EZH2-Mediated PTEN Silencing Promotes AKT-Dependent Afatinib Resistance in Radiation-Resistant Cervical Cancer Cells
Abstract
Highlights
- Radiation-acquired HeLaR cervical cancer cells exhibit significant afatinib resistance.
- EZH2 epigenetically silences PTEN, leading to sustained AKT activation.
- Pharmacologic inhibition of EZH2 or PI3K/AKT restores afatinib sensitivity.
- In vivo, combination therapy with an EZH2 inhibitor and afatinib suppresses tumor growth without toxicity.
- The EZH2–PTEN–AKT axis represents a potential therapeutic target in recurrent and radioresistant cervical cancer.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Reagents
2.3. Ethics Statement
2.4. Cell Culture and Establishment of Radioresistant Cervical Cancer Cell Line
2.5. Viability Assay
2.6. Western Blot Analysis
2.7. Methylation-Specific Polymerase Chain Reaction for PTEN
2.8. Chromatin Immunoprecipitation Assays
2.9. Antitumor Activity in the Xenograft Model
2.10. Statistical Analysis
3. Results
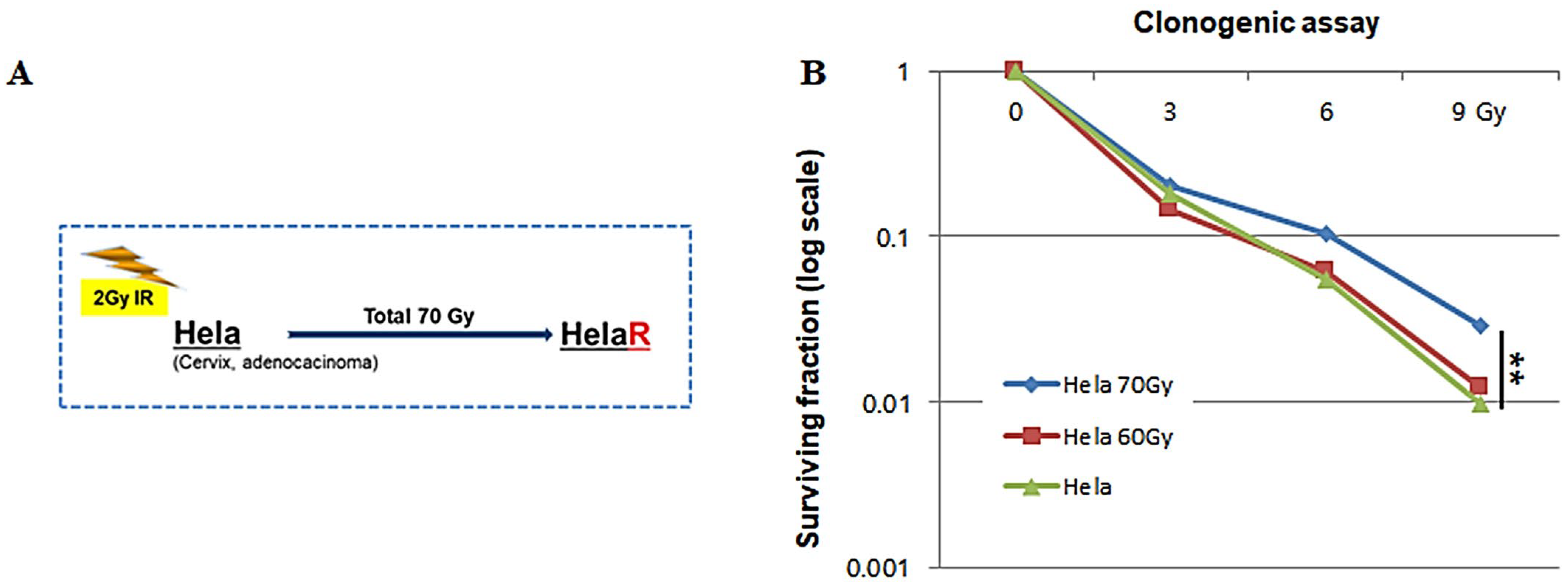
3.1. Establishment of the HeLaR Cell Line and Confirmation of Radioresistance
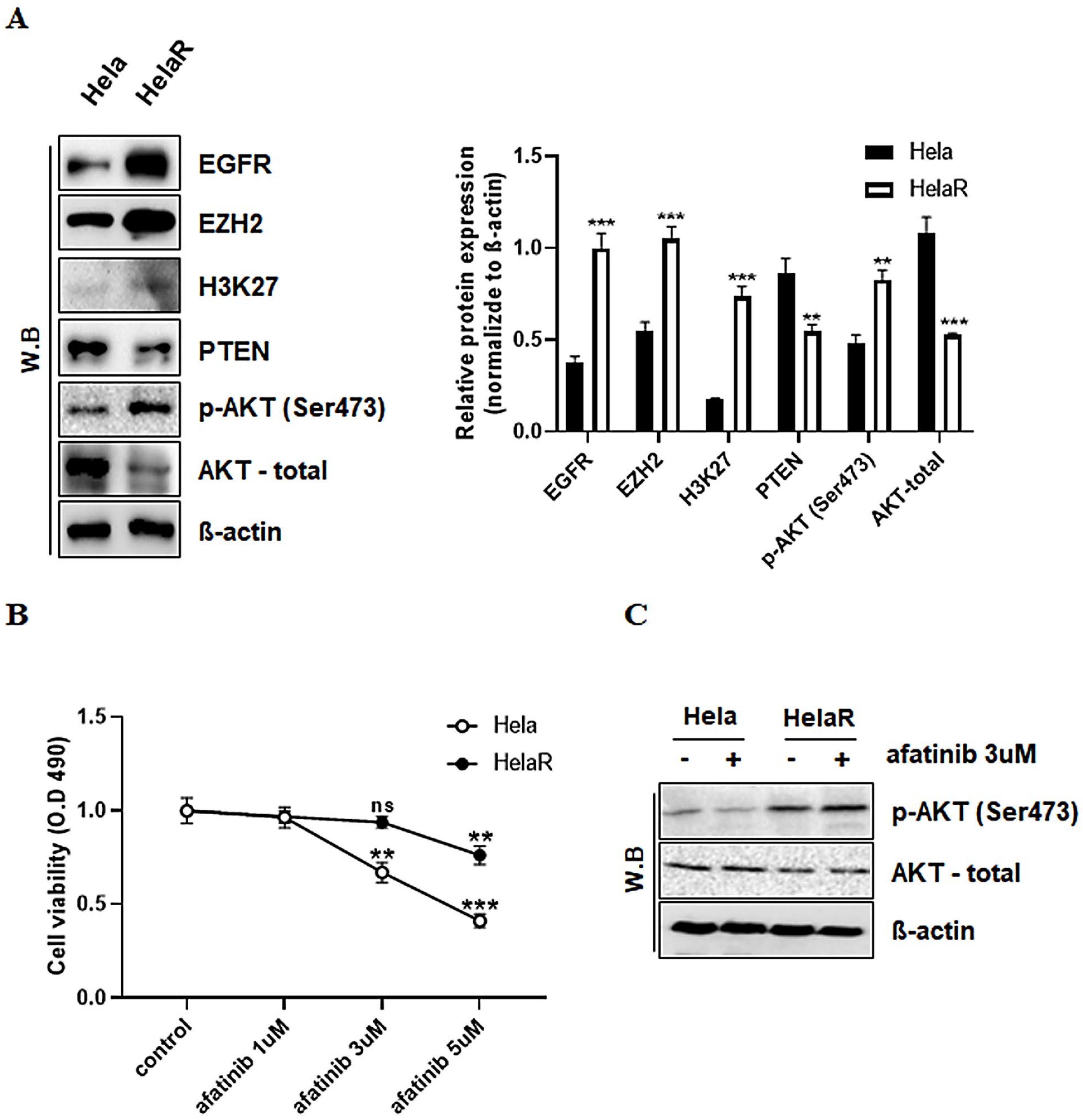
3.2. Alterations in EGFR Signaling and Reduced Sensitivity to Afatinib in HeLaR Cells
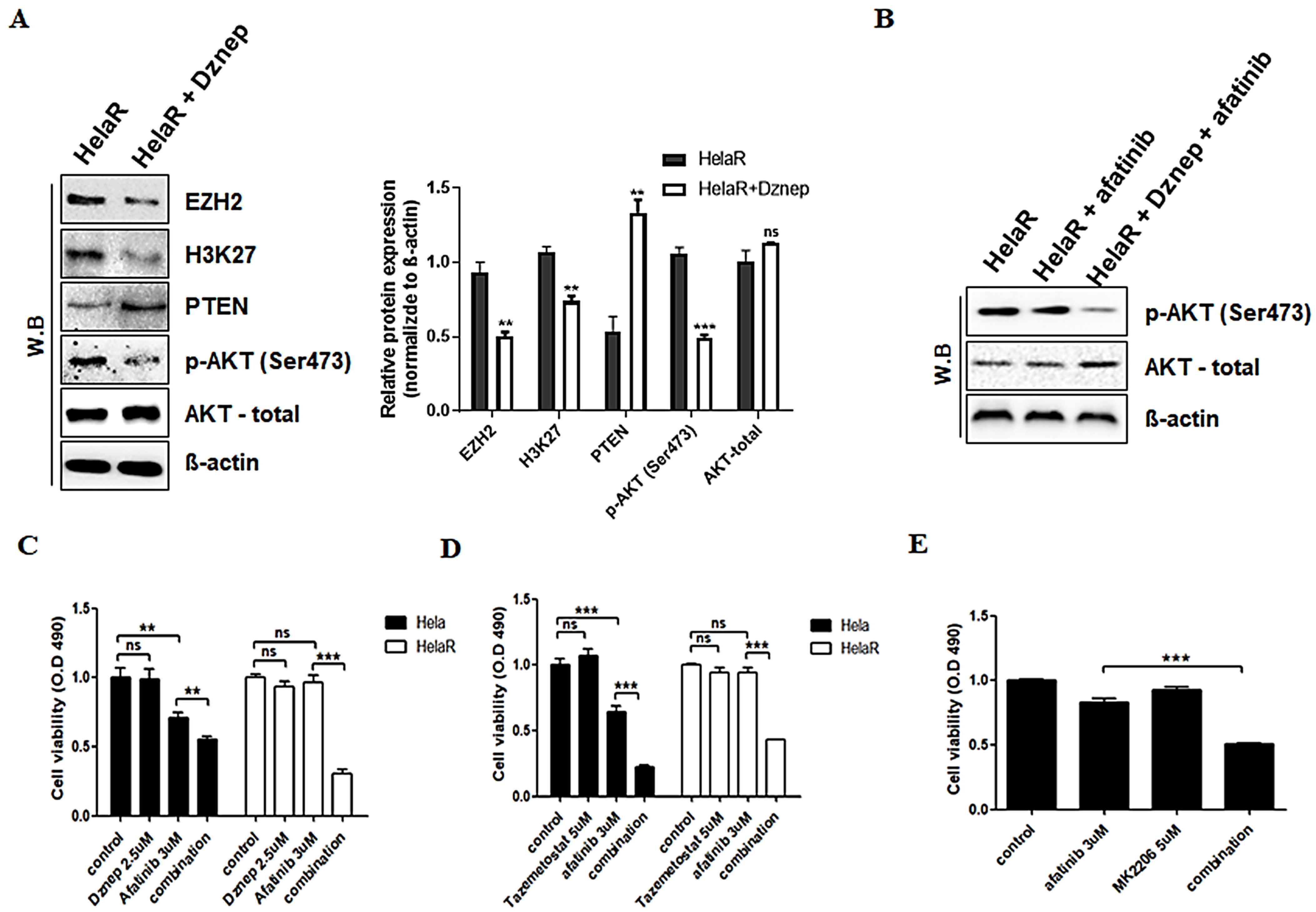
3.3. Reversal of Afatinib Resistance by EZH2 Inhibition
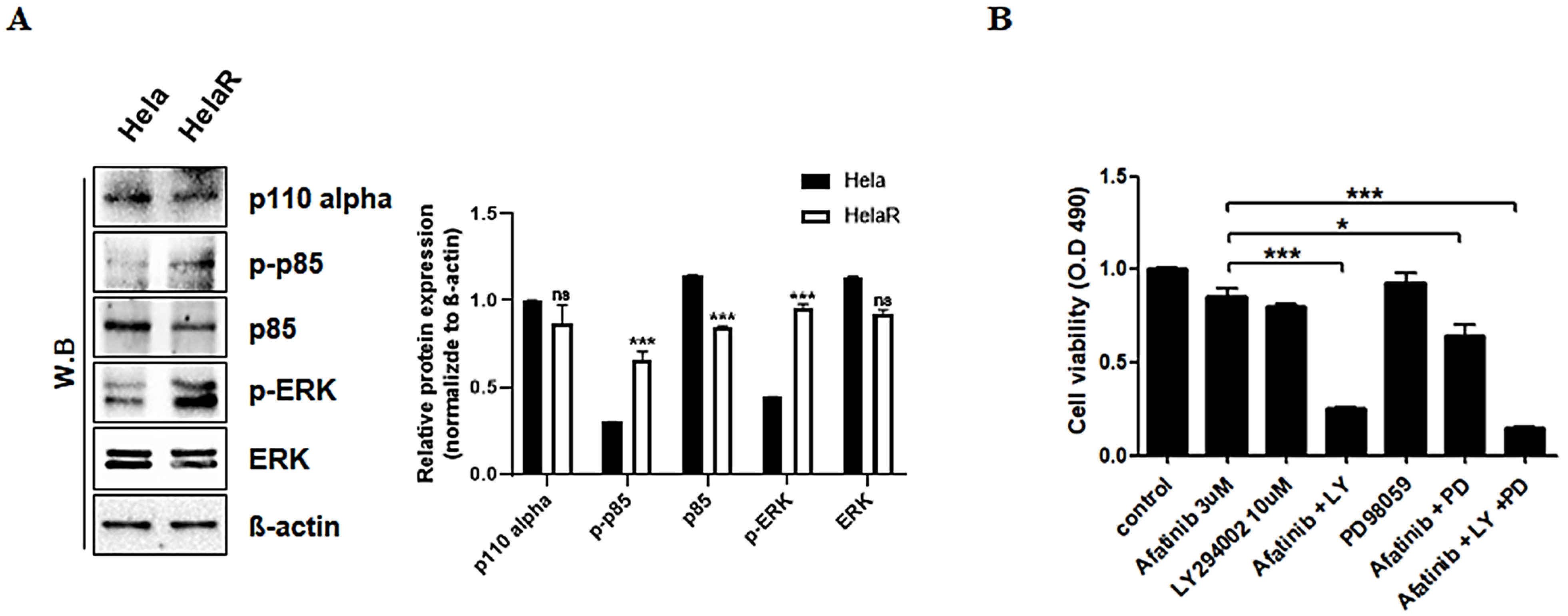
3.4. Role of PI3K and ERK Pathways in HeLaR Cell Survival
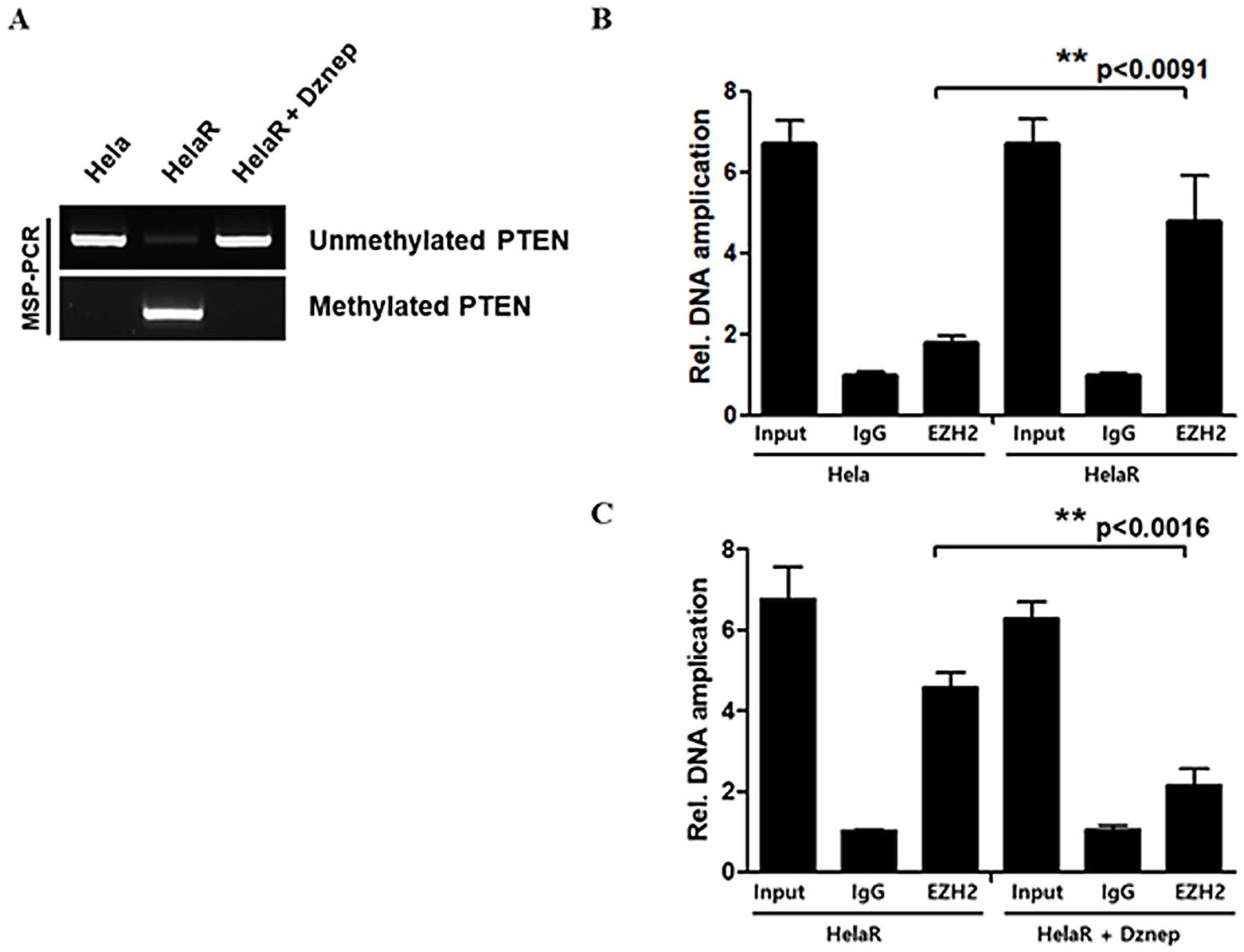
3.5. Epigenetic Silencing of PTEN by EZH2
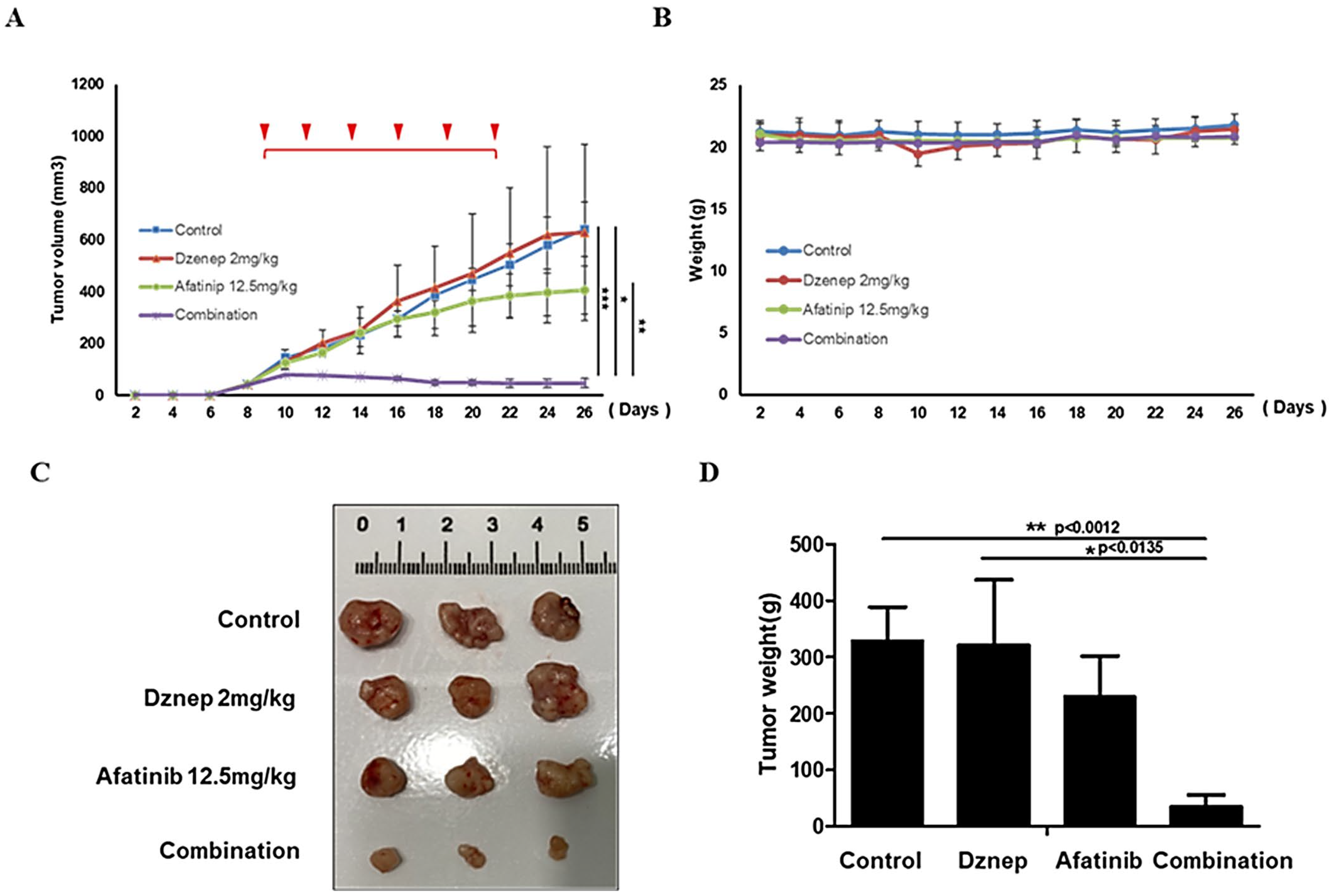
3.6. In Vivo Validation in Xenograft Models
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT | Protein kinase B |
| BSA | Bovine serum albumin |
| ChIP | Chromatin immunoprecipitation |
| CO2 | Carbon dioxide |
| DMSO | Dimethyl sulfoxide |
| DNA | Deoxyribonucleic acid |
| Dznep | 3-Deazaneplanocin A |
| ECL | Enhanced chemiluminescence |
| EGFR | Epidermal growth factor receptor |
