Outcomes of Living-Donor Liver Transplantation for Cholangiocarcinoma Versus Hepatocellular Carcinoma in the United States
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Design and Patient Population
2.3. Study Outcome
2.4. Statistical Methods
3. Results
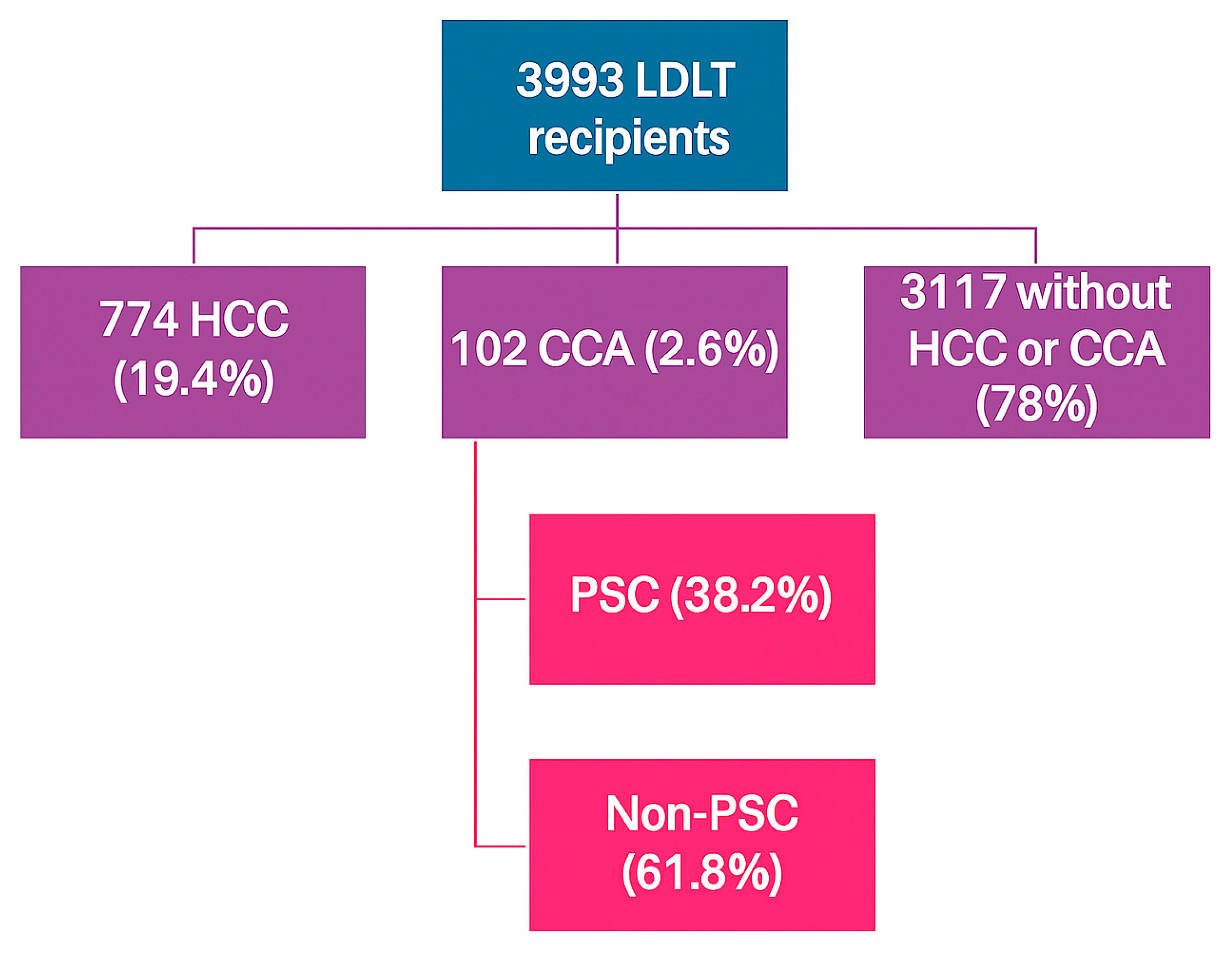
3.1. Baseline Characteristics of LDLT Recipients with CCA Versus HCC
3.2. Percentage of LDLT by Indication and UNOS Region
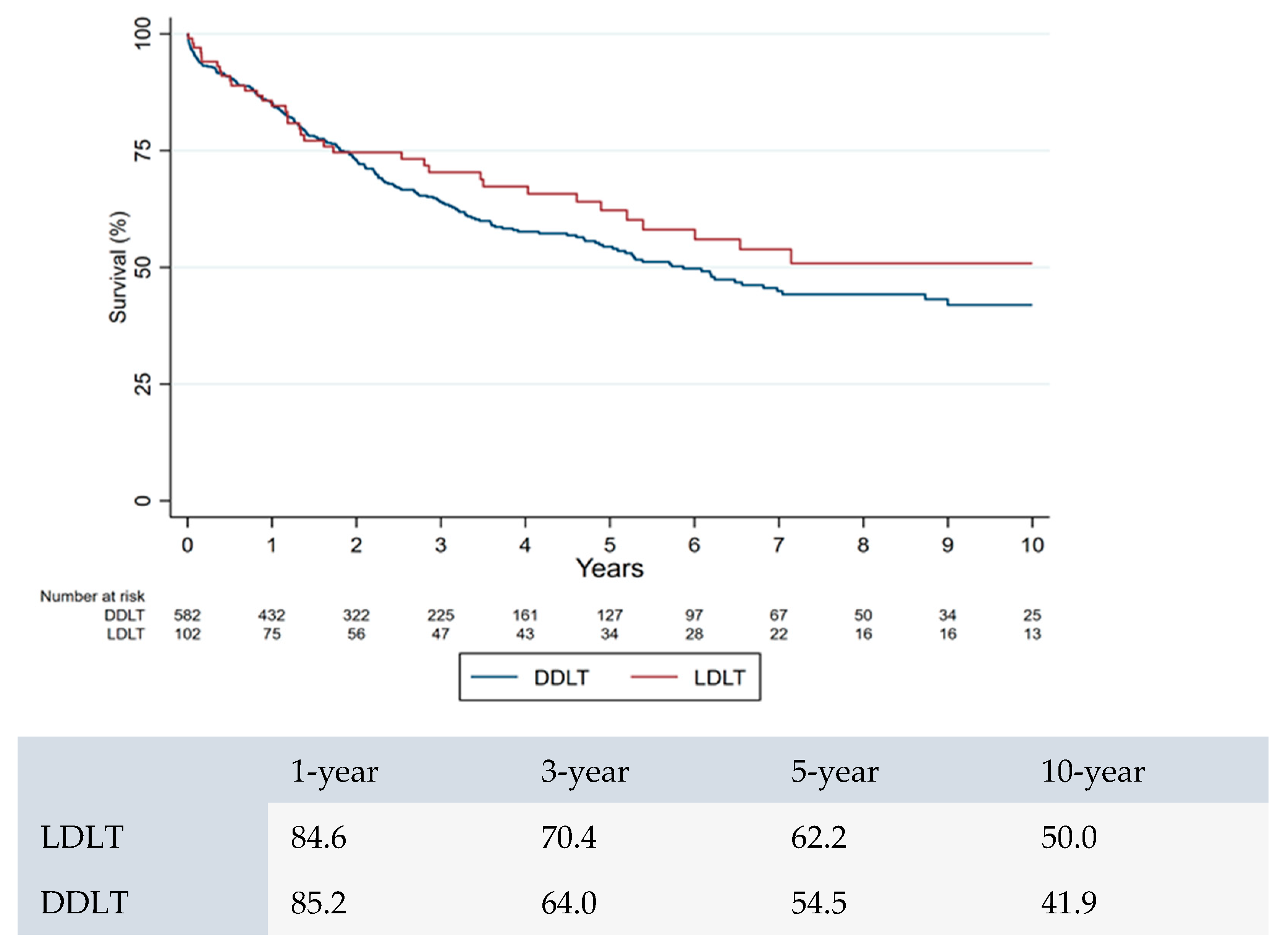
3.3. Survival Analysis Between CCA Versus HCC Patients After LDLT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALD | Alcohol-Associated Liver Disease |
| CA 19-9 | Carbohydrate Antigen 19-9 |
| CCA | Cholangiocarcinoma |
| dCCA | Distal Cholangiocarcinoma |
| iCCA | Intrahepatic Cholangiocarcinoma |
| pCCA | Perihilar Cholangiocarcinoma |
| FISH | Fluorescence In Situ Hybridization |
| HCC | Hepatocellular Carcinoma |
| LT | Liver Transplant |
| LDLT | Living-Donor Liver Transplant |
| MASLD | Metabolic-Dysfunction-Associated Steatotic Liver Disease |
| OPTN | Organ Procurement and Transplantation Network |
| PSC | Primary Sclerosing Cholangitis |
| TIPS | Transjugular Intrahepatic Portosystemic Shunt |
| UNOS | United Network for Organ Sharing |
| US | United States |
References
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef]
- DeOliveira, M.L.; Cunningham, S.C.; Cameron, J.L.; Kamangar, F.; Winter, J.M.; Lillemoe, K.D.; Choti, M.A.; Yeo, C.J.; Schulick, R.D. Cholangiocarcinoma: Thirty-one-year experience with 564 patients at a single institution. Ann. Surg. 2007, 245, 755–762. [Google Scholar] [CrossRef]
- Nakeeb, A.; Pitt, H.A.; Sohn, T.A.; Coleman, J.; Abrams, R.A.; Piantadosi, S.; Hruban, R.H.; Lillemoe, K.D.; Yeo, C.J.; Cameron, J.L. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann. Surg. 1996, 224, 463–473; discussion 473–475. [Google Scholar] [CrossRef]
