AI-Assisted Simple Scoring Algorithm Was Helpful in the Risk Assessment of Cardiac Involvement in Patients with Pulmonary Sarcoidosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants and Diagnosis for Pulmonary Sarcoidosis
- Pathological evaluation (the finding of non-necrotizing granulomatous inflammation in one or more tissue samples) and exclusion of other granulomatous diseases;
- Löfgren‘s syndrome (erythema nodosum, fever, arthritis, and bilateral hilar adenopathy with or without parenchymal disease) + spontaneous radiological regression;
- Typical radiological features + hypercalcemia + renal failure.
2.2. Staging of Pulmonary Sarcoidosis and Diagnosis in Other Organs
2.3. Screening and Diagnostic Procedures of CS
2.4. Statistical Analysis
3. Results
4. Discussion
5. Strengths of the Study
6. Study Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | artificial intelligence |
| ATS | American Thoracic Society |
| BPM | beats per minute |
| BTS | British Thoracic Society |
| CMR | cardiac magnetic resonance |
| CS | cardiac sarcoidosis |
| CS(+) | cardiac sarcoidosis conformed by cardiac magnetic resonance |
| CS(-) | cardiac sarcoidosis excluded on cardiac magnetic resonance |
| CT | computed tomography |
| ECG | electrocardiogram |
| ESC | European Society of Cardiology |
| ECV | extracellular volume mapping |
| GLS | global longitudinal strain |
| HRS | Heart Rhythm Study |
| IL-2 | interleukin 2 |
| IL-12 | interleukin 12 |
| INF-γ | interferon-γ (INF-γ) |
| JCS | Japanese Circulation Society |
| LGE | late gadolinium enhancement |
| LVEF | left ventricular ejection fraction |
| MRI | magnetic resonance imaging |
| nsVT | non-sustained ventricular tachycardia |
| PS | pulmonary sarcoidosis |
| ROC | receiver-operated characteristic |
| STIR | short tau inversion recovery |
| SVES | supraventricular extrasystole |
| SVT | supraventricular tachycardia |
| TAPSE | tricuspid annular plane systolic excursion |
| TNFα | tumor necrosis factor α |
| TTE | transthoracic echocardiography |