| ERK | Extracellular signal-regulated kinase |
| EZH2 | Enhancer of zeste homolog 2 |
| FBS | Fetal bovine serum |
| Gy | Gray (unit of radiation dose) |
| H3K27me3 | Trimethylation of histone H3 at lysine 27 |
| HRP | Horseradish peroxidase |
| IgG | Immunoglobulin G |
| IRB | Institutional Review Board |
| MAPK | Mitogen-activated protein kinase |
| MSP | Methylation-specific polymerase chain reaction |
| MTS | 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium assay |
| OD490 | Optical density at 490 nm |
| PBS | Phosphate-buffered saline |
| PCR | Polymerase chain reaction |
| PI3K | Phosphoinositide 3-kinase |
| PRC2 | Polycomb repressive complex 2 |
| PTEN | Phosphatase and tensin homolog |
| qPCR | Quantitative polymerase chain reaction |
| RPMI | Roswell Park Memorial Institute medium |
| SDS-PAGE | Sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| SD | Standard deviation |
| TBST | Tris-buffered saline with Tween 20 |
| TKI | Tyrosine kinase inhibitor |
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global Cancer Statistics, 2012. Cancer Epidemiol. Biomark. Prev. 2015, 26, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Willers, H.; Held, K.D. Introduction to Clinical Radiation Biology. Cancer J. 2013, 19, 200–207. [Google Scholar] [CrossRef] [PubMed]
- McDermott, N.; Meunier, A.; Mooney, B.; Sheahan, K.; Muldoon, C.; McClean, B.; Clynes, M.; Farrell, M.; O’Connor, R. Radiotherapy and Drug Resistance in Cancer. Cancer Lett. 2014, 353, 232–240. [Google Scholar] [CrossRef]
- Xu, M.J.; Johnson, D.E.; Grandis, J.R. EGFR-Targeted Therapies in the Post-Genomic Era. Oncogene 2010, 29, 6043–6054. [Google Scholar] [CrossRef] [PubMed]
- Normanno, N.; De Luca, A.; Bianco, C.; Strizzi, L.; Mancino, M.; Maiello, M.R.; Carotenuto, A.; De Feo, G.; Caponigro, F.; Salomon, D.S. Epidermal Growth Factor Receptor (EGFR) Signaling in Cancer. Gene 2006, 366, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Wee, P.; Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Restaino, S.; Pellecchia, G.; Arcieri, M.; Bogani, G.; Taliento, C.; Greco, P.; Driul, L.; Chiantera, V.; Ercoli, A.; Fanfani, F.; et al. Management for Cervical Cancer Patients: A Comparison of the Guidelines from the International Scientific Societies (ESGO–NCCN–ASCO–AIOM–FIGO–BGCS–SEOM–ESMO–JSGO). Cancers 2024, 16, 2541. [Google Scholar] [PubMed]
- Kim, K.H.; Roberts, C.W.M. Targeting EZH2 in Cancer. Nat. Med. 2016, 22, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wu, Z.J.; Groner, A.C.; He, H.H.; Cai, C.; Lis, R.T.; Wu, X.; Stack, E.C.; Loda, M.; Liu, T.; et al. EZH2 Oncogenic Activity in Castration-Resistant Prostate Cancer Cells Is Polycomb-Independent. Science 2012, 338, 1465–1469. [Google Scholar] [CrossRef]
- González, M.E.; Dupriez, D.; Djabali, M.; Li, M.; Bhatia, N.; Howard, C.; Liu, L.; Reuther, G.W.; Roussel, M.F.; Pui, C.H. Histone Methyltransferases as Regulators of Cancer Progression. Cancer Res. 2011, 71, 2360–2370. [Google Scholar] [PubMed]