- Rizvi, S.; Khan, S.A.; Hallemeier, C.L.; Kelley, R.K.; Gores, G.J. Cholangiocarcinoma—Evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2018, 15, 95–111. [Google Scholar] [CrossRef]
- Eletta, O.A.; Panayotova, G.G.; Lunsford, K.E. Liver Transplant for Intrahepatic Cholangiocarcinoma. Surg. Clin. N. Am. 2024, 104, 215–225. [Google Scholar] [CrossRef]
- Shaib, Y.; El-Serag, H.B. The epidemiology of cholangiocarcinoma. Semin. Liver Dis. 2004, 24, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Merino, N.; Aix, S.P.; Cortés-Funes, H. Chemotherapy for cholangiocarcinoma: An update. World J. Gastrointest. Oncol. 2013, 5, 171–176. [Google Scholar] [CrossRef]
- Bile Duct Cancer Survival Rates. Cholangiocarcinoma Survival Rates. February 2023. Available online: https://www.cancer.org/cancer/bile-duct-cancer/detection-diagnosis-staging/survival-by-stage.html (accessed on 1 December 2024).
- Groot Koerkamp, B.; Wiggers, J.K.; Allen, P.J.; Besselink, M.G.; Blumgart, L.H.; Busch, O.R.; Coelen, R.J.; D’Angelica, M.I.; DeMatteo, R.P.; Gouma, D.J.; et al. Recurrence Rate and Pattern of Perihilar Cholangiocarcinoma after Curative Intent Resection. J. Am. Coll. Surg. 2015, 221, 1041–1049. [Google Scholar] [CrossRef]
- Öztürk, N.B.; Jamil, L.H. An assessment of risk factors for recurrence and survival for patients undergoing liver resection for intrahepatic cholangiocarcinoma. Eur. J. Gastroenterol. Hepatol. 2024, 36, 766–774. [Google Scholar] [CrossRef]
- Nagino, M.; Ebata, T.; Yokoyama, Y.; Igami, T.; Sugawara, G.; Takahashi, Y.; Nimura, Y. Evolution of surgical treatment for perihilar cholangiocarcinoma: A single-center 34-year review of 574 consecutive resections. Ann. Surg. 2013, 258, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Vidili, G.; Rengo, M.; Bujanda, L.; Ponz-Sarvisé, M.; Lamarca, A. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int. 2019, 39 (Suppl. S1), 98–107. [Google Scholar] [CrossRef]