| VES | ventricular extrasystole |
| VF | ventricular fibrillation |
References
- Schupp, J.C.; Freitag-Wolf, S.; Bargagli, E.; Mihailović-Vučinić, V.; Rottoli, P.; Grubanovic, A.; Müller, A.; Jochens, A.; Tittmann, L.; Schnerch, J.; et al. Phenotypes of organ involvement in sarcoidosis. Eur. Respir. J. 2018, 5, 1700991. [Google Scholar] [CrossRef]
- Markatis, E.; Afthinos, A.; Antonakis, E.; Papanikolaou, I.C. Cardiac sarcoidosis: Diagnosis and management. Rev. Cardiovasc. Med. 2020, 1, 321–338. [Google Scholar] [CrossRef]
- Baughman, R.P.; Teirstein, A.S.; Judson, M.A.; Rossman, M.D.; Yeager, H., Jr.; Bresnitz, E.A.; DePalo, L.; Hunninghake, G.; Iannuzzi, M.C.; Johns, C.J.; et al. Case Control Etiologic Study of Sarcoidosis (ACCESS) research group. Clinical characteristics of patients in a case control study of sarcoidosis. Am. J. Respir. Crit. Care Med. 2001, 164, 1885–1889. [Google Scholar] [CrossRef]
- Błasińska, K.; Jędrych, M.E.; Opoka, L.; Tomkowski, W.; Szturmowicz, M. Imaging Plays a Key Role in the Diagnosis and Control of the Treatment of Bone Sarcoidosis. Biomedicines 2023, 11, 1866. [Google Scholar] [CrossRef]
- Zimna, K.; Szturmowicz, M.; Sobiecka, M.; Błasińska, K.; Bartosiewicz, M.; Tomkowski, W.Z. Sudden Vision Loss Due to Optic Neuritis-An Uncommon Presentation of Neurosarcoidosis. Diagnostics 2023, 13, 2579. [Google Scholar] [CrossRef]
- Rossides, M.; Darlington, P.; Kullberg, S.; Arkema, E.V. Sarcoidosis: Epidemiology and clinical insights. J. Intern. Med. 2023, 293, 668–680. [Google Scholar] [CrossRef]
- Santulli, G. Cardiac Sarcoidosis: Updated Insights on Epidemiology and Diagnostic Criteria. Am. J. Cardiol. 2023, 204, 425–427. [Google Scholar] [CrossRef]
- Kouranos, V.; Sharma, R. Cardiac sarcoidosis: State-of-the-art review. Heart 2021, 107, 1591–1599. [Google Scholar] [CrossRef]
- Zipse, M.M. Editorial commentary: Cardiac sarcoidosis in contemporary practice: Forward progress, but clinical quandaries persist. Trends Cardiovasc. Med. 2023, 33, 456–457. [Google Scholar] [CrossRef]
