Edoxaban Safety and Effectiveness in Real-Life Patients with Heart Failure and Atrial Fibrillation: EMAYIC Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Patients, and Endpoints
2.2. Statistical Considerations
3. Results
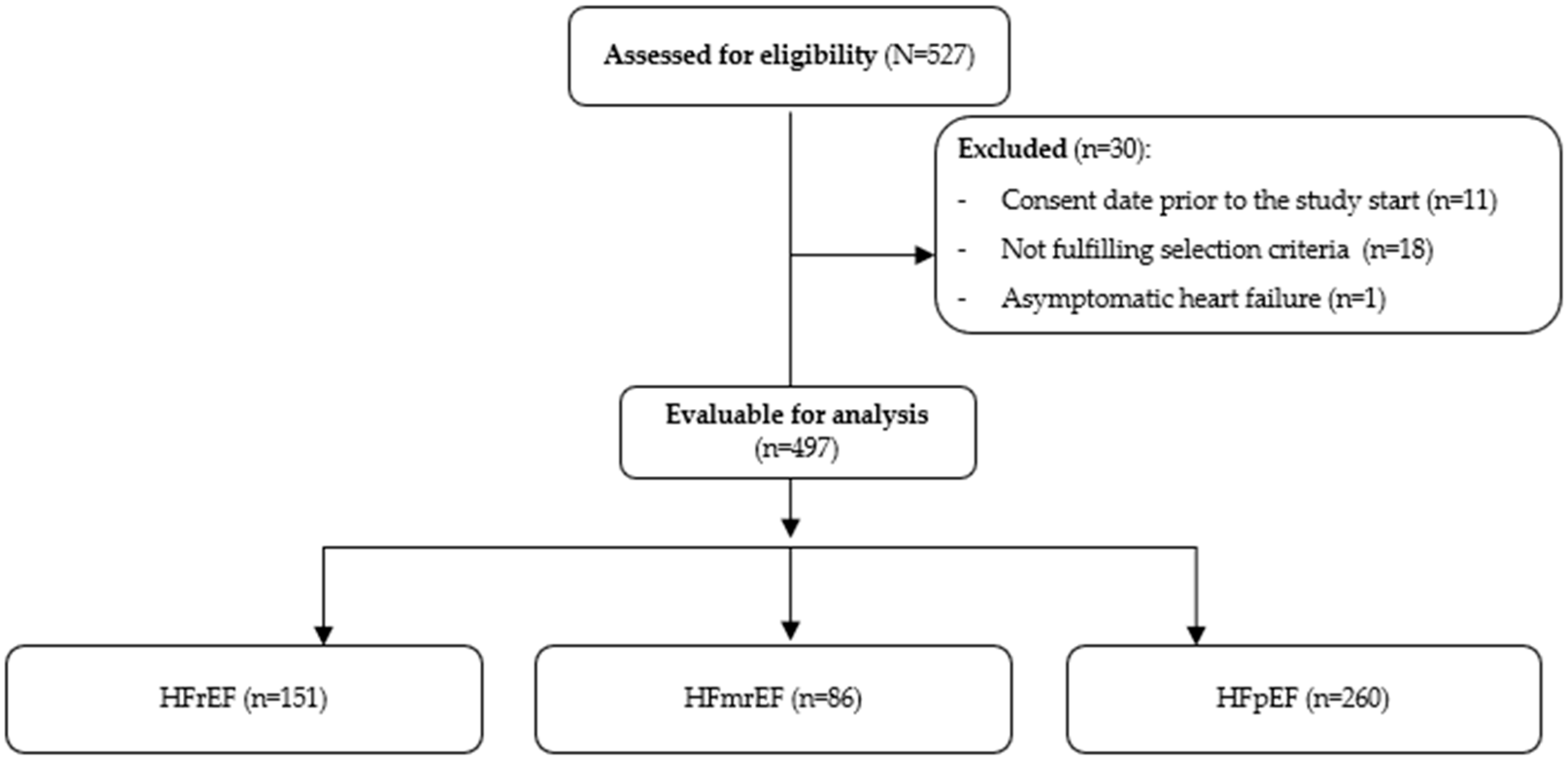
3.1. Patients
3.2. Medical Therapy
3.3. Follow up
3.4. Safety
3.5. Effectiveness
3.6. Other Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | American College of Cardiology |
| ACC | American Heart Association |
| AEs | Adverse events |
| AF | Atrial fibrillation |
| BMI | Body mass index |
| CAD | Coronary artery disease |
| CKD | Chronic kidney disease |
| CKD-EPI | Chronic Kidney Disease Epidemiology Collaboration |
| CRNM | Clinically relevant nonmajor |
| CRNMB | Clinically relevant nonmajor bleeding |
| DOAC | Direct-acting oral anticoagulants |
| DPB | Diastolic blood pressure |
| eGFR | Estimated glomerular filtration rate |
| ESC | European Society of Cardiology |
| HF | Heart failure |
| HF FEVI | Heart Failure With Preserved Ejection Fraction |
| HFmrEF | Heart failure with mid-range ejection fraction |
| HFpEF | Heart failure with preserved ejection fraction |
| HFrEF | Heart failure with reduced ejection fraction |
| ICH | Intracranial hemorrhage |
| INR | International normalized ratio |
| IQR | Interquartile range |
| ISTH | International Society on Thrombosis and Haemostasis |
| LVEF | Left ventricular ejection fraction |
| NVAF | Non-valvular atrial fibrillation |
| NYHA | New York Heart Association |
| OAC | Oral anticoagulation |
| SBP | Systolic blood pressure |
| SD | Standard deviation |
| SE | Systemic embolism |
| TIA | Transient ischemic attack |
| TTR | Therapeutic range |
| VKA | Vitamin K antagonists |
References
- Diaz, J.; Martinez, F.; Calderon, J.M.; Fernandez, A.; Sauri, I.; Uso, R.; Trillo, J.L.; Redon, J.; Forner, M.J. Incidence and impact of atrial fibrillation in heart failure patients: Real-world data in a large community. ESC Heart Fail. 2022, 9, 4230–4239. [Google Scholar] [CrossRef]
- Yang, E.; Vaishnav, J.; Song, E.; Lee, J.; Schulman, S.; Calkins, H.; Berger, R.; Russell, S.D.; Sharma, K. Atrial fibrillation is an independent risk factor for heart failure hospitalization in heart failure with preserved ejection fraction. ESC Heart Fail. 2022, 9, 2918–2927. [Google Scholar] [CrossRef]
- Odutayo, A.; Wong, C.X.; Hsiao, A.J.; Hopewell, S.; Altman, D.G.; Emdin, C.A. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: Systematic review and meta-analysis. BMJ 2016, 354, i4482. [Google Scholar] [CrossRef]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef]