- Goldaracena, N.; Gorgen, A.; Sapisochin, G. Current status of liver transplantation for cholangiocarcinoma. Liver Transplant. 2018, 24, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Halder, R.; Amaraneni, A.; Shroff, R.T. Cholangiocarcinoma: A review of the literature and future directions in therapy. HepatoBiliary Surg. Nutr. 2022, 11, 555–566. [Google Scholar] [CrossRef]
- Meyer, C.G.; Penn, I.; James, L. Liver transplantation for cholangiocarcinoma: Results in 207 patients1. Transplantation 2000, 69, 1633–1637. [Google Scholar] [CrossRef]
- Rea, D.J.; Heimbach, J.K.; Rosen, C.B.; Haddock, M.G.; Alberts, S.R.; Kremers, W.K.; Gores, G.J.; Nagorney, D.M. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann. Surg. 2005, 242, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.K.; Taner, T.; Heimbach, J.K.; Gores, G.J.; Rosen, C.B. Liver Transplantation for Peri-hilar Cholangiocarcinoma. J. Gastrointest. Surg. 2020, 24, 2679–2685. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Haddock, M.G.; Alberts, S.R.; Nyberg, S.L.; Ishitani, M.B.; Rosen, C.B.; Gores, G.J. Transplantation for hilar cholangiocarcinoma. Liver Transplant. 2004, 10, S65–S68. [Google Scholar] [CrossRef]
- Lehrke, H.D.D.; Heimbach, J.K.; Wu, T.-T.; Jenkins, S.M.; Gores, G.J.; Rosen, C.B.; Mounajjed, T. Prognostic Significance of the Histologic Response of Perihilar Cholangiocarcinoma to Preoperative Neoadjuvant Chemoradiation in Liver Explants. Am. J. Surg. Pathol. 2016, 40, 510–518. [Google Scholar] [CrossRef]
- Murad, S.D.; Kim, W.R.; Harnois, D.M.; Douglas, D.D.; Burton, J.; Kulik, L.M.; Botha, J.F.; Mezrich, J.D.; Chapman, W.C.; Schwartz, J.J.; et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology 2012, 143, 88–98.e3. [Google Scholar] [CrossRef]
