderly patients: The GENERATIONS trial, a pharmacodynamic and pharmacokinetic study in stable coronary artery disease patients. J. Am. Coll. Cardiol. 2013, 62, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Roe, M.T.; Goodman, S.G.; Ohman, E.M.; Stevens, S.R.; Hochman, J.S.; Gottlieb, S.; Martinez, F.; Dalby, A.J.; Boden, W.E.; White, H.D.; et al. Elderly patients with acute coronary syndromes managed without revascularization: Insights into the safety of long-term dual antiplatelet therapy with reduced-dose prasugrel versus standard dose clopidogrel. Circulation 2013, 128, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Savonitto, S.; Ferri, L.A.; Piatti, L.; Grosseto, D.; Piovaccari, G.; Morici, N.; Bossi, I.; Sganzerla, P.; Tortorella, G.; Cacucci, M.; et al. Comparison of reduced-dose prasugrel and standard-dose clopidogrel in elderly patients with acute coronary syndromes undergoing early percutaneous revascularization. Circulation 2018, 137, 2435–2445. [Google Scholar] [CrossRef]
- Capodanno, D.; Alfonso, F.; Levine, G.N.; Valgimigli, M.; Angiolillo, D.J. ACC/AHA versus ESC guidelines on dual antiplatelet therapy: JACC guideline comparison. J. Am. Coll. Cardiol. 2018, 72, 2915–2931. [Google Scholar] [CrossRef]
- Schmucker, J.; Fach, A.; Mata Marin, L.A.; Retzlaff, T.; Osteresch, R.; Kollhorst, B.; Hambrecht, R.; Pohlabeln, H.; Wienbergen, H. Efficacy and safety of ticagrelor in comparison to clopidogrel in elderly patients with ST-segment elevation myocardial infarctions. J. Am. Heart Assoc. 2019, 8, e012530. [Google Scholar] [CrossRef] [PubMed]
- Szummer, K.; Montez-Rath, M.E.; Alfredsson, J.; Erlinge, D.; Lindahl, B.; Hofmann, R.; Ravn-Fischer, A.; Svensson, P.; Jernberg, T. Comparison between ticagrelor and clopidogrel in elderly patients with an acute coronary syndrome—Insights from the SWEDEHEART registry. Circulation 2020, 42, 1700–1708. [Google Scholar] [CrossRef] [PubMed]
- Gimbel, M.; Qaderdan, K.; Willemsen, L.; Hermanides, R.; Bergmeijer, T.; de Very, E.; Heestermans, T.; Tjon Joe Gin, M.; Waalewijn, R.; Hofma, S.; et al. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-STelevation acute coronary syndrome (POPular AGE): The randomised, open-label, non-inferiority trial. Lancet 2020, 395, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; van Klaveren, D.; James, S.; Heg, D.; Räber, L.; Feres, F.; Pilgrim, T.; Hong, M.K.; Kim, H.S.; Colombo, A.; et al. PRECISE-DAPT Study Investigators. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: A pooled analysis of individual-patient datasets from clinical trials. Lancet 2017, 389, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Völschow, B.; Goßling, A.; Kellner, C.; Neumann, J.T. Frailty prevalence, invasive treatment frequency, and in-hospital outcome in patients hospitalized for acute coronary syndrome in Germany (2005–2022): A nationwide registry study. Lancet Reg. Health Eur. 2024, 49, 101168. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Bhatt, D.L.; Steg, P.G.; Storey, R.F.; Cohen, M.; Im, K.; Oude Ophuis, T.; Budaj, A.; Goto, S.; López-Sendón, J.; et al. Ischaemic risk and efficacy of ticagrelor in relation to time from P2Y12 inhibitor withdrawal in patients with prior myocardial infarction: Insights from PEGASUS-TIMI 54. Eur. Heart J. 2016, 37, 1133–1142. [Google Scholar] [CrossRef]