- Mamas, M.A.; Caldwell, J.C.; Chacko, S.; Garratt, C.J.; Fath-Ordoubadi, F.; Neyses, L. A meta-analysis of the prognostic significance of atrial fibrillation in chronic heart failure. Eur. J. Heart Fail. 2009, 11, 676–683. [Google Scholar] [CrossRef]
- Schrage, B.; Geelhoed, B.; Niiranen, T.J.; Gianfagna, F.; Vishram-Nielsen, J.K.K.; Costanzo, S.; Söderberg, S.; Ojeda, F.M.; Vartiainen, E.; Donati, M.B.; et al. Comparison of Cardiovascular Risk Factors in European Population Cohorts for Predicting Atrial Fibrillation and Heart Failure, Their Subsequent Onset, and Death. J. Am. Heart Assoc. 2020, 9, e015218. [Google Scholar] [CrossRef]
- Kotecha, D.; Chudasama, R.; Lane, D.A.; Kirchhof, P.; Lip, G.Y. Atrial fibrillation and heart failure due to reduced versus preserved ejection fraction: A systematic review and meta-analysis of death and adverse outcomes. Int. J. Cardiol. 2016, 203, 660–666. [Google Scholar] [CrossRef]
- Hsu, J.C.; Freeman, J.V. Underuse of Vitamin K Antagonist and Direct Oral Anticoagulants for Stroke Prevention in Patients With Atrial Fibrillation: A Contemporary Review. Clin. Pharmacol. Ther. 2018, 104, 301–310. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef]
- Connolly, S.J.; Eikelboom, J.; Joyner, C.; Diener, H.C.; Hart, R.; Golitsyn, S.; Flaker, G.; Avezum, A.; Hohnloser, S.H.; Diaz, R.; et al. Apixaban in patients with atrial fibrillation. N. Engl. J. Med. 2011, 364, 806–817. [Google Scholar] [CrossRef]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Špinar, J.; et al. Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef]
- Rose, A.J.; Hylek, E.M.; Ozonoff, A.; Ash, A.S.; Reisman, J.I.; Berlowitz, D.R. Patient characteristics associated with oral anticoagulation control: Results of the Veterans AffaiRs Study to Improve Anticoagulation (VARIA). J. Thromb. Haemost. 2010, 8, 2182–2191. [Google Scholar] [CrossRef]
- Witt, D.M.; Delate, T.; Clark, N.P.; Martell, C.; Tran, T.; Crowther, M.A.; Garcia, D.A.; Ageno, W.; Hylek, E.M. Twelve-month outcomes and predictors of very stable INR control in prevalent warfarin users. J. Thromb. Haemost. 2010, 8, 744–749. [Google Scholar] [CrossRef]
- van Diepen, S.; Hellkamp, A.S.; Patel, M.R.; Becker, R.C.; Breithardt, G.; Hacke, W.; Halperin, J.L.; Hankey, G.J.; Nessel, C.C.; Singer, D.E.; et al. Efficacy and safety of rivaroxaban in patients with heart failure and nonvalvular atrial fibrillation: Insights from ROCKET AF. Circ. Heart Fail. 2013, 6, 740–747. [Google Scholar] [CrossRef]