- De Vreede, I.; Steers, J.L.; Burch, P.A.; Rosen, C.B.; Gunderson, L.L.; Haddock, M.G.; Burgart, L.; Gores, G.J. Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoirradiation for cholangiocarcinoma. Liver Transplant. 2000, 6, 309–316. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Gores, G.J.; Haddock, M.G.; Alberts, S.R.; Nyberg, S.L.; Ishitani, M.B.; Rosen, C.B. Liver transplantation for unresectable perihilar cholangiocarcinoma. Semin. Liver Dis. 2004, 24, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Gores, G.J.; Haddock, M.G.; Alberts, S.R.; Pedersen, R.; Kremers, W.; Nyberg, S.L.; Ishitani, M.B.; Rosen, C.B. Predictors of disease recurrence following neoadjuvant chemoradiotherapy and liver transplantation for unresectable perihilar cholangiocarcinoma. Transplantation 2006, 82, 1703–1707. [Google Scholar] [CrossRef] [PubMed]
- Sonnenday, C.J. Liver Transplantation for Hilar Cholangiocarcinoma. Surg. Clin. N. Am. 2024, 104, 183–196. [Google Scholar] [CrossRef]
- Kaido, T.; Uemoto, S. Does living donation have advantages over deceased donation in liver transplantation? J. Gastroenterol. Hepatol. 2010, 25, 1598–1603. [Google Scholar] [CrossRef]
- Penn, I. Hepatic transplantation for primary and metastatic cancers of the liver. Surgery 1991, 110, 726. [Google Scholar]
- Castaldo, E.T.; Pinson, C.W. Liver transplantation for non-hepatocellular carcinoma malignancy. HPB 2007, 9, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.; Heimbach, J.; Gores, G. Surgery for cholangiocarcinoma: The role of liver transplantation. HPB 2008, 10, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Gores, G.J. Liver transplantation for cholangiocarcinoma. Liver Transpl. 2015, 21 (Suppl. S1), S32–S33. [Google Scholar] [CrossRef]
- Hill, A.; Olumba, F.; Chapman, W. Transplantation for Hepatocellular Carcinoma. Surg. Clin. N. Am. 2024, 104, 103–111. [Google Scholar] [CrossRef]
- Organ Procurement and Transplantation Network Policy. 2021. Available online: https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf (accessed on 1 January 2025).
