Three-Dimensional Photogrammetric Assessment of Facial Symmetry Improvement Following Botulinum Toxin Treatment in Patients with Facial Palsy: An Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
- Age > 18 years;
- Unilateral peripheral facial palsy lasting more than one year;
- Confirmed indication and treatment request for facial symmetrization with botulinum toxin injections.
- Age < 18 years;
- Facial scar or skin lesion that could affect facial symmetry measurements;
- History of facial surgery leading to altered facial dynamics or volumes, including facial rehabilitation techniques;
- Neurodegenerative or neuromuscular disease affecting facial movements;
- Facial palsy < 12 months’ duration (to ensure that only patients without potential for spontaneous recovery were included);
- Botulinum toxin injection to the face within the previous 6 months;
- Contraindications to botulinum toxin injection (pregnancy or breastfeeding, known hypersensitivity or allergy, neuromuscular or autoimmune disease, myasthenia gravis, and Lambert–Eaton myasthenic syndrome).
2.2. Facial Palsy Assessment
2.3. Botulinum Toxin Injection Procedure
2.4. Image Acquisition
- 1.
- Neutral: Face at rest and without expression.
- 2.
- Surprise (“raise your eyebrows”): Eyebrows elevated, appearance of frontal wrinkles, and widening of the palpebral fissures.
- 3.
- Frown (“furrow your brows”): Partial closure of the palpebral fissures and appearance of glabellar wrinkles.
- 4.
- “Mona Lisa” smile: Slight, subtle smile without tooth exposure, and mild elevation of the oral commissures.
- 5.
- Forced smile: Full, maximal, and exaggerated smile showing the teeth.
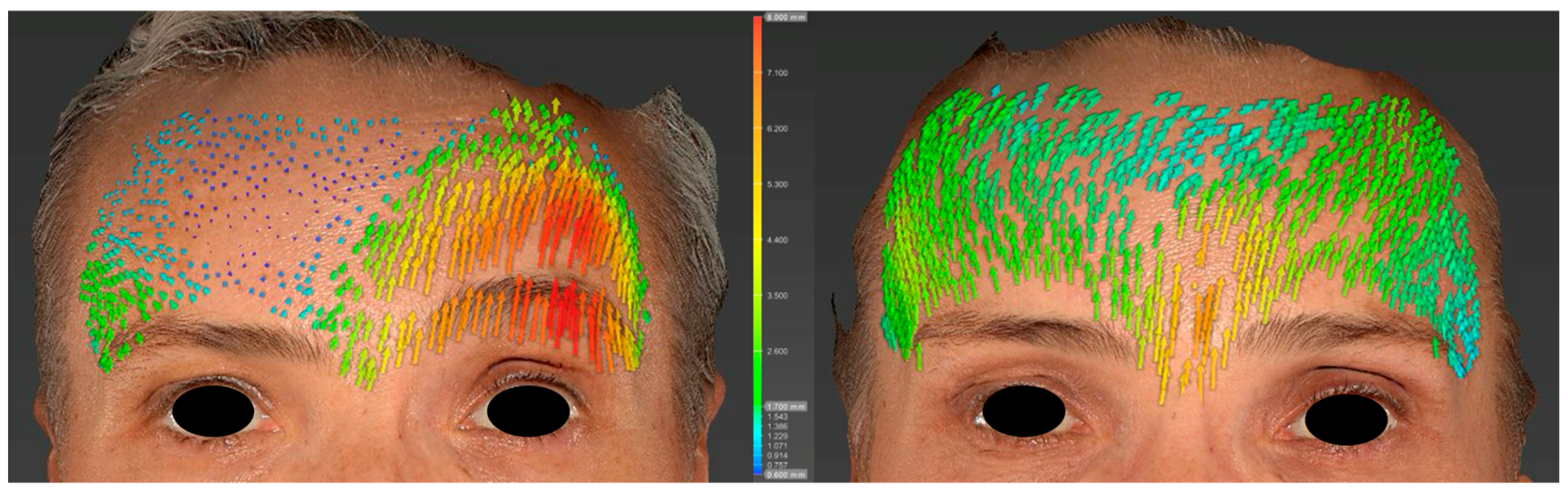
2.5. Analysis
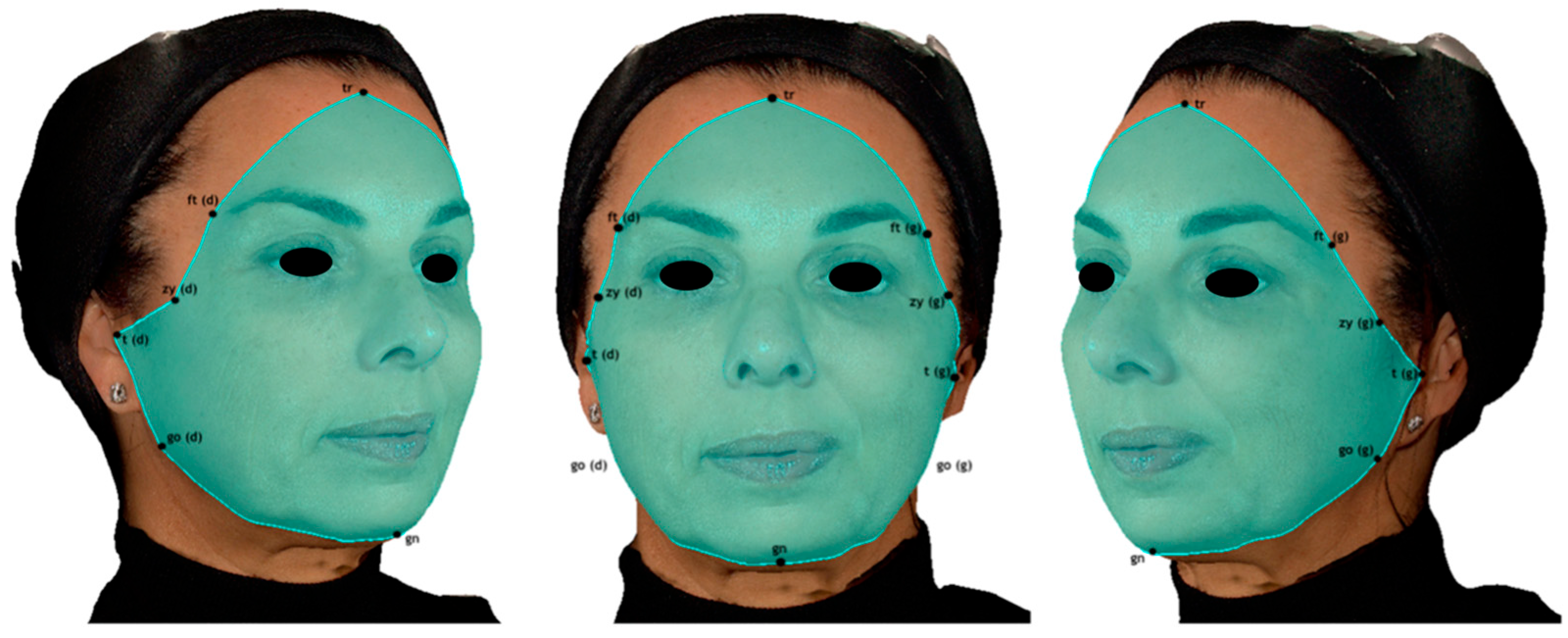
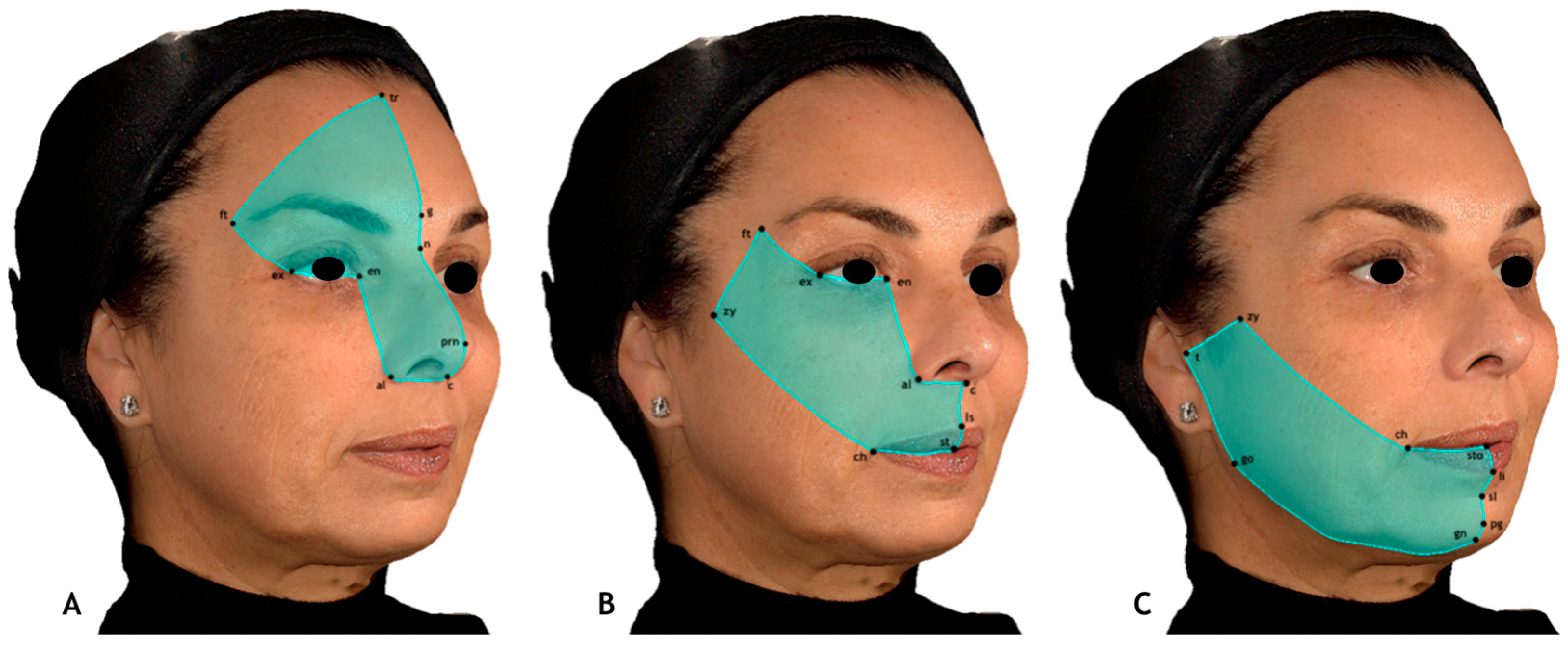
- Upper third: Trichion, glabella, pronasale, columella, alare, endocanthion, exocanthion, and frontotemporal points.