- McMurray, J.J.; Ezekowitz, J.A.; Lewis, B.S.; Gersh, B.J.; van Diepen, S.; Amerena, J.; Bartunek, J.; Commerford, P.; Oh, B.H.; Harjola, V.P.; et al. Left ventricular systolic dysfunction, heart failure, and the risk of stroke and systemic embolism in patients with atrial fibrillation: Insights from the ARISTOTLE trial. Circ. Heart Fail. 2013, 6, 451–460. [Google Scholar] [CrossRef]
- Ferreira, J.; Ezekowitz, M.D.; Connolly, S.J.; Brueckmann, M.; Fraessdorf, M.; Reilly, P.A.; Yusuf, S.; Wallentin, L. Dabigatran compared with warfarin in patients with atrial fibrillation and symptomatic heart failure: A subgroup analysis of the RE-LY trial. Eur. J. Heart Fail. 2013, 15, 1053–1061. [Google Scholar] [CrossRef]
- Magnani, G.; Giugliano, R.P.; Ruff, C.T.; Murphy, S.A.; Nordio, F.; Metra, M.; Moccetti, T.; Mitrovic, V.; Shi, M.; Mercuri, M.; et al. Efficacy and safety of edoxaban compared with warfarin in patients with atrial fibrillation and heart failure: Insights from ENGAGE AF-TIMI 48. Eur. J. Heart Fail. 2016, 18, 1153–1161. [Google Scholar] [CrossRef]
- Xiong, Q.; Lau, Y.C.; Senoo, K.; Lane, D.A.; Hong, K.; Lip, G.Y. Non-vitamin K antagonist oral anticoagulants (NOACs) in patients with concomitant atrial fibrillation and heart failure: A systemic review and meta-analysis of randomized trials. Eur. J. Heart Fail. 2015, 17, 1192–1200. [Google Scholar] [CrossRef]
- Savarese, G.; Giugliano, R.P.; Rosano, G.M.; McMurray, J.; Magnani, G.; Filippatos, G.; Dellegrottaglie, S.; Lund, L.H.; Trimarco, B.; Perrone-Filardi, P. Efficacy and Safety of Novel Oral Anticoagulants in Patients With Atrial Fibrillation and Heart Failure: A Meta-Analysis. JACC Heart Fail. 2016, 4, 870–880. [Google Scholar] [CrossRef]
- Gómez Doblas, J.J.; Cepeda-Rodrigo, J.M.; Agra Bermejo, R.; Blanco Labrador, E.; Blasco, M.T.; Carrera Izquierdo, M.; Lekuona, I.; Recio Mayoral, A.; Rafols, C.; Manito, N. Outcomes and factors associated with mortality in patients with atrial fibrillation and heart failure: FARAONIC study. Clin. Cardiol. 2023, 46, 1390–1397. [Google Scholar] [CrossRef]