- Piccolo, R.; Magnani, G.; Ariotti, S.; Gargiulo, G.; Marino, M.; Santucci, A.; Franzone, A.; Tebaldi, M.; Heg, D.; Windecker, S.; et al. Ischaemic and bleeding outcomes in elderly patients undergoing a prolonged versus shortened duration of dual antiplatelet therapy after percutaneous coronary intervention: Insights from the PRODIGY randomised trial. EuroIntervention 2017, 13, 78–86. [Google Scholar] [CrossRef]
- Schulz-Schupke, S.; Byrne, R.A.; Ten Berg, J.M.; Neumann, F.J.; Han, Y.; Adriaenssens, T.; Tölg, R.; Seyfarth, M.; Maeng, M.; Zrenner, B.; et al. ISAR-SAFE: A randomized, double-blind, placebo-controlled trial of 6 vs. 12 months of clopidogrel therapy after drug-eluting stenting. Eur. Heart J. 2015, 36, 1252–1263. [Google Scholar] [CrossRef]
- Giustino, G.; Baber, U.; Sartori, S.; Mehran, R.; Mastoris, I.; Kini, A.S.; Sharma, S.K.; Pocock, S.J.; Dangas, G.D. Duration of dual antiplatelet therapy after drug-eluting stent implantation: A systematic review and meta-analysis of randomized controlled trials. J. Am. Coll. Cardiol. 2015, 65, 1298–1310. [Google Scholar] [CrossRef]
- Gitto, M.; Baber, U.; Sartori, S.; Vogel, B.; Angiolillo, D.J.; Briguori, C.; Cohen, D.J.; Collier, T.; Dudek, D.; Oliva, A.; et al. Ticagrelor monotherapy versus ticagrelor plus aspirin in patients with chronic coronary syndrome and high ischaemic risk: A post hoc analysis of the TWILIGHT trial. EuroIntervention 2025, 21, 550–559. [Google Scholar] [CrossRef]
- Kunadian, V.; Gitto, M.; Vogel, B.; Sartori, S.; Angiolillo, D.J.; Bhatt, D.L.; Chehab, B.M.; Feng, Y.; de la Torre Hernandez, J.M.; Krucoff, M.W.; et al. 1-Month or 3-Month DAPT in Women and Men at High Bleeding Risk Undergoing PCI. JACC Cardiovasc. Interv. 2025, 18, 866–878. [Google Scholar] [CrossRef] [PubMed]
- Jin, I.T.; Kim, Y.; Heo, S.J.; Lee, Y.J.; Lee, S.J.; Hong, S.J.; Ahn, C.M.; Kim, J.S.; Cho, D.K.; Ko, Y.G.; et al. Comparison of Short-term and Standard Duration Dual Antiplatelet Therapy in Elderly Patients: A Pooled Analysis of Five Korean Randomized Clinical Trials. Korean Circ. J. 2025, 55, e121. [Google Scholar] [CrossRef] [PubMed]
- Nana, M.; Sutton, N.R.; Kochar, A.; Rymer, J.A.; Lowenstern, A.M.; Gackenbach, G.; Hummel, S.L.; Goyal, P.; Rich, M.W.; Kirkpatrick, J.N.; et al. Assessment and management of older aults undergoing PCI, Part 1. A JACC: Advances Expert Panel. JACC Adv. 2023, 2, 100389. [Google Scholar] [CrossRef]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.P.; Costa, F.; Jeppsson, A.; Jüni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur. Heart J. 2018, 39, 213–254. [Google Scholar] [CrossRef]
- Chen, Q.; Lapane, K.; Nunes, A.P.; Tjia, J.; Hugunin, J.; Alcusky, M. Prevalence and the factors associated with oral anticoagulant use among nursing home residents. J. Clin. Pharm. Ther. 2021, 46, 1714–1728. [Google Scholar] [CrossRef]