- Li, L.; Liu, J.; Delohery, T.; O’Brien, P.; Wang, Y.; Han, M.; Wei, L.; Chen, X.; Lin, J.; Zhang, T. Loss of PTEN Expression in Cancer Progression. Oncotarget 2017, 8, 21729–21740. [Google Scholar] [CrossRef]
- Hollander, M.C.; Blumenthal, G.M.; Dennis, P.A. PTEN Loss in the Continuum of Common Cancers, Rare Syndromes and Mouse Models. Nat. Rev. Cancer 2011, 11, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Xu, M.; Hua, R.; Yang, Y.; Li, Q.; Feng, Y.; Liu, T.; Guo, W. Clinical Applications of EZH2 Inhibitors in Cancer Therapy. Biomark. Res. 2018, 6, 8. [Google Scholar] [CrossRef]
- Debeb, B.G.; Lacerda, L.; Xu, W.; Larson, R.; Solley, T.; Krishnamurthy, S.; Reuben, J.M.; Ueno, N.T.; Woodward, W.A. Histone Deacetylase Inhibitors in Cancer Therapy. J. Exp. Clin. Cancer Res. 2014, 33, 52. [Google Scholar] [CrossRef]
- Liang, M.; Zhou, J.; Wang, F.; Zhang, X.; Sun, Y.; Liu, Y.; Zhao, L.; Chen, H.; Li, C.; Zhao, Y. Epigenetic Modifications in Cervical Cancer: Therapeutic Potential of Targeting EZH2. J. Cancer Res. Clin. Oncol. 2024, 150, 1959–1974. [Google Scholar]