- Middle third: Endocanthion, alare, columella, subnasale, labiale superius, stomion, cheilion, zygion, frontotemporal points, and exocanthion.
- Lower third: Stomion, labiale inferius, sublabiale, pogonion, gnathion, gonion, tragion, zygion, and cheilion.
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shamil, E.; Noriega, M.; Moin, S.; Ko, T.K.; Tan, D.J.Y.; Meller, C.; Andrews, P.; Lekakis, G. Psychological Aspects of Facial Palsy. Facial Plast. Surg. FPS 2024, 40, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Dring, J.A.E.; Reeves, E.; Hotton, M. Social anxiety in people with facial palsy: The role of fear of negative evaluation and appearance-fixing behaviour. Br. J. Health Psychol. 2025, 30, e70018. [Google Scholar] [CrossRef]
- Knoedler, L.; Knoedler, S.; Chartier, C.; Panayi, A.C.; Orgill, D.P.; Moog, P.; Oezdemir, B.; von Isenburg, S.; Studier-Fischer, A.; Prantl, L.; et al. The Rise of Facial Palsy on Social Media Over the Last 5 Years. J. Craniofacial Surg. 2023, 34, 564–570. [Google Scholar] [CrossRef]
- Benichou, L.; Labbe, D.; Le Louarn, C.; Guerreschi, P. Séquelles de paralysie faciale et toxine botulique. Ann. Chir. Plast. Esthétique 2015, 60, 377–392. [Google Scholar] [CrossRef]
- Cooper, L.; Lui, M.; Nduka, C. Botulinum toxin treatment for facial palsy: A systematic review. J. Plast. Reconstr. Aesthetic Surg. JPRAS 2017, 70, 833–841. [Google Scholar] [CrossRef]
- Yener, A.; Acharya, V.; Andrews, P.; Meller, C.; Shamil, E. The Role of Botulinum Toxin A Neuromodulator in the Management of Synkinesis in Facial Palsy. Facial Plast. Surg. FPS 2025, 41, 395–400. [Google Scholar] [CrossRef]