- Heeringa, J.; van der Kuip, D.A.M.; Hofman, A.; Kors, J.A.; van Herpen, G.; Stricker, B.H.C.; Stijnen, T.; Lip, G.Y.H.; Witteman, J.C.M. Prevalence, incidence and lifetime risk of atrial fibrillation: The Rotterdam study. Eur. Heart J. 2006, 27, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Hylek, E.M.; Phillips, K.A.; Chang, Y.; Henault, L.E.; Selby, J.V.; Singer, D.E. Prevalence of diagnosed atrial fibrillation in adults national implications for rhythm management and stroke prevention: The anticoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA 2001, 285, 2370–2375. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the european association for cardio-thoracic surgery (EACTS): The task force for the diagnosis and management of atrial fibrillation of the european society of cardiology (ESC) developed with the special contribution of the european heart rhythm association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Ortel, T.L.; Neumann, I.; Ageno, W.; Beyth, R.; Clark, N.P.; Cuker, A.; Hutten, B.A.; Jaff, M.R.; Manja, V.; Schulman, S.; et al. American society of hematology 2020 guidelines for management of venous thromboembolism: Treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020, 4, 4693–4738. [Google Scholar] [CrossRef]
- Steffel, J.; Collins, R.; Antz, M.; Cornu, P.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; Rowell, N.; et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Europace 2021, 23, 1612–1676. [Google Scholar] [CrossRef]
- Stuby, J.; Haschke, M.; Tritschler, T.; Aujesky, D. Oral anticoagulant therapy in older adults. Thromb. Res. 2024, 238, 1–10. [Google Scholar] [CrossRef]
- Lin, L.; Lim, W.S.; Zhou, H.J.; Khoo, A.L.; Tan, K.T.; Chew, A.P.; Foo, D.; Chin, J.J.; Lim, B.P. Clinical and safety outcomes of oral antithrombotics for stroke prevention in atrial fibrillation: A systematic review and network meta-analysis. J. Am. Med. Dir. Assoc. 2015, 16, 1103.e1–1103.e19. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, T.; Chen, X.; Tian, W.; Ma, D.; Zhang, J.; Li, Q.; Chen, Z.; Ju, J.; Xu, H.; et al. Efficacy and safety of oral anticoagulants in older adult patients with atrial fibrillation: Pairwise and network meta-analyses. J. Am. Med. Dir. Assoc. 2023, 24, 1233–1239.e26. [Google Scholar] [CrossRef]
- Grymonprez, M.; De Backer, T.L.; Bertels, X.; Steurbaut, S.; Lahousse, L. Long-term comparative effectiveness and safety of dabigatran, rivaroxaban, apixaban and edoxaban in patients with atrial fibrillation: A nationwide cohort study. Front. Pharmacol. 2023, 14, 1125576. [Google Scholar] [CrossRef]
- Okumura, K.; Akao, M.; Yoshida, T.; Kawata, M.; Okazaki, O.; Akashi, S.; Eshima, K.; Tanizawa, K.; Fukuzawa, M.; Hayashi, T.; et al. Low dose edoxaban in very elderly patients with atrial fibrillation. N. Engl. J. Med. 2020, 383, 1735–1745. [Google Scholar] [CrossRef]
- Akashi, S.; Oguri, M.; Ikeno, E.; Manita, M.; Taura, J.; Watanabe, S.; Hayashi, T.; Akao, M.; Okumura, K.; Akishita, M.; et al. Outcomes and safety of very-low-dose edoxaban in frail patients with atrial fibrillation in the ELDERCARE-AF randomized clinical trial. JAMA Netw. Open 2022, 5, e2228500. [Google Scholar] [CrossRef] [PubMed]
- AGS. American geriatrics society 2023 updated AGS beers criteria® for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 2023, 71, 2052–2081. [Google Scholar] [CrossRef]
- Joosten, L.P.; van Doorn, S.; van de Ven, P.M.; Köhlen, B.T.G.; Nierman, M.C.; Koek, H.L.; Hemels, M.E.W.; Huisman, M.V.; Kruip, M.; Faber, L.M.; et al. Safety of switching from a vitamin K antagonist to a non-vitamin K antagonist oral anticoagulant in frail older patients with atrial fibrillation: Results of the FRAIL-AF randomized controlled trial. Circulation 2023, 149, 279–289. [Google Scholar] [CrossRef]