- Humar, A.; Ganesh, S.; Jorgensen, D.; Tevar, A.; Ganoza, A.; Molinari, M.; Hughes, C. Adult Living Donor Versus Deceased Donor Liver Transplant (LDLT Versus DDLT) at a Single Center: Time to Change Our Paradigm for Liver Transplant. Ann. Surg. 2019, 270, 444–451. [Google Scholar] [CrossRef]
- Blechacz, B.; Komuta, M.; Roskams, T.; Gores, G.J. Clinical diagnosis and staging of cholangiocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 512–522. [Google Scholar] [CrossRef]
- Kitajima, T.; Hibi, T.; Moonka, D.; Sapisochin, G.; Abouljoud, M.S.; Nagai, S. Center Experience Affects Liver Transplant Outcomes in Patients with Hilar Cholangiocarcinoma. Ann. Surg. Oncol. 2020, 27, 5209–5221. [Google Scholar] [CrossRef]
- Krasnodębski, M.; Grąt, M.; Jastrzębski, M.; Szczęśniak, M.; Morawski, M.; Zając, K.; Patkowski, W.; Zieniewicz, K. Unsatisfactory Long-term Results of Liver Transplant in Patients with Intrahepatic Cholangiocarcinoma. Transplant. Proc. 2020, 52, 2463–2467. [Google Scholar] [CrossRef]
- Lee, D.D.; Croome, K.P.; Musto, K.R.; Melendez, J.; Tranesh, G.; Nakhleh, R.; Taner, C.B.; Nguyen, J.H.; Patel, T.; Harnois, D.M. Liver transplantation for intrahepatic cholangiocarcinoma. Liver Transplant. 2018, 24, 634–644. [Google Scholar] [CrossRef]
- Sapisochin, G.; de Lope, C.R.; Gastaca, M.; de Urbina, J.O.; Suarez, M.A.; Santoyo, J.; Castroagudín, J.F.; Varo, E.; López-Andujar, R.; Palacios, F.; et al. “Very early” intrahepatic cholangiocarcinoma in cirrhotic patients: Should liver transplantation be reconsidered in these patients? Am. J. Transplant. 2014, 14, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Sapisochin, G.; Facciuto, M.; Rubbia-Brandt, L.; Marti, J.; Mehta, N.; Yao, F.; Vibert, E.; Cherqui, D.; Grant, D.; Hernandez-Alejandro, R.; et al. Liver transplantation for “very early” intrahepatic cholangiocarcinoma: International retrospective study supporting a prospective assessment. Hepatology 2016, 64, 1178–1188. [Google Scholar] [CrossRef]
- Sapisochin, G.; Ivanics, T.; Heimbach, J. Liver Transplantation for Intrahepatic Cholangiocarcinoma: Ready for Prime Time? Hepatology 2021, 75, 455–472. [Google Scholar] [CrossRef] [PubMed]
- Sapisochin, G.M.; Javle, M.; Lerut, J.; Ohtsuka, M.; Ghobrial, M.; Hibi, T.; Kwan, N.M.; Heimbach, J. Liver Transplantation for Cholangiocarcinoma and Mixed Hepatocellular Cholangiocarcinoma: Working Group Report From the ILTS Transplant Oncology Consensus Conference. Transplantation 2020, 104, 1125–1130. [Google Scholar] [CrossRef]
- Lunsford, K.E.; Javle, M.; Heyne, K.; Shroff, R.T.; Abdel-Wahab, R.; Gupta, N.; Mobley, C.M.; Saharia, A.; Victor, D.W.; Nguyen, D.T.; et al. Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: A prospective case-series. Lancet Gastroenterol. Hepatol. 2018, 3, 337–348. [Google Scholar] [CrossRef]
- McMillan, R.R.; Javle, M.; Kodali, S.; Saharia, A.; Mobley, C.; Heyne, K.; Hobeika, M.J.; Lunsford, K.E.; Victor, D.W.; Shetty, A.; et al. Survival following liver transplantation for locally advanced, unresectable intrahepatic cholangiocarcinoma. Am. J. Transplant. 2022, 22, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Novel Protocol: Living-Donor Liver Transplant to Treat Hilar Cholangiocarcinoma. October 2023. Available online: https://www.upmcphysicianresources.com/news/100123-hilar (accessed on 1 January 2025).
- Andraus, W.; Ochoa, G.; de Martino, R.B.; Pinheiro, R.S.N.; Santos, V.R.; Lopes, L.D.; Júnior, R.M.A.; Waisberg, D.R.; Santana, A.C.; Tustumi, F.; et al. The role of living donor liver transplantation in treating intrahepatic cholangiocarcinoma. Front. Oncol. 2024, 14, 1404683. [Google Scholar] [CrossRef] [PubMed]
- Liver Transplant for Stable, Advanced Intrahepatic Cholangiocarcinoma. NCT04195503; Ongoing, Last Updated 10 October 2024. Available online: https://www.clinicaltrials.gov/study/NCT04195503?term=NCT04195503&rank=1 (accessed on 1 January 2025).