- Carré, F.; Amar, J.; Tankéré, F.; Foirest, C. Botulinum Toxin Injections to Manage Sequelae of Peripheral Facial Palsy. Toxins 2024, 16, 161. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis Pecora, C.; Shitara, D. Botulinum Toxin Type A to Improve Facial Symmetry in Facial Palsy: A Practical Guideline and Clinical Experience. Toxins 2021, 13, 159. [Google Scholar] [CrossRef]
- Makhdoom, N.; Chaudry, A.S.; Wong, Z.Y. The use of botulinum toxin to improve cosmesis in patients with facial asymmetry following facial palsy/synkinesis—A literature review. J. Plast. Reconstr. Aesthetic Surg. JPRAS 2025, 100, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Mat Lazim, N.; Ismail, H.; Abdul Halim, S.; Nik Othman, N.A.; Haron, A. Comparison of 3 Grading Systems (House-Brackmann, Sunnybrook, Sydney) for the Assessment of Facial Nerve Paralysis and Prediction of Neural Recovery. Medeni. Med. J. 2023, 38, 111–119. [Google Scholar] [CrossRef]
- Dindaroğlu, F.; Kutlu, P.; Duran, G.S.; Görgülü, S.; Aslan, E. Accuracy and reliability of 3D stereophotogrammetry: A comparison to direct anthropometry and 2D photogrammetry. Angle Orthod. 2016, 86, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Pradel, R.; Savoldelli, C.; Rios, O.; Kestemont, P.; Lerhe, B. Facial Painting and 3D Stereophotogrammetric Analysis of Facial Dynamics: A Reliable Anatomical Educational Method. J. Clin. Med. 2024, 13, 2304. [Google Scholar] [CrossRef] [PubMed]
- Savoldelli, C.; Benat, G.; Castillo, L.; Chamorey, E.; Lutz, J.C. Accuracy, repeatability and reproducibility of a handheld three-dimensional facial imaging device: The Vectra H1. J. Stomatol. Oral Maxillofac. Surg. 2019, 120, 289–296. [Google Scholar] [CrossRef]
- Andrade, L.M.; Rodrigues da Silva, A.M.B.; Magri, L.V.; Rodrigues da Silva, M.A.M. Repeatability Study of Angular and Linear Measurements on Facial Morphology Analysis by Means of Stereophotogrammetry. J. Craniofacial Surg. 2017, 28, 1107–1111. [Google Scholar] [CrossRef]