- Greco, A.; Laudani, C.; Spagnolo, M.; Agnello, F.; Faro, D.C.; Finocchiaro, S.; Legnazzi, M.; Mauro, M.S.; Mazzone, P.M.; Occhipinti, G.; et al. Pharmacology and Clinical Development of Factor XI Inhibitors. Circulation 2023, 147, 897–913. [Google Scholar] [CrossRef]
- Presume, J.; Ferreira, J.; Ribeiras, R. Factor XI Inhibitors: A New Horizon in Anticoagulation Therapy. Cardiol. Ther. 2024, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Harrington, J.; Piccini, J.P.; Alexander, J.H.; Granger, C.B.; Patel, M.R. Clinical Evaluation of Factor XIa Inhibitor Drugs. J. Am. Coll. Cardiol. 2023, 81, 771–779. [Google Scholar] [CrossRef]
- Muscente, F.; De Caterina, R. The new in anticoagulation: Factor XI inhibitors. Eur. Heart J. Suppl. 2023, 25 (Suppl. B), B65–B68. [Google Scholar] [CrossRef]
- Piccini, J.P.; Caso, V.; Connolly, S.J.; Fox, K.A.A.; Oldgren, J.; Jones, W.S.; Gorog, D.A.; Durdil, V.; Viethen, T.; Neumann, C.; et al. Safety of the oral factor XIa inhibitor asundexian compared with apixaban in patients with atrial fibrillation (PACIFIC-AF): A multicentre, randomised, double-blind, double-dummy, dose-finding phase 2 study. Lancet 2022, 399, 1383–1390. [Google Scholar] [CrossRef]
- Piccini, J.P.; Patel, M.R.; Steffel, J.; Ferdinand, K.; Van Gelder, I.C.; Russo, A.M.; Ma, C.S.; Goodman, S.G.; Oldgren, J.; Hammett, C.; et al. Asundexian versus Apixaban in Patients with Atrial Fibrillation. N. Engl. J. Med. 2025, 392, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Molina, C.A.; Toyoda, K.; Bereczki, D.; Bangdiwala, S.I.; Kasner, S.E.; Lutsep, H.L.; Tsivgoulis, G.; Ntaios, G.; Czlonkowska, A.; et al. Safety and efficacy of factor XIa inhibition with milvexian for secondary stroke prevention (AXIOMATIC-SSP): A phase 2, international, randomised, double-blind, placebo-controlled, dose-finding trial. Lancet Neurol. 2024, 23, 46–59. [Google Scholar] [CrossRef]
- Weitz, J.I.; Strony, J.; Ageno, W.; Gailani, D.; Hylek, E.M.; Lassen, M.R.; Mahaffey, K.W.; Notani, R.S.; Roberts, R.; Segers, A.; et al. Milvexian for the prevention of venous thromboembolism. N. Engl. J. Med. 2021, 385, 2161–2172. [Google Scholar] [CrossRef] [PubMed]
- Verhamme, P.; Yi, B.A.; Segers, A.; Salter, J.; Bloomfield, D.; Büller, H.R.; Raskob, G.E.; Weitz, J.I. ANT-005 TKA Investigators. Abelacimab for prevention of venous thromboembolism. N. Engl. J. Med. 2021, 385, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Ruff, C.T.; Patel, S.M.; Giugliano, R.P.; Morrow, D.A.; Hug, B.; Kuder, J.F.; Goodrich, E.L.; Chen, S.A.; Goodman, S.G.; Joung, B.; et al. Abelacimab versus Rivaroxaban in Patients with Atrial Fibrillation. N. Engl. J. Med. 2025, 392, 361–371. [Google Scholar] [CrossRef]
- Janssen Research & Development, LLC. An Open-Label, Randomized, Crossover Study to Evaluate the Relative Oral Bioavailability, Pharmacokinetics, and Food Effect After Single Dose (for Part 1, Part 3, Part 4 and Part 5) or Multiple-Dose (for Part 2) Administration of JNJ-70033093 (Milvexian) in Healthy Adult Participants Using Tablet, Capsule and Dispersed Tablet Formulations. 2022. Available online: https://clinicaltrials.gov/study/NCT04844424 (accessed on 1 September 2023).