- Schnabel, R.B.; Ameri, P.; Siller-Matula, J.M.; Diemberger, I.; Gwechenberger, M.; Pecen, L.; Manu, M.C.; Souza, J.; De Caterina, R.; Kirchhof, P. Outcomes of patients with atrial fibrillation on oral anticoagulation with and without heart failure: The ETNA-AF-Europe registry. Europace 2023, 25, euad280. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.P.; Mentz, R.J.; Mebazaa, A.; Voors, A.A.; Butler, J.; Roessig, L.; Fiuzat, M.; Zannad, F.; Pitt, B.; O’Connor, C.M.; et al. Patient selection in heart failure with preserved ejection fraction clinical trials. J. Am. Coll. Cardiol. 2015, 65, 1668–1682. [Google Scholar] [CrossRef] [PubMed]
- The Criteria Committee of the New York Heart Association. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels, 9th ed.; Little, Brown & Co.: Boston, MA, USA, 1944; pp. 253–256. [Google Scholar]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2020, 42, 373–498. [Google Scholar] [CrossRef]
- Pisters, R.; Lane, D.A.; Nieuwlaat, R.; de Vos, C.B.; Crijns, H.J.; Lip, G.Y. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest 2010, 138, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; Kearon, C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef]
- Kaatz, S.; Ahmad, D.; Spyropoulos, A.C.; Schulman, S. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2015, 13, 2119–2126. [Google Scholar] [CrossRef]
- De Caterina, R.; Kelly, P.; Monteiro, P.; Deharo, J.C.; de Asmundis, C.; López-de-Sá, E.; Weiss, T.W.; Waltenberger, J.; Steffel, J.; de Groot, J.R.; et al. Characteristics of patients initiated on edoxaban in Europe: Baseline data from edoxaban treatment in routine clinical practice for patients with atrial fibrillation (AF) in Europe (ETNA-AF-Europe). BMC Cardiovasc. Disord. 2019, 19, 165. [Google Scholar] [CrossRef]
- Tsuji, K.; Sakata, Y.; Nochioka, K.; Miura, M.; Yamauchi, T.; Onose, T.; Abe, R.; Oikawa, T.; Kasahara, S.; Sato, M.; et al. Characterization of heart failure patients with mid-range left ventricular ejection fraction-a report from the CHART-2 Study. Eur. J. Heart Fail. 2017, 19, 1258–1269. [Google Scholar] [CrossRef]
- Rickenbacher, P.; Kaufmann, B.A.; Maeder, M.T.; Bernheim, A.; Goetschalckx, K.; Pfister, O.; Pfisterer, M.; Brunner-La Rocca, H.P. Heart failure with mid-range ejection fraction: A distinct clinical entity? Insights from the Trial of Intensified versus standard Medical therapy in Elderly patients with Congestive Heart Failure (TIME-CHF). Eur. J. Heart Fail. 2017, 19, 1586–1596. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef]
- Pan, Y.; Li, D.; Ma, J.; Shan, L.; Wei, M. NT-proBNP test with improved accuracy for the diagnosis of chronic heart failure. Medicine 2017, 96, e9181. [Google Scholar] [CrossRef]