- Nordenswan, H.-K.; Pöyhönen, P.; Lehtonen, J.; Ekström, K.; Uusitalo, V.; Niemelä, M.; Vihinen, T.; Kaikkonen, K.; Haataja, P.; Kerola, T.; et al. Incidence of Sudden Cardiac Death and Life-Threatening Arrhythmias in Clinically Manifest Cardiac Sarcoidosis With and Without Current Indications for an Implantable Cardioverter Defibrillator. Circulation 2022, 146, 964–975. [Google Scholar] [CrossRef]
- Murtagh, G.; Laffin, L.J.; Patel, K.V.; Patel, A.V.; Bonham, C.A.; Yu, Z.; Addetia, K.; El-Hangouche, N.; Maffesanti, F.; Mor-Avi, V.; et al. Improved detection of myocardial damage in sarcoidosis using longitudinal strain in patients with preserved left ventricular ejection fraction. Echocardiography 2016, 33, 1344–1352. [Google Scholar] [CrossRef]
- Patel, M.R.; Cawley, P.J.; Heitner, J.F.; Klem, I.; Parker, M.A.; Jaroudi, W.A.; Meine, T.J.; White, J.B.; Elliott, M.D.; Kim, H.W.; et al. Detection of myocardial damage in patients with sarcoidosis. Circulation 2009, 120, 1969–1977. [Google Scholar] [CrossRef]
- Silverman, K.J.; Hutchins, G.M.; Bulkley, B.H. Cardiac sarcoid: A clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation 1978, 58, 1204–1211. [Google Scholar] [CrossRef]
- Crouser, E.D.; Maier, L.A.; Wilson, K.C.; Bonham, C.A.; Morgenthau, A.S.; Patterson, K.C.; Abston, E.; Bernstein, R.C.; Blankstein, R.; Chen, E.S.; et al. Diagnosis and Detection of Sarcoidosis. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care. Med. 2020, 201, e26–e51. [Google Scholar] [CrossRef]
- Thillai, M.; Atkins, C.P.; Crawshaw, A.; Hart, S.P.; Ho, L.P.; Kouranos, V.; Patterson, K.C.; Screaton, N.J.; Whight, J.; Wells, A.U. BTS Clinical Statement on pulmonary sarcoidosis. Thorax 2021, 76, 4–20. [Google Scholar] [CrossRef]
- Friedrich, M.G.; Sechtem, U.; Schulz-Menger, J.; Holmvang, G.; Alakija, P.; Cooper, L.T.; White, J.A.; Abdel-Aty, H.; Gutberlet, M.; Prasad, S.; et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J. Am. Coll. Cardiol. 2009, 53, 1475–1487. [Google Scholar] [CrossRef]