- Badreldin, H.A.; Alsuhebany, N.; Alzahrani, M.; Alshehri, A.M.; Aldoughaim, M.; Alqifari, S.; Yassin, O.; Alfehaid, L.; Alqahtani, T. A leap forward in anticoagulation with FXI and FXIa Inhibition. Curr. Res. Pharmacol. Drug Discov. 2024, 6, 100179. [Google Scholar] [CrossRef]
- Dunlay, S.M.; Chamberlain, A.M. Multimorbidity in Older Patients with Cardiovascular Disease. Curr. Cardiovasc. Risk Rep. 2016, 10, 3. [Google Scholar] [CrossRef]
- Castiello, D.S.; Buongiorno, F.; Manzi, L.; Narciso, V.; Forzano, I.; Florimonte, D.; Sperandeo, L.; Canonico, M.E.; Avvedimento, M.; Paolillo, R.; et al. Procedural and Antithrombotic Therapy Optimization in Patients with Atrial Fibrillation Undergoing Percutaneous Coronary Intervention: A Narrative Review. J. Cardiovasc. Dev. Dis. 2025, 12, 142. [Google Scholar] [CrossRef]
- Lip, G.Y.; Huber, K.; Andreotti, F.; Arnesen, H.; Airaksinen, J.K.; Cuisset, T.; Kirchhof, P.; Marín, F. Antithrombotic management of atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing coronary stenting: Executive summary—A Consensus Document of the European Society of Cardiology Working Group on Thrombosis, endorsed by the European Heart Rhythm Association (EHRA) and the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 2010, 31, 1311–1318. [Google Scholar] [CrossRef]
- Lamberts, M.; Olesen, J.B.; Ruwald, M.H.; Hansen, C.M.; Karasoy, D.; Kristensen, S.L.; Køber, L.; Torp-Pedersen, C.; Gislason, G.H.; Hansen, M.L. Bleeding After Initiation of Multiple Antithrombotic Drugs, Including Triple Therapy, in Atrial Fibrillation Patients Following Myocardial Infarction and Coronary Intervention. Circulation 2012, 126, 1185–1193. [Google Scholar] [CrossRef]
- Hess, C.N.; Peterson, E.D.; Peng, S.A.; de Lemos, J.A.; Fosbol, E.L.; Thomas, L.; Bhatt, D.L.; Saucedo, J.F.; Wang, T.Y. Use and Outcomes of Triple Therapy Among Older Patients With Acute Myocardial Infarction and Atrial Fibrillation. J. Am. Coll. Cardiol. 2015, 66, 616–627. [Google Scholar] [CrossRef]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef]
- Dewilde, W.J.; Oirbans, T.; Verheugt, F.W.; Kelder, J.C.; De Smet, B.J.; Herrman, J.P.; Adriaenssens, T.; Vrolix, M.; Heestermans, A.A.; Vis, M.M.; et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: An open-label, randomised, controlled trial. Lancet 2013, 381, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.M.; Mehran, R.; Bode, C.; Halperin, J.; Verheugt, F.W.; Wildgoose, P.; Birmingham, M.; Ianus, J.; Burton, P.; van Eickels, M.; et al. Prevention of Bleeding in Patients with Atrial Fibrillation Undergoing PCI. N. Engl. J. Med. 2016, 375, 2423–2434. [Google Scholar] [CrossRef]
- Cannon, C.P.; Bhatt, D.L.; Oldgren, J.; Lip, G.Y.H.; Ellis, S.G.; Kimura, T.; Maeng, M.; Merkely, B.; Zeymer, U.; Gropper, S.; et al. Dual Antithrombotic Therapy with Dabigatran after PCI in Atrial Fibrillation. N. Engl. J. Med. 2017, 377, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Vranckx, P.; Valgimigli, M.; Eckardt, L.; Tijssen, J.; Lewalter, T.; Gargiulo, G.; Batushkin, V.; Campo, G.; Lysak, Z.; Vakaliuk, I.; et al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): A randomised, open-label, phase 3b trial. Lancet 2019, 394, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.D.; Heizer, G.; Aronson, R.; Vora, A.N.; Massaro, T.; Mehran, R.; Goodman, S.G.; Windecker, S.; Darius, H.; Li, J.; et al. Antithrombotic Therapy after Acute Coronary Syndrome or PCI in Atrial Fibrillation. N. Engl. J. Med. 2019, 380, 1509–1524. [Google Scholar] [CrossRef]