- Richards, M.; Di Somma, S.; Mueller, C.; Nowak, R.; Peacock, W.F.; Ponikowski, P.; Mockel, M.; Hogan, C.; Wu, A.H.; Clopton, P.; et al. Atrial fibrillation impairs the diagnostic performance of cardiac natriuretic peptides in dyspneic patients: Results from the BACH Study (Biomarkers in ACute Heart Failure). JACC Heart Fail. 2013, 1, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Westenbrink, B.D.; Alings, M.; Granger, C.B.; Alexander, J.H.; Lopes, R.D.; Hylek, E.M.; Thomas, L.; Wojdyla, D.M.; Hanna, M.; Keltai, M.; et al. Anemia is associated with bleeding and mortality, but not stroke, in patients with atrial fibrillation: Insights from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Am. Heart J. 2017, 185, 140–149. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Ishii, M.; Iguchi, M.; Masunaga, N.; Tezuka, Y.; Doi, K.; Esato, M.; Chun, Y.; Wada, H.; Hasegawa, K.; et al. Association of anemia with stroke/systemic embolism, bleeding, and cardiovascular death in patients with atrial fibrillation: The Fushimi AF Registry. Eur. Heart J. 2017, 38, ehx501. [Google Scholar] [CrossRef]
- Lavalle, C.; Pierucci, N.; Mariani, M.V.; Piro, A.; Borrelli, A.; Grimaldi, M.; Rossillo, A.; Notarstefano, P.; Compagnucci, P.; Dello Russo, A.; et al. Italian Registry in the Setting of Atrial Fibrillation Ablation with Rivaroxaban—IRIS. Minerva Cardiol. Angiol. 2024, 72, 625–637. [Google Scholar] [CrossRef]
- Stolfo, D.; Fabris, E.; Lund, L.H.; Savarese, G.; Sinagra, G. From mid-range to mildly reduced ejection fraction heart failure: A call to treat. Eur. J. Intern. Med. 2022, 103, 29–35. [Google Scholar] [CrossRef]
- Fonarow, G.C.; Stough, W.G.; Abraham, W.T.; Albert, N.M.; Gheorghiade, M.; Greenberg, B.H.; O’Connor, C.M.; Sun, J.L.; Yancy, C.W.; Young, J.B. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: A report from the OPTIMIZE-HF Registry. J. Am. Coll. Cardiol. 2007, 50, 768–777. [Google Scholar] [CrossRef]
- Sweitzer, N.K.; Lopatin, M.; Yancy, C.W.; Mills, R.M.; Stevenson, L.W. Comparison of clinical features and outcomes of patients hospitalized with heart failure and normal ejection fraction (> or =55%) versus those with mildly reduced (40% to 55%) and moderately to severely reduced (<40%) fractions. Am. J. Cardiol. 2008, 101, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.S.; Tay, W.T.; Teng, T.H.K.; Vedin, O.; Benson, L.; Dahlstrom, U.; Savarese, G.; Lam, C.S.P.; Lund, L.H. A comprehensive population-based characterization of heart failure with mid-range ejection fraction. Eur. J. Heart Fail. 2017, 19, 1624–1634. [Google Scholar] [CrossRef]