- Holtzclaw, A.W.; Mrsic, Z.; Church, T.L.; Shumar, J.N.; Liotta, R.A.; Aslam, S.N.; Fontana, J.R.; Nations, J.A.; Lazarus, A.; Browning, R.F.; et al. Optimizing routine screening for cardiac sarcoidosis through use of commonly available studies. Respir. Med. 2021, 178, 106331. [Google Scholar] [CrossRef]
- Birnie, D.H.; Sauer, W.H.; Bogun, F.; Cooper, J.M.; Culver, D.A.; Duvernoy, C.S.; Judson, M.A.; Kron, J.; Mehta, D.; Nielsen, J.C.; et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014, 11, 1305–1323. [Google Scholar] [CrossRef]
- Cheng, R.K.; Kittleson, M.M.; Beavers, C.J.; Birnie, D.H.; Blankstein, R.; Bravo, P.E.; Gilotra, N.A.; Judson, M.A.; Patton, K.K.; Rose-Bovino, L.; et al. Diagnosis and Management of Cardiac Sarcoidosis: A Scientific Statement from the American Heart Association. Circulation 2024, 149, e1197–e1216. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef]
- Sharma, R.; Kouranos, V.; Cooper, L.T.; Metra, M.; Ristic, A.; Heidecker, B.; Baksi, J.; Wicks, E.; Merino, J.L.; Klingel, K.; et al. Management of cardiac sarcoidosis. Eur. Heart J. 2024, 45, 2697–2726. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, J.; Sato, T.; Ohira, H.; Yoshikawa, S.; Hattori, T.; Manabe, O.; Oyama-Manabe, N.; Tsuneta, S.; Kimura, H.; Takenaka, S.; et al. Prevalence, incidence, and clinical features of cardiac involvement in patients with pulmonary sarcoidosis. Respir. Med. 2025, 238, 107954. [Google Scholar] [CrossRef]
- Bakker, A.L.M.; Mathijssen, H.; Huitema, M.P.; Kapteijns, L.; Grutters, J.C.; Veltkamp, M.; Keijsers, R.G.; Akdim, F.; van Es, H.W.; Peper, J.; et al. Holter Monitoring and Cardiac Biomarkers in Screening for Cardiac Sarcoidosis. Lung 2024, 203, 10. [Google Scholar] [CrossRef]
- Martusewicz-Boros, M.M.; Boros, P.W.; Wiatr, E.; Kempisty, A.; Piotrowska-Kownacka, D.; Roszkowski-Śliż, K. Cardiac Sarcoidosis: Is it More Common in Men? Lung 2016, 194, 61–66. [Google Scholar] [CrossRef]