- Andreou, I.; Briasoulis, A.; Pappas, C.; Ikonomidis, I.; Alexopoulos, D. Ticagrelor Versus Clopidogrel as Part of Dual or Triple Antithrombotic Therapy: A Systematic Review and Meta-Analysis. Cardiovasc. Drugs Ther. 2018, 32, 287–294. [Google Scholar] [CrossRef]
- Gargiulo, G.; Goette, A.; Tijssen, J.; Eckardt, L.; Lewalter, T.; Vranckx, P.; Valgimigli, M. Safety and efficacy outcomes of double vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: A systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials. Eur. Heart J. 2019, 40, 3757–3767. [Google Scholar]
- Chandrasekhar, J.; Baber, U.; Sartori, S.; Goel, R.; Nicolas, J.; Vogel, B.; Snyder, C.; Kini, A.; Briguori, C.; Witzenbichler, B.; et al. Antithrombotic strategy variability in atrial fibrillation and obstructive coronary disease revascularised with percutaneous coronary intervention: Primary results from the AVIATOR 2 international registry. EuroIntervention 2022, 18, e656–e665. [Google Scholar] [CrossRef]
- De Luca, L.; Bolognese, L.; Rubboli, A.; Vetrano, A.; Callerame, M.; Rivetti, L.; Gonzini, L.; Gabrielli, D.; Di Lenarda, A.; Gulizia, M.M. Combinations of antithrombotic therapies prescribed after percutaneous coronary intervention in patients with acute coronary syndromes and atrial fibrillation: Data from the nationwide MATADOR-PCI registry. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, e45–e47. [Google Scholar] [CrossRef] [PubMed]
- Sciahbasi, A.; De Rosa, S.; Gargiulo, G.; Giacoppo, D.; Calabrò, P.; Talarico, G.P.; Zilio, F.; Talanas, G.; Tebaldi, M.; Andò, G.; et al. Management of Patients Treated With Oral Anticoagulant Therapy Undergoing Percutaneous Coronery Intervention With Stent Implantation: The PERSEO Registry. J. Cardiovasc. Pharmacol. 2024, 84, 457–467. [Google Scholar] [CrossRef]
- Minardi, S.; De Rosa, S.; Salvi, N.; Andò, G.; Talanas, G.; D’Angelo, C.; Moretti, C.; Mazza, T.M.; Cortese, B.; Musumeci, G.; et al. Clinical characteristics, therapeutic strategies, and outcomes in elderly patients on oral anticoagulant therapy undergoing percutaneous coronary interventions: Post-hoc analysis of the PERSEO Registry. J. Geriatr. Cardiol. 2025, 22, E1–E8. [Google Scholar] [CrossRef] [PubMed]
- Verburg, A.; Bor, W.L.; Küçük, I.T.; Henriques, J.P.S.; Vink, M.A.; Ruifrok, W.T.; Plomp, J.; Heestermans, T.A.C.M.; Schotborgh, C.E.; Vlaar, P.J.; et al. Temporary omission of oral anticoagulation in atrial fibrillation patients undergoing percutaneous coronary intervention: Rationale and design of the WOEST-3 randomised trial. EuroIntervention 2024, 20, e898–e904. [Google Scholar] [CrossRef] [PubMed]
- De Luca, L.; Rubboli, A.; Lettino, M.; Tubaro, M.; Leonardi, S.; Casella, G.; Valente, S.; Rossini, R.; Sciahbasi, A.; Natale, E.; et al. ANMCO position paper on antithrombotic treatment of patients with atrial fibrillation undergoing intracoronary stenting and/or acute coronary syndromes. Eur. Heart J. Suppl. 2022, 24 (Suppl. C), C254–C271. [Google Scholar] [CrossRef] [PubMed]
- Sciahbasi, A.; Gargiulo, G.; Talarico, G.P.; Cesaro, A.; Zilio, F.; De Rosa, S.; Talanas, G.; Tebaldi, M.; Andò, G.; Rigattieri, S.; et al. Design of PERSEO Registry on the management of patients treated with oral anticoagulants and coronary stent. J. Cardiovasc. Med. 2022, 23, 738–743. [Google Scholar] [CrossRef]
| Condition | Mechanism | Clinical Implications | |
|---|---|---|---|