- Huang, Y.; Zhang, W.; Fan, Y.; Zhao, Q.; Li, Y.; Liu, H.; Xu, X.; Chen, J.; Zhou, X.; Zhang, Y. Role of EZH2 in Radioresistance of Cervical Cancer. Front. Oncol. 2021, 11, 586771. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xu, Y.; Lin, C.; Wang, H.; Zhang, X.; Chen, H.; Zhao, L.; Chen, J.; Zhao, Y.; Sun, Y.; et al. EZH2 Inhibition and Its Potential in Overcoming Drug Resistance. Front. Oncol. 2022, 12, 999643. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Wang, S.; Yang, Y.; Li, T.; Zhao, H.; Zhou, X.; Liu, J.; Zhang, Y.; Chen, J.; Wang, H. Targeting the PI3K/AKT Pathway in Therapy Resistance. Nat. Commun. 2020, 11, 5242. [Google Scholar]
- Xu, M. Epigenetic Regulation and Therapeutic Resistance in Cancer. Expert Rev. Mol. Med. 2024, 26, e5. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.-H.; Kim, S.C.; Park, S.; Park, J.W.; Lee, S.-H. EZH2-Mediated PTEN Silencing Promotes AKT-Dependent Afatinib Resistance in Radiation-Resistant Cervical Cancer Cells. J. Clin. Med. 2025, 14, 7329. https://doi.org/10.3390/jcm14207329
Lee W-H, Kim SC, Park S, Park JW, Lee S-H. EZH2-Mediated PTEN Silencing Promotes AKT-Dependent Afatinib Resistance in Radiation-Resistant Cervical Cancer Cells. Journal of Clinical Medicine. 2025; 14(20):7329. https://doi.org/10.3390/jcm14207329
Chicago/Turabian StyleLee, Won-Hyoek, Seong Cheol Kim, Sungchan Park, Jeong Woo Park, and Sang-Hun Lee. 2025. "EZH2-Mediated PTEN Silencing Promotes AKT-Dependent Afatinib Resistance in Radiation-Resistant Cervical Cancer Cells" Journal of Clinical Medicine 14, no. 20: 7329. https://doi.org/10.3390/jcm14207329
APA StyleLee, W.-H., Kim, S. C., Park, S., Park, J. W., & Lee, S.-H. (2025). EZH2-Mediated PTEN Silencing Promotes AKT-Dependent Afatinib Resistance in Radiation-Resistant Cervical Cancer Cells. Journal of Clinical Medicine, 14(20), 7329. https://doi.org/10.3390/jcm14207329