- Heimbach, J.K.; Sanchez, W.; Rosen, C.B.; Gores, G.J. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB 2011, 13, 356–360. [Google Scholar] [CrossRef] [PubMed]

| CCA (n = 102) | HCC (n = 774) | p-Value | |
|---|---|---|---|
| Age, years (mean, SD) | 51.0 (11.9) | 59.1 (9.9) | <0.001 |
| Gender, Male (n, %) | 70 (68.6%) | 507 (65.5%) | 0.53 |
| Race | <0.001 | ||
| White | 98.1 (96%) | 553 (71.4%) | |
| Black | 1 (0.9%) | 30 (3.8%) | |
| Hispanic | 2 (1.9%) | 132 (17.0%) | |
| Asian | 0 (0.0%) | 53 (6.8%) | |
| Other | 1 (0.9%) | 6 (0.7%) | |
| PSC | 39 (38.2%) | 25 (3.2%) | <0.001 |
| MELD (Mean, SD) | 12.0 (6.0) | 13.9 (5.8) | 0.66 |
| Diabetes | 9 (8.8%) | 228 (29.4%) | <0.001 |
| Ascites | 21 (20.5%) | 413 (53.3%) | <0.001 |
| Hepatic encephalopathy | 9 (8.8%) | 318 (41.0%) | <0.001 |
| TIPS | 1 (1.0%) | 69 (8.9%) | <0.001 |
| Donor Age | 37.3 (10.3) | 38.5 (11.6) | 0.30 |
| Donor gender, Male | 54 (52.9%) | 359 (46.3%) | 0.21 |
| Hazard Ratio | 95% Confidence Interval | p Value | ||
|---|---|---|---|---|
| Age | 1.238 | 1.146 | 1.337 | <0.001 |
| Non-HCC/CCA | Reference | |||
| HCC | 1.345 | 1.113 | 1.624 | 0.002 |
| CCA | 2.978 | 2.123 | 4.177 | <0.001 |
| Creatinine | 1.1291 | 1.163 | 1.432 | <0.001 |
| Diabetes | 1.306 | 1.092 | 1.562 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurakar, M.; Ozgur, O.S.; Donmez, A.E.; Ozturk, N.B.; Bilgili, M.M.; Parraga, X.; Alsaqa, M.; Abdulrazzak, E.; Lingamsetty, S.S.P.; Bonder, A.; et al. Outcomes of Living-Donor Liver Transplantation for Cholangiocarcinoma Versus Hepatocellular Carcinoma in the United States. J. Clin. Med. 2025, 14, 7306. https://doi.org/10.3390/jcm14207306
Gurakar M, Ozgur OS, Donmez AE, Ozturk NB, Bilgili MM, Parraga X, Alsaqa M, Abdulrazzak E, Lingamsetty SSP, Bonder A, et al. Outcomes of Living-Donor Liver Transplantation for Cholangiocarcinoma Versus Hepatocellular Carcinoma in the United States. Journal of Clinical Medicine. 2025; 14(20):7306. https://doi.org/10.3390/jcm14207306
Chicago/Turabian StyleGurakar, Merve, Ozge Sila Ozgur, Ayse Eylul Donmez, Nazli Begum Ozturk, Mehmet M. Bilgili, Ximena Parraga, Marwan Alsaqa, Eyad Abdulrazzak, Shanmukh Sai Pavan Lingamsetty, Alan Bonder, and et al. 2025. "Outcomes of Living-Donor Liver Transplantation for Cholangiocarcinoma Versus Hepatocellular Carcinoma in the United States" Journal of Clinical Medicine 14, no. 20: 7306. https://doi.org/10.3390/jcm14207306
APA StyleGurakar, M., Ozgur, O. S., Donmez, A. E., Ozturk, N. B., Bilgili, M. M., Parraga, X., Alsaqa, M., Abdulrazzak, E., Lingamsetty, S. S. P., Bonder, A., Gurakar, A., & Saberi, B. (2025). Outcomes of Living-Donor Liver Transplantation for Cholangiocarcinoma Versus Hepatocellular Carcinoma in the United States. Journal of Clinical Medicine, 14(20), 7306. https://doi.org/10.3390/jcm14207306