- Codari, M.; Pucciarelli, V.; Stangoni, F.; Zago, M.; Tarabbia, F.; Biglioli, F.; Sforza, C. Facial thirds–based evaluation of facial asymmetry using stereophotogrammetric devices: Application to facial palsy subjects. J. Cranio-Maxillofac. Surg. 2017, 45, 76–81. [Google Scholar] [CrossRef]
- Biglio, A.; Rossetti, G.; Gibelli, D.M.; Dolci, C.; Cappella, A.; Allevi, F.; Vaira, L.A.; De Riu, G.; Sforza, C.; Biglioli, F. Three-dimensional evaluation of symmetry in facial palsy reanimation using stereophotogrammetric devices: A series of 15 cases. J. Cranio-Maxillofac. Surg. 2023, 51, 766–771. [Google Scholar] [CrossRef]
- Samsudin, W.S.W.; Sundaraj, K. Evaluation and Grading Systems of Facial Paralysis for Facial Rehabilitation. J. Phys. Ther. Sci. 2013, 25, 515–519. [Google Scholar] [CrossRef]
- Waubant, A.; Franco-Vidal, V.; Ribadeau Dumas, A. Validation de la version française de l’échelle faciale de Sunnybrook. Ann. Françaises D’oto-Rhino-Laryngol. Pathol. Cervico-Faciale 2022, 139, 119–122. [Google Scholar] [CrossRef]
- Lorenc, Z.P.; Kenkel, J.M.; Fagien, S.; Hirmand, H.; Nestor, M.S.; Sclafani, A.P.; Sykes, J.M.; Waldorf, H.A. A review of AbobotulinumtoxinA (Dysport). Aesthetic Surg. J. 2013, 33 (Suppl. S1), 13S–17S. [Google Scholar] [CrossRef]
- de Maio, M.; Bento, R.F. Botulinum Toxin in Facial Palsy: An Effective Treatment for Contralateral Hyperkinesis. Plast. Reconstr. Surg. 2007, 120, 917–927. [Google Scholar] [CrossRef]
- Kornreich, D.; Mitchell, A.A.; Webb, B.D.; Cristian, I.; Jabs, E.W. Quantitative Assessment of Facial Asymmetry Using Three-Dimensional Surface Imaging in Adults: Validating the Precision and Repeatability of a Global Approach. Cleft Palate-Craniofacial J. 2016, 53, 126–131. [Google Scholar] [CrossRef]