| Thrombotic Risk Factors | Increased platelet reactivity | ↑ ADP-induced platelet aggregation | Enhanced thrombus formation |
| Reduced antiplatelet activity | ↓ PGI2; ↓ circulating PGI2 | Higher platelet aggregation | |
| Endothelial dysfunction | ↓ NO production | Reduced antiplatelet effect | |
| Increased intraplatelet ROS production | ↑ NO degradation | Prothrombotic state | |
| Altered coagulation cascade | ↑ Procoagulant factors | Hypercoagulability | |
| Chronic inflammatory state | Persistent activation platelets | Sustained prothrombotic state | |
| Reduced fibrinolytic activity | ↑ PAI-1 | Impaired clot dissolution | |
| Condition | Mechanism | ClinicalImplications | |
| Bleeding Risk Factors | Vascular functional changes | ↑ capillary fragility | Increased hemorrhagic risk |
| Hepatic impairment | ↓ drug metabolism and clearance | Accumulation of drugs | |
| Hypoalbuminemia | altered distribution | Enhanced drug activity | |
| Anemia | ↓ platelet function | ↓hemostatic response | |
| Previous GI bleeding | ↑ mucosal vulnerability | Recurrence of bleeding | |
| Falls | ↓ balance, vision, proprioception | Traumatic hemorrhage | |
| Condition | Mechanism | ClinicalImplications | |
| Combined thrombotic and ischemic risk factors | Hypertension | ↑ atherosclerosis and vasculopathy | Ischemic and hemorrhagic stroke |
| Polypharmacy | Drug discontinuation/underuse | Therapeutic failure | |
| Renal impairment | Vascular disease + ↓ clearance of drugs | Ischemic and hemorrhagic events | |
| Previous vascular events | ↑ thrombotic risk + fragile vasculature | Ischemic and hemorrhagic events |
| Apixaban | Edoxaban | Rivaroxaban | Dabigatran | |
|---|---|---|---|---|
| Age ≥ 80 years | 2.5 mg BID if age ≥ 80 and ≤60 kg, or serum Cr ≥ 1.5 mg/dL | General caution advised | Caution in ≥75 years | Consider lower dose (110 mg BID) in ≥80 years |
| Low body weight | 2.5 mg BID if ≤60 kg and age ≥ 80, or serum Cr ≥ 1.5 mg/dL | 30 mg OD if ≤60 kg | General caution | Careful monitoring |
| Renal impairment (CrCl 15–50 mL/min) | 2.5 mg BID if serum Cr ≥ 1.5 mg/dL and age ≥ 80, or ≤60 kg | 30 mg OD if CrCl 15–50 mL/min | 15 mg OD if CrCl 15–49 mL/min | Consider reduction to 110 mg BID |
| Concomitant P-gp or CYP3A4 inhibitors | Caution advised | 30 mg OD | Caution advised | Caution advised |
| Frailty | Preferred agent | Suitable ts | Use with caution | Less preferred |
| Cognitive impairment | Well tolerated | Preferred for OD dose | Preferred for OD dose | Less preferred |
| High GI bleeding risk/history of GI pathology | Lower risk | Higher risk with high-dose | Higher risk | Higher risk vs. apixaban |
| Main Author | Study | Year | Main Results |
|---|---|---|---|
| Piccini J.P. et al. (PACIFIC-AF) [84] | Randomized | 2022 | Asundexian reduced bleeding compared to Apixaban |
| Piccini J.P. et al. (OCEANIC-AF) [85] | Randomized | 2025 | Asundexian was associated with higher incidence of stroke or SE. |
| Sharma M. et al. (AXIOMATIC-SSP) [86] | Randomized | 2024 | Milvexian added to dual antiplatelet therapy did not increase the risk of major bleeding |
| Weitz J. et al. (AXIOMATIC-TKR) [87] | Randomized | 2021 | Milvexian reduces VTE rates with low bleeding risk compared to Enoxaparin |
| VerhammeP. et al. (ANT-005 TKA) [88] | Randomized | 2021 | Single intravenous dose of Abelacimab reduced bleeding compared to Enoxaparin. |
| Ruff C.T. et al. (AZALEA-TIMI 71) [89] | Randomized | 2025 | Abelacimab reduced bleeding events compared to Rivaroxaban |