- Terasaki, F.; Azuma, A.; Anzai, T.; Ishizaka, N.; Ishida, Y.; Isobe, M.; Inomata, T.; Ishibashi-Ueda, H.; Eishi, Y.; Kitakaze, M.; et al. Japanese Circulation Society Joint Working Group. JCS 2016 Guideline on Diagnosis and Treatment of Cardiac Sarcoidosis. Digest Version. Circ. J. 2019, 83, 2329–2388. [Google Scholar] [CrossRef]
- Ribeiro Neto, M.L.; Jellis, C.L.; Joyce, E.; Callahan, T.D.; Hachamovitch, R.; Culver, D.A. Update in Cardiac Sarcoidosis. Ann. Am. Thorac. Soc. 2019, 16, 341–350. [Google Scholar] [CrossRef]
- Darlington, P.; Gabrielsen, A.; Cederlund, K.; Kullberg, S.; Grunewald, J.; Eklund, A.; Sörensson, P. Diagnostic approach for cardiac involvement in sarcoidosis. Sarcoidosis Vasc. Diffuse Lung Dis. 2019, 36, 11–17. [Google Scholar]
- Suzuki, T.; Kanda, T.; Kubota, S.; Imai, S.; Muratam, K. Holter monitoring as a non-invasive indicator of cardiac involvement in sarcoidosis. Chest 1994, 106, 1021–1024. [Google Scholar] [CrossRef]
- Freeman, A.M.; Curran-Everett, D.; Weinberger, H.D.; Fenster, B.E.; Buckner, J.K.; Gottschall, E.B.; Sauer, W.H.; Maier, L.A.; Hamzeh, N.Y. Predictors of cardiac sarcoidosis using commonly available cardiac studies. Am. J. Cardiol. 2013, 112, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Quijano-Campos, J.C.; Williams, L.; Agarwal, S.; Tweed, K.; Parker, R.; Lalvani, A.; Chiu, Y.-D.; Dorey, K.; Devine, T.; Stoneman, V.; et al. CASPA (CArdiac Sarcoidosis in PApworth) improving the diagnosis of cardiac involvement in patients with pulmonary sarcoidosis: Protocol for a prospective observational cohort study. BMJ Open Respir. Res. 2020, 7, e000608. [Google Scholar] [CrossRef] [PubMed]
- Slart, R.H.J.A.; Glaudemans, A.W.J.M.; Lancellotti, P.; Hyafil, F.; Blankstein, R.; Schwartz, R.G.; Jaber, W.A.; Russell, R.; Gimelli, A.; Rouzet, F.; et al. A joint procedural position statement on imaging in cardiac sarcoidosis: From the Cardiovascular and Inflammation & Infection Committees of the European Association of Nuclear Medicine, the European Association of Cardiovascular Imaging, and the American Society of Nuclear Cardiology. J. Nucl. Cardiol. Off. Publ. Am. Soc. Nucl. Cardiol. 2018, 25, 298–319. [Google Scholar]