- Lund, L.H.; Claggett, B.; Liu, J.; Lam, C.S.; Jhund, P.S.; Rosano, G.M.; Swedberg, K.; Yusuf, S.; Granger, C.B.; Pfeffer, M.A.; et al. Heart failure with mid-range ejection fraction in CHARM: Characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. Eur. J. Heart Fail. 2018, 20, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, I.; Leung, K.S.K.; Tse, G.; Lee, A.P.W. Novel Mechanisms in Heart Failure With Preserved, Midrange, and Reduced Ejection Fraction. Front. Physiol. 2019, 10, 874. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Overall | HFrEF | HFmrEF | HFpEF | p-Value |
|---|---|---|---|---|---|
| N (%) | 497 (100.0) | 151 (30.4) | 86 (17.3) | 260 (52.3) | |
| Age, median (IQR), years a | 76.3 (67.6–82.5) | 71.0 (63.2–78.3) | 77.2 (69.0–81.4) | 78.4 (70.8–84.9) | <0.001 * |
| Gender, male, n (%) b | 288 (57.9) | 122 (80.8) | 51 (59.3) | 115 (44.2) | <0.001 Ω |
| BMI, median (IQR), Kg/m2 | 28.7 (25.2–31.1) a | 28.0 (25.4–31.2) | 28.1 (25.8–30.4) b | 27.7 (24.8–31.2) c | 0.867 * |
| Smoking, n (%) | <0.001 Ω | ||||
| Never smoker | 295 (59.4) | 63 (41.7) | 52 (60.5) | 180 (69.2) | |
| Ex-smoker | 152 (30.6) | 65 (43.0) | 27 (31.4) | 60 (23.1) | |
| Active smoker | 50 (10.1) | 23 (15.2) | 7 (8.1) | 20 (7.7) | |
| Alcohol consumption, n (%) d | 30 (6.0) | 16 (10.6) | 3 (3.5) | 11 (4.2) | 0.018 Ω |
| Comorbidities, n (%) e | |||||
| Arterial hypertension | 371 (77.5) f | 110 (73.3) g | 62 (74.7) h | 199 (80.9) i | |
| Congestive HF | 361 (75.4) f | 130 (86.7) g | 64 (77.1) h | 167 (67.9) i | |
| Dyslipidemia | 266 (55.5) f | 89 (59.3) g | 45 (54.2) h | 132 (53.7) i | |
| CKD (eGFR < 60 mL/min) | 172 (35.9) f | 56 (37.3) g | 27 (32.5) h | 89 (36.2) i | |
| Diabetes mellitus | 163 (34.0) f | 57 (38.0) g | 30 (36.1) h | 76 (30.9) i | |
| Ischemic cardiomyopathy | 101 (21.1) f | 54 (36.0) g | 19 (22.9) h | 28 (11.4) i | |
| Myocardial infarction | 70 (69.3) | 42 (77.8) | 11 (57.9) | 17 (60.7) | 0.138 Ω |
| CAD | 29 (77.5) f | 52 (34.7) g | 19 (22.9) h | 28 (11.4) i | |
| Valvular heart disease | 94 (18.9) | 24 (15.9) | 15 (17.4) | 55 (21.2) | 0.393 Ω |
| Mitral | 49 (52.1) | 14 (58.3) | 7 (46.7) | 28 (50.9) | |
| Aortic | 37 (39.4) | 9 (37.5) | 7 (46.7) | 21 (38.2) | |
| Prior ischemic stroke | 20 (4.2) f | 7 (4.7) g | 3 (3.6) | 10 (4.1) i | 0.636 † |
| Prior TIA | 4 (18.2) | 0 (0.0) | 1 (33.3) | 3 (25.0) | |
| Anemia | 47 (9.5) | 9 (6.0) | 13 (15.1) | 25 (9.6) | 0.068 Ω |