| LIBREXIA-AF | Randomized | Ongoing | Milvexian vs. Apixaban in patients with Atrial Fibrillation |
| Main Author | Study | Year | Main Results |
|---|---|---|---|
| Lamberts M., et al. [95] | Registry | 2012 | High risk of bleeding with TAT after MI/PCI |
| Hess C.N. et al. [96] | Registry | 2015 | Higher rates of bleeding with TAT without difference in MI, death, or stroke vs. DAPT |
| Dewilde W.J.M. et al. M. et al. (WOEST) [98] | Randomized | 2013 | Less bleeding with clopidogrel without aspirin and no increase in the rate of thrombotic events |
| Gibson C.M. et al. (PIONEER-AF PCI) [99] | Randomized | 2016 | Rivaroxaban plus P2Y12 inhibitor reduced bleeding compared with VKA plus DAPT |
| Cannon C.P. et al. (REDUAL-PCI) [100] | Randomized | 2017 | Dabigatran + P2Y12 inhibitors reduces bleeding vs. TAT with warfarin |
| VranckxP. et al. (ENTRUST-AF PCI) [101] | Randomized | 2019 | Edoxaban + P2Y12 inhibitors was non inferior for bleeding vs. TAT with warfarin |
| Lopes L.D. et al. (AUGUSTUS) [102] | Randomized | 2019 | Apixaban + P2Y12 inhibitors reduces bleeding vs. TT with VKA |
| Andreou I., et al. [103] | Meta-analysis | 2018 | Ticagrelor in TAT increases bleeding complications compared to clopidogrel. |
| Gargiulo G., et al. [104] | Meta-analysis | 2019 | DAT reduces bleeding compared to TAT. |
| Chandrasekhar J. et al. (AVIATOR 2) [105] | Registry | 2022 | TAT was the most common strategy without differences in outcomes compared to DAT. |
| De Luca L. et al. (MATADOR-PCI) [106] | Registry | 2021 | TAT is yet frequently utilized for long periods |
| Sciahbasi A. et al. (PERSEO) [107] | Registry | 2024 | DAT reduces bleedings compared to TAT. |
| Minardi S. et al. (PERSEO-Elderly) [108] | Registry | 2025 | Higher risk of MACCE and bleeding in elderly patients on OAT post PCI |
| Verburg A. et al. (WOEST-3) [109] | Randomized | ongoing | 1-month DAPT without oral anticoagulant therapy post PCI followed by DAT vs. SOC |
| Windecker S. et al. (MATRIX-2) (ClinicalTrials.gov Identifier: NCT05955365) | Randomized | ongoing | 1-month monotherapy with oral P2Y12 inhibitors post PCI followed by DOAC vs. SOC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciahbasi, A.; Minardi, S.; Salvi, N.; Infusino, F.; Granatelli, A. Antithrombotic Therapy in the Elderly with Cardiovascular Disease: Walking the Tightrope Between Efficacy and Bleeding Risk—A Narrative Review. J. Clin. Med. 2025, 14, 7340. https://doi.org/10.3390/jcm14207340
Sciahbasi A, Minardi S, Salvi N, Infusino F, Granatelli A. Antithrombotic Therapy in the Elderly with Cardiovascular Disease: Walking the Tightrope Between Efficacy and Bleeding Risk—A Narrative Review. Journal of Clinical Medicine. 2025; 14(20):7340. https://doi.org/10.3390/jcm14207340
Chicago/Turabian StyleSciahbasi, Alessandro, Simona Minardi, Nicolò Salvi, Fabio Infusino, and Antonino Granatelli. 2025. "Antithrombotic Therapy in the Elderly with Cardiovascular Disease: Walking the Tightrope Between Efficacy and Bleeding Risk—A Narrative Review" Journal of Clinical Medicine 14, no. 20: 7340. https://doi.org/10.3390/jcm14207340
APA StyleSciahbasi, A., Minardi, S., Salvi, N., Infusino, F., & Granatelli, A. (2025). Antithrombotic Therapy in the Elderly with Cardiovascular Disease: Walking the Tightrope Between Efficacy and Bleeding Risk—A Narrative Review. Journal of Clinical Medicine, 14(20), 7340. https://doi.org/10.3390/jcm14207340