- Taylor, H.O.; Morrison, C.S.; Linden, O.; Phillips, B.; Chang, J.; Byrne, M.E.; Sullivan, S.R.; Forrest, C.R. Quantitative facial asymmetry: Using three-dimensional photogrammetry to measure baseline facial surface symmetry. J. Craniofacial Surg. 2014, 25, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, T.; Xi, T.; Schreurs, R.; Bergé, S.; Maal, T. Quantification of facial asymmetry: A comparative study of landmark-based and surface-based registrations. J. Cranio-Maxillofac. Surg. 2016, 44, 1131–1136. [Google Scholar] [CrossRef]
- Ostwald, J.; Berssenbrügge, P.; Dirksen, D.; Runte, C.; Wermker, K.; Kleinheinz, J.; Jung, S. Measured symmetry of facial 3D shape and perceived facial symmetry and attractiveness before and after orthognathic surgery. J. Cranio-Maxillofac. Surg. 2015, 43, 521–527. [Google Scholar] [CrossRef]
- Djordjevic, J.; Toma, A.M.; Zhurov, A.I.; Richmond, S. Three-dimensional quantification of facial symmetry in adolescents using laser surface scanning. Eur. J. Orthod. 2014, 36, 125–132. [Google Scholar] [CrossRef]
- Primozic, J.; Perinetti, G.; Zhurov, A.; Richmond, S.; Ovsenik, M. Assessment of facial asymmetry in growing subjects with a three-dimensional laser scanning system. Orthod. Craniofacial Res. 2012, 15, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Islam, S.M.S.; Murray, K.; Goonewardene, M.S. Facial asymmetry assessment in adults using three-dimensional surface imaging. Prog. Orthod. 2015, 16, 36. [Google Scholar] [CrossRef]
- Meyer-Marcotty, P.; Stellzig-Eisenhauer, A.; Bareis, U.; Hartmann, J.; Kochel, J. Three-dimensional perception of facial asymmetry. Eur. J. Orthod. 2011, 33, 647–653. [Google Scholar] [CrossRef]
- Alqattan, M.; Djordjevic, J.; Zhurov, A.I.; Richmond, S. Comparison between landmark and surface-based three-dimensional analyses of facial asymmetry in adults. Eur. J. Orthod. 2015, 37, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ozsoy, U. Comparison of Different Calculation Methods Used to Analyze Facial Soft Tissue Asymmetry: Global and Partial 3-Dimensional Quantitative Evaluation of Healthy Subjects. J. Oral Maxillofac. Surg. 2016, 74, 1847.e1–1847.e9. [Google Scholar] [CrossRef]