- Kurmann, R.; Mankad, S.V.; Mankad, R. Echocardiography in Sarcoidosis. Curr. Cardiol. Rep. 2018, 20, 118. [Google Scholar] [CrossRef] [PubMed]
- Swigris, J.J.; Olson, A.L.; Huie, T.J.; Fernandez-Perez, E.R.; Solomon, J.; Sprunger, D.; Brown, K.K. Sarcoidosis-related mortality in the United States from 1988 to 2007. Am. J. Respir. Crit. Care Med. 2011, 183, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Barssoum, K.; Altibi, A.M.; Rai, D.; Kumar, A.; Kharsa, A.; Chowdhury, M.; Thakkar, S.; Shahid, S.; Abdelazeem, M.; Abuzaid, A.S.; et al. Speckle tracking echocardiography can predict subclinical myocardial involvement in patients with sarcoidosis: A meta-analysis. Echocardiography 2020, 37, 2061–2070. [Google Scholar] [CrossRef]
- Di Stefano, C.; Bruno, G.; Arciniegas Calle, M.C.; Acharya, G.A.; Fussner, L.M.; Ungprasert, P.; Cooper, L.T.; Blauwet, L.A.; Ryu, J.H.; Pellikka, P.A.; et al. Diagnostic and predictive value of speckle tracking echocardiography in cardiac sarcoidosis. BMC Cardiovasc. Disord. 2020, 20, 21. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2017, 19, 75. [Google Scholar]
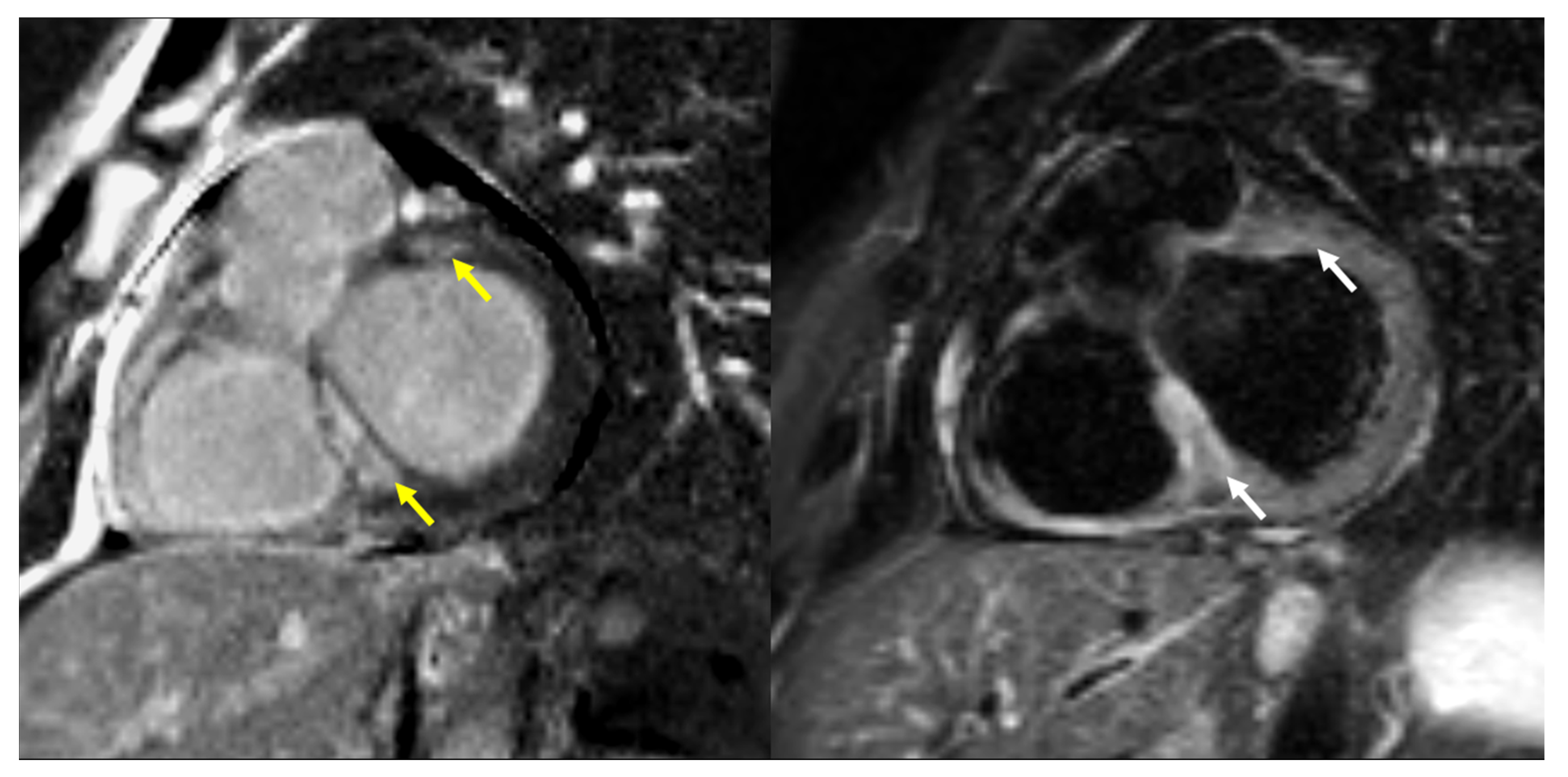
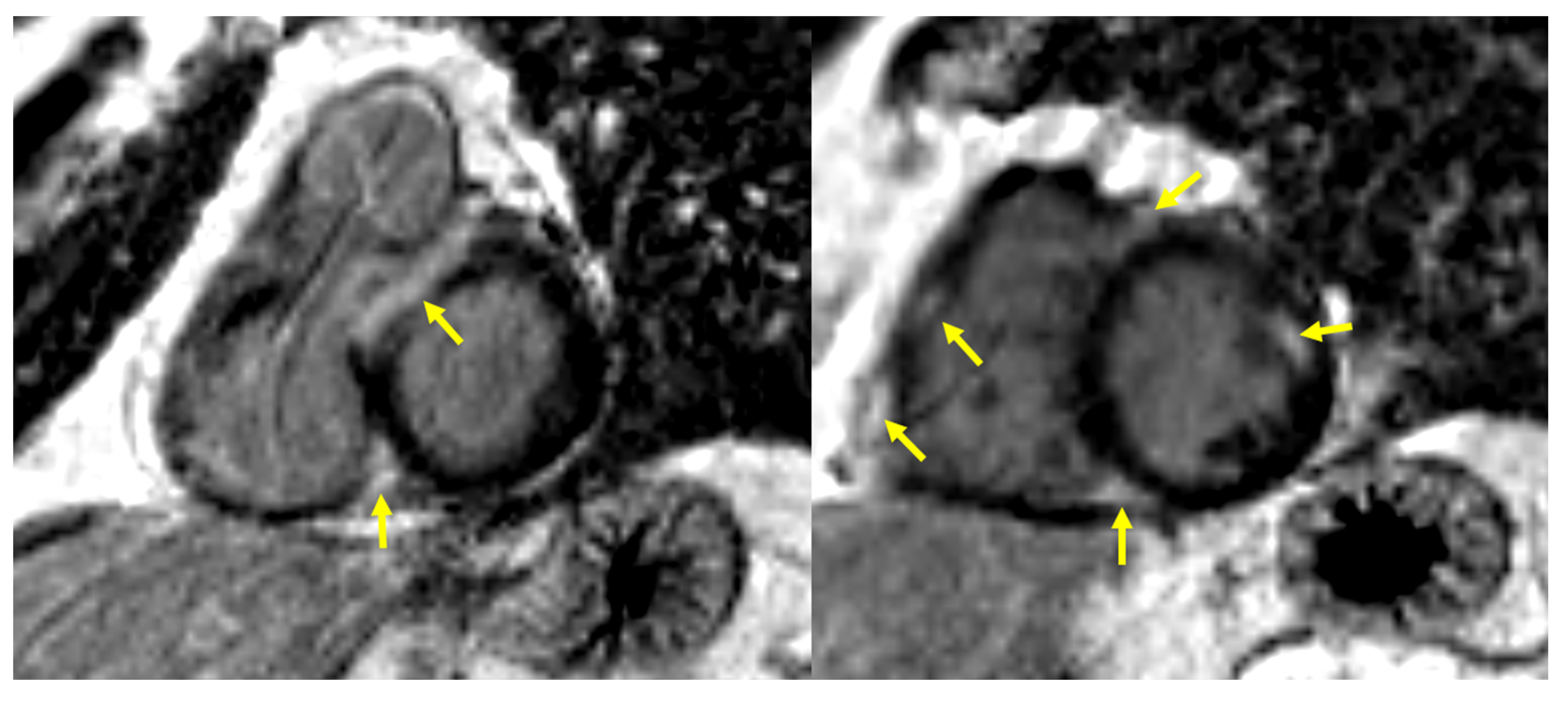
| Parameter | Whole Group (N° 92) | Cardiac Involvement on CMR | p | |
|---|---|---|---|---|
| Yes (N° 48) | No (N° 44) | |||
| Age, mean (±SD) | 43.86 (±10.00) | 43.04 (±10.6) | 44.7 (±9.1) | 0.416 |
| Gender (N°, %) | 0.368 | |||
| M | 65 (71) | 36 (75) | 29 (66) | |
| F | 27 (29) | 12 (25) | 15 (34) | |
| Lung involvement | 0.0509 | |||
| Stage (N°,%) | ||||
| I | 14 (15) | 3 (6) | 11 (25) | |
| II | 60 (65) | 34 (71) | 26 (59) | |
| III | 7 (8) | 3 (6) | 4 (9) | |
| IV | 11 (12) | 8 (17) | 3 (7) | |
| Other organs, symptoms (N°, %) | 25 (27) | 17 (35) | 8 (18) | 0.063 |
| Hypercalcemia | 7 (8) | 6 (13) | 1 (2) | 0.113 |
| Liver, spleen | 15 (16) | 13 (27) | 2 (4.5) | 0.0009 |
| Eye | 2 (2) | 0 | 2 (4.5) | 0.437 |
| Skin | 2 (2) | 1 (2) | 1 (2) | 0.514 |
| Bones | 1 (1) | 1 (2) | 0 | 0.96 |
| Parameter | Whole Group (N° 92) | Cardiac Involvement on CMR | p | |
|---|---|---|---|---|
| Yes (N° 48) | No (N° 44) | |||
| Palpitations/syncope N° (%) | 22 (24) | 14 (29) | 8 (18) | 0.234 |
| Chest pain N° (%) | 25 (27) | 11 (23) | 14 (32) | 0.359 |
| Dyspnea N° (%) | 32 (35) | 19 (40) | 13 (30) | 0.383 |
| No symptoms N° (%) | 33 (36) | 17 (35) | 16 (36) | >0.999 |
| Parameter | Whole Group (N° 92) | Cardiac Involvement on CMR | p | |
|---|---|---|---|---|
| Yes (N° 48) | No (N° 44) | |||
| LVEF%, mean (±SD) | 58 (±8) | 58 (±9.4) | 58 (±6.3) | 0.544 |
| LVEF% < 50 N° (%) | 6 (6.5) | 4 (8.3) | 2 (4.6) | 0.679 |
| TAPSE, mean (±SD) | 23.74 (±3.09) | 23.81 (±3.45) | 23.66 (±2.68) | 0.961 |
| Increased echogenicity N° (%) | 46 (50) | 27 (56) | 19 (43) | 0.297 |
| Pericardial effusion N° (%) | 12 (13) | 7 (15) | 5 (11) | 0.761 |
| Parameter | Whole Group | Cardiac Involvement on CMR | p | ||
|---|---|---|---|---|---|
| Yes | No | ||||
| ECG | N° | 92 | 48 | 44 | 0.0594 |
| Abnormal, N° (%) | 39 (42) | 25 (52) | 14 (32) | ||
| Holter ECG | N° | 75 | 45 | 30 | 0.009 |
| Abnormal, N° (%) | 37 (49) | 28 (62) | 9 (30) | ||
| Single-Factor Analysis | Multiple Factor Analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| age | 0.9828 | 0.9420 to 1.024 | 0.412 | 1.034 | 0.9749 to 1.100 | 0.274 |
| ECHO LVEF | 0.9953 | 0.9446 to 1.048 | 0.855 | 1.039 | 0.9571 to 1.130 | 0.356 |
| Holter ECG | 3.843 | 1.469 to 10.71 | 0.008 | 4.197 | 1.463 to 12.94 | 0.009 |
| Liver/spleen | 5.076 | 1.492 to 23.45 | 0.017 | 6.535 | 1.460 to 47.28 | 0.027 |
| ECG | 2.329 | 1.006 to 5.553 | 0.051 | 1.931 | 0.6594 to 5.768 | 0.230 |
| Hypercalcemia | 6.143 | 0.9913 to 118.6 | 0.099 | 3.063 | 0.3770 to 65.08 | 0.348 |
| Parameter | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Palpitations/syncope | 29% | 82% | 64% | 51% |
| Chest pain | 23% | 68% | 44% | 45% |
| Dyspnea | 40% | 71% | 59% | 52% |
| ECG | 52% | 68% | 64% | 57% |