| Patient | Age | Sex | Side of Palsy | Etiology |
|---|---|---|---|---|
| 1 | 43 | M | Right | Parotid tumor |
| 2 | 88 | F | Right | Acoustic neuroma |
| 3 | 60 | F | Left | Acoustic neuroma |
| 4 | 62 | M | Right | Bell’s palsy |
| 5 | 50 | F | Left | Parotid tumor |
| 6 | 41 | F | Right | Bell’s palsy |
| 7 | 54 | F | Left | Bell’s palsy |
| 8 | 64 | F | Right | Herpes zoster oticus |
| 9 | 46 | F | Right | Bell’s palsy |
| 10 | 45 | F | Right | Bell’s palsy |
| 11 | 65 | F | Left | Acoustic neuroma |
| 12 | 44 | F | Left | Acoustic neuroma |
| 13 | 72 | M | Left | Bell’s palsy |
| 14 | 57 | M | Right | Bell’s palsy |
| 15 | 70 | F | Right | Trauma |
| 16 | 47 | F | Right | Bell’s palsy |
| Facial Region | Mean RMS Values (Pre-Injection) | Mean RMS Values (Post-Injection) | p-Value | Effect Size (Cohen’s d) |
|---|---|---|---|---|
| Upper third | 1.06 (0.38) | 0.93 (0.30) | <0.001 | 0.369 |
| Middle third | 1.37 (0.43) | 1.11 (0.41) | <0.001 | 0.622 |
| Lower third | 1.42 (0.57) | 1.28 (0.53) | <0.001 | 0.267 |
| Whole face | 1.51 (0.42) | 1.35 (0.43) | <0.001 | 0.381 |
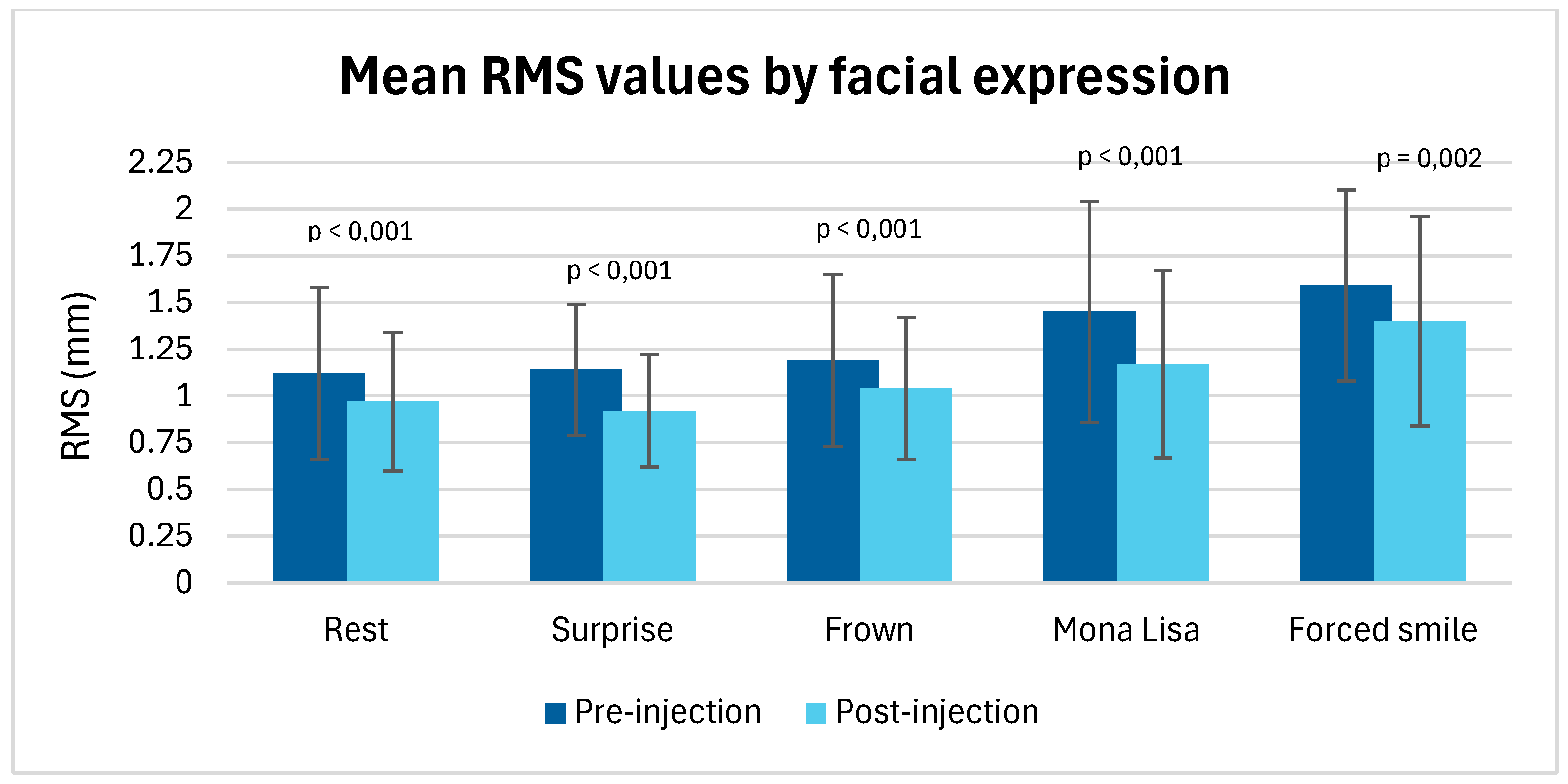
| Expression | Mean RMS Values (Pre-Injection) | Mean RMS Values (Post-Injection) | p-Value | Effect Size (Cohen’s d) |
|---|---|---|---|---|
| Rest | 1.12 (0.46) | 0.97 (0.37) | p < 0.001 | 0.613 |
| Surprise | 1.14 (0.35) | 0.92 (0.3) | p < 0.001 | 1.270 |
| Frown | 1.19 (0.46) | 1.04 (0.38) | p < 0.001 | 0.832 |
| Mona Lisa smile | 1.45 (0.59) | 1.17 (0.50) | p < 0.001 | 0.870 |
| Forced smile | 1.59 (0.51) | 1.40 (0.56) | p = 0.02 | 0.496 |
| Patient | House & Brackmann (Pre-Injection) | House & Brackmann (Post-Injection) | Sunnybrook (Pre-Injection) | Sunnybrook (Post-Injection) |
|---|---|---|---|---|
| 1 | 4 | 3 | 34 | 49 |
| 2 | 5 | 3 | 15 | 58 |
| 3 | 5 | 4 | 14 | 34 |
| 4 | 4 | 3 | 43 | 62 |
| 5 | 2 | 1 | 85 | 96 |
| 6 | 4 | 3 | 33 | 58 |
| 7 | 4 | 3 | 37 | 66 |
| 8 | 3 | 3 | 50 | 68 |
| 9 | 5 | 4 | 25 | 29 |
| 10 | 5 | 5 | 15 | 29 |
| 11 | 4 | 6 | 13 | 29 |
| 12 | 4 | 4 | 32 | 31 |
| 13 | 3 | 3 | 48 | 51 |
| 14 | 4 | 3 | 39 | 45 |
| 15 | 3 | 2 | 55 | 83 |
| 16 | 4 | 3 | 40 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradel, R.; Lerhe, B.; Kestemont, P.; Helmer, C.; Savoldelli, C.; Rios, O. Three-Dimensional Photogrammetric Assessment of Facial Symmetry Improvement Following Botulinum Toxin Treatment in Patients with Facial Palsy: An Observational Study. J. Clin. Med. 2025, 14, 7298. https://doi.org/10.3390/jcm14207298
Pradel R, Lerhe B, Kestemont P, Helmer C, Savoldelli C, Rios O. Three-Dimensional Photogrammetric Assessment of Facial Symmetry Improvement Following Botulinum Toxin Treatment in Patients with Facial Palsy: An Observational Study. Journal of Clinical Medicine. 2025; 14(20):7298. https://doi.org/10.3390/jcm14207298
Chicago/Turabian StylePradel, Robin, Barbara Lerhe, Philippe Kestemont, Charlotte Helmer, Charles Savoldelli, and Olina Rios. 2025. "Three-Dimensional Photogrammetric Assessment of Facial Symmetry Improvement Following Botulinum Toxin Treatment in Patients with Facial Palsy: An Observational Study" Journal of Clinical Medicine 14, no. 20: 7298. https://doi.org/10.3390/jcm14207298
APA StylePradel, R., Lerhe, B., Kestemont, P., Helmer, C., Savoldelli, C., & Rios, O. (2025). Three-Dimensional Photogrammetric Assessment of Facial Symmetry Improvement Following Botulinum Toxin Treatment in Patients with Facial Palsy: An Observational Study. Journal of Clinical Medicine, 14(20), 7298. https://doi.org/10.3390/jcm14207298