| Holter ECG | 62% | 70% | 76% | 55% |
| Echocardiography—increased echogenicity | 56% | 57% | 59% | 54% |
| Echocardiography—increased echogenicity and/or decreased systolic function | 69% | 34% | 53% | 43% |
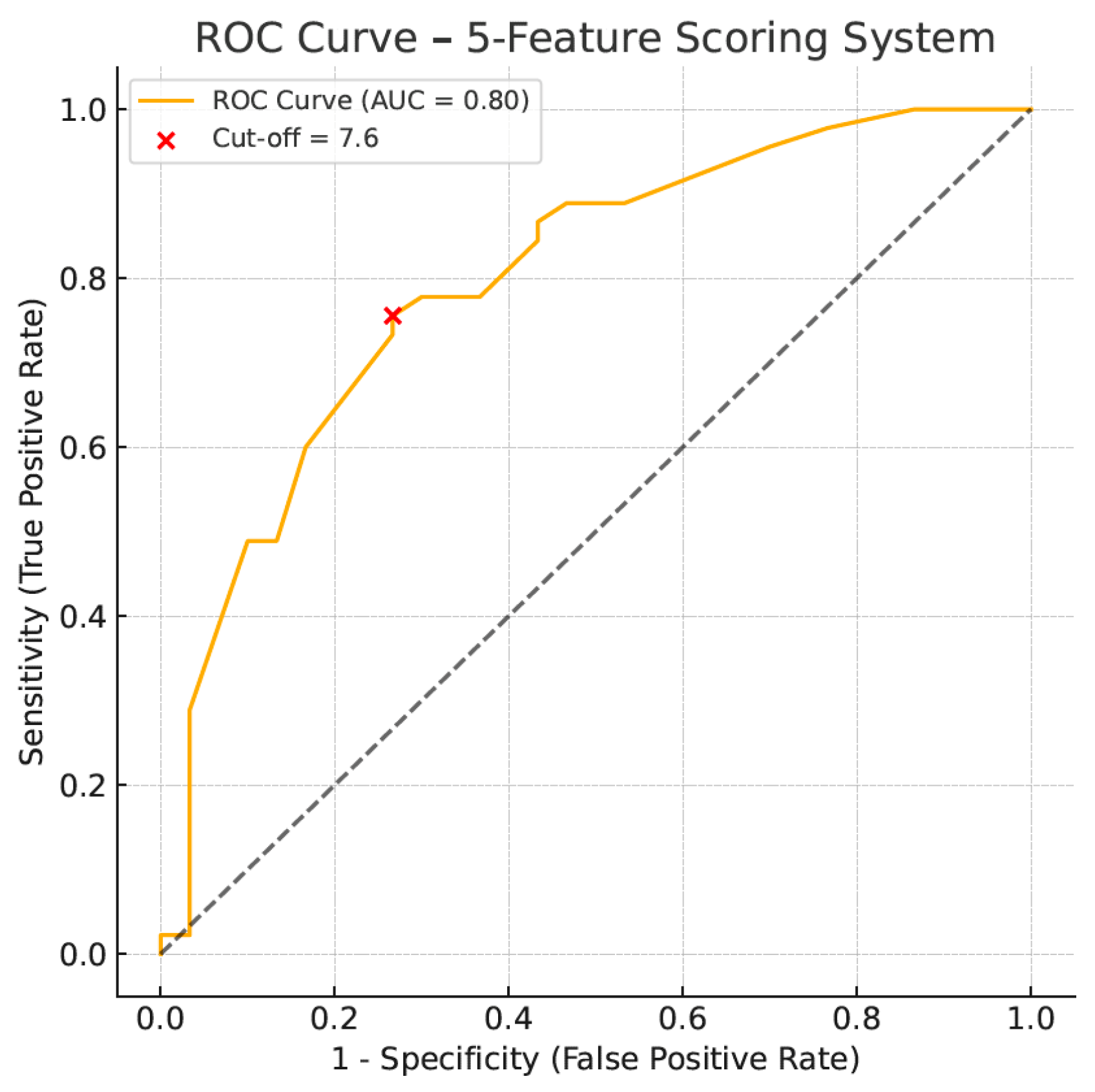
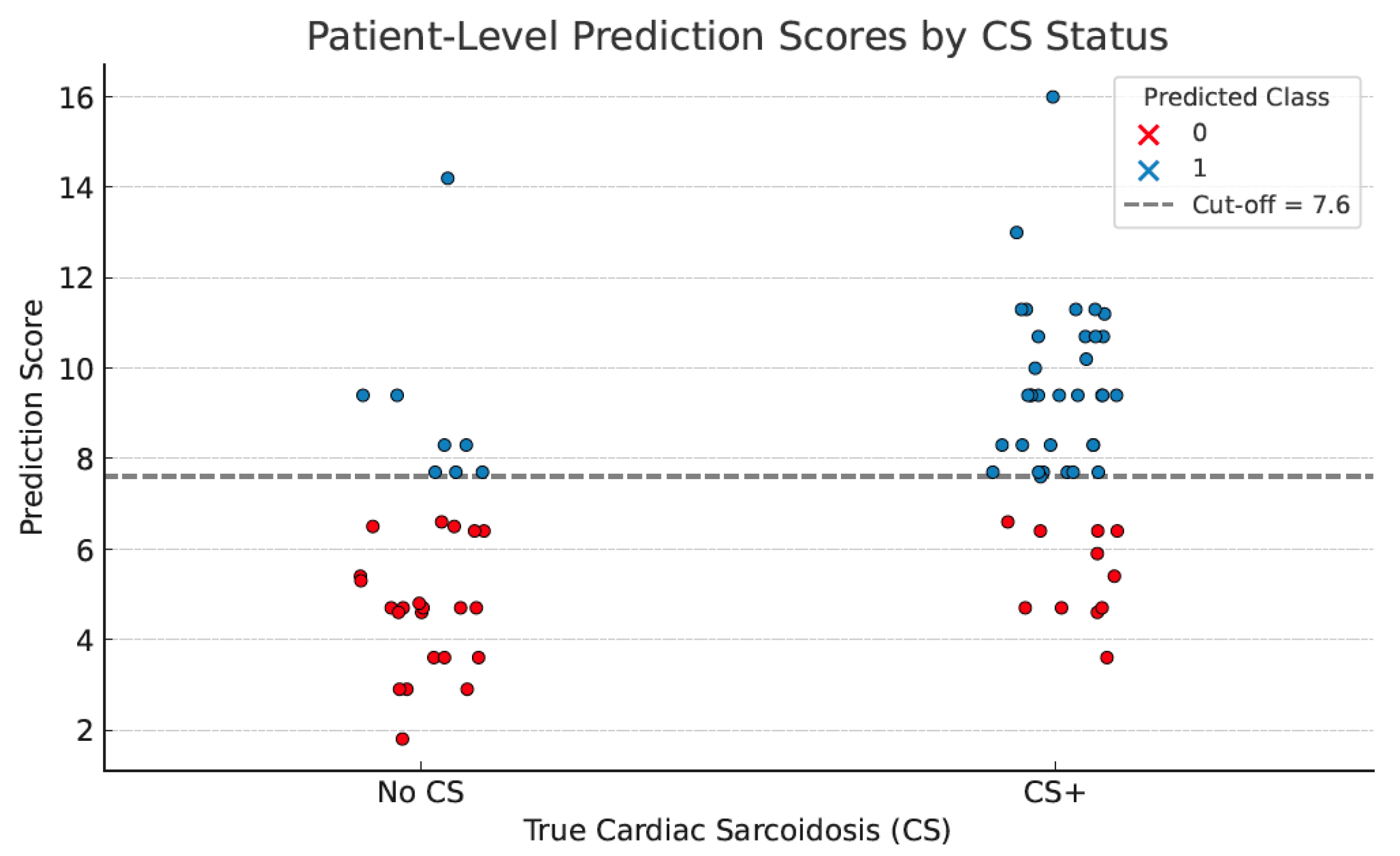
| AI assisted scoring system | 76% | 73% | 81% | 67% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dybowska, M.; Tomkowski, W.Z.; Lewandowska, K.B.; Piotrowska-Kownacka, D.; Sobiecka, M.; Kempisty, A.; Opoka, L.; Radwan-Rohrenschef, P.; Wyrostkiewicz, D.; Szturmowicz, M. AI-Assisted Simple Scoring Algorithm Was Helpful in the Risk Assessment of Cardiac Involvement in Patients with Pulmonary Sarcoidosis. J. Clin. Med. 2025, 14, 7290. https://doi.org/10.3390/jcm14207290
Dybowska M, Tomkowski WZ, Lewandowska KB, Piotrowska-Kownacka D, Sobiecka M, Kempisty A, Opoka L, Radwan-Rohrenschef P, Wyrostkiewicz D, Szturmowicz M. AI-Assisted Simple Scoring Algorithm Was Helpful in the Risk Assessment of Cardiac Involvement in Patients with Pulmonary Sarcoidosis. Journal of Clinical Medicine. 2025; 14(20):7290. https://doi.org/10.3390/jcm14207290
Chicago/Turabian StyleDybowska, Malgorzata, Witold Z. Tomkowski, Katarzyna B. Lewandowska, Dorota Piotrowska-Kownacka, Malgorzata Sobiecka, Anna Kempisty, Lucyna Opoka, Piotr Radwan-Rohrenschef, Dorota Wyrostkiewicz, and Monika Szturmowicz. 2025. "AI-Assisted Simple Scoring Algorithm Was Helpful in the Risk Assessment of Cardiac Involvement in Patients with Pulmonary Sarcoidosis" Journal of Clinical Medicine 14, no. 20: 7290. https://doi.org/10.3390/jcm14207290
APA StyleDybowska, M., Tomkowski, W. Z., Lewandowska, K. B., Piotrowska-Kownacka, D., Sobiecka, M., Kempisty, A., Opoka, L., Radwan-Rohrenschef, P., Wyrostkiewicz, D., & Szturmowicz, M. (2025). AI-Assisted Simple Scoring Algorithm Was Helpful in the Risk Assessment of Cardiac Involvement in Patients with Pulmonary Sarcoidosis. Journal of Clinical Medicine, 14(20), 7290. https://doi.org/10.3390/jcm14207290