| Labile INR (TTR < 60%) | 133 (27.8) f | 47 (31.3) g | 21 (25.3) h | 65 (26.4) i | |
| Bleeding predisposition | 28 (5.6) | 7 (4.6) | 6 (7.0) | 15 (5.8) | 0.747 Ω |
| Bleeding history (in previous year) | 17 (3.4) | 3 (2.0) | 5 (5.8) | 9 (3.5) | 0.296 Ω |
| Major bleeding | 7 (41.2) | 1 (33.3) | 2 (40.0) | 4 (44.4) | 0.564 † |
| Moderate-severe dementia | 5 (1.0) | 2 (1.3) | 1 (1.2) | 2 (0.8) | 0.848 † |
| Clinical characteristics | |||||
| Blood pressure, median (IQR), mmHg | |||||
| SBP | 126.0 (115.0–137.0) | 119.0 (108.0–129.0) | 129.5 (117.8–140.0) | 130.0 (120.0–140.0) | <0.001 * |
| DPB | 75.0 (69.0–82.0) | 72.0 (64.8–80.0) | 76.0 (70.0–84.3) | 77.0 (70.0–83.0) | 0.002 * |
| Type of NVAF, n (%) | 0.496 Ω | ||||
| Paroxysmal | 126 (25.4) | 38 (25.2) | 23 (26.7) | 65 (25.0) | |
| Persistent | 125 (25.2) | 40 (26.5) | 25 (29.1) | 60 (23.1) | |
| Long-standing persistent | 28 (5.6) | 12 (7.9) | 5 (5.8) | 11 (4.2) | |
| Permanent | 218 (43.9) | 61 (40.4) | 33 (38.4) | 124 (47.7) | |
| CHAD2DS2-VASc score, mean (SD) | 4.0 (1.5) | 3.8 (1.6) | 4.1 (1.5) | 4.1 (1.5) | 0.213 * |
| HAS-BLED score, mean (SD) | 1.5 (0.9) j | 1.5 (1.0) k | 1.6 (1.0) | 1.6 (0.9) | 0.579 * |
| HF LVEF (%), median (IQR) | 50.0 (35.0–60.0) | 30.0 (25.0–35.0) | 45.0 (42.0–45.0) | 60.0 (55.0–62.0) | <0.001 * |
| NYHA Functional Classification, n (%) | 0.602 † | ||||
| Class I | 107 (22.9) | 35 (23.8) | 24 (28.9) | 48 (20.2) | |
| Class II | 291 (62.2) | 92 (62.6) | 49 (59.0) | 150 (63.0) | |
| Class III | 63 (13.5) | 17 (11.6) | 10 (12.0) | 36 (15.1) | |
| Class IV | 7 (1.5) | 3 (2.0) | 0 (0.0) | 4 (1.7) | |
| Laboratory | |||||
| Creatinine clearance, median (IQR), mL/min | 64.0 (49.0–80.0) l | 62.0 (48.0–79.0) m | 66.0 (52.0–80.0) | 65.0 (48.0–81.0) n | 0.659 € |
| Cockroft-Gault, median (IQR), mL/min/m2 | 60.8 (45.4–82.9) | 64.9 (47.8–93.1) | 62.4 (49.3–86.5) | 57.7 (43.4–78.8) | 0.052 |
| CKD-EPI, median (IQR), mL/min/1.73 m2 | 64.1 (47.3–79.9) | 61.5 (46.7–76.7) | 67.3 (51.5–80.9) | 63.8 (46.3–80.4) | 0.269 * |
| NT-pro-BNP, median (IQR) | 1884.0 (1009.0–3371.5) | 2272.5 (1178.3–4078.8) | 1750.0 (934.0–3480.5) | 1708–0 (994.3–2857.0) | 0.005 * |
| Characteristics | Overall | HFrEF | HFmrEF | HFpEF | p-Value |
|---|---|---|---|---|---|
| Bleeding outcomes | |||||
| Major or CRNM bleeding, n % | 31 (6.6) | 11 (7.5) | 3 (3.6) | 17 (7.1) | 0.474 |
| Major bleeding | |||||
| Number of bleeding events, n (%) | 11 (2.4) a | 5 (3.4) b | 1 (1.2) c | 5 (2.1) d | 0.602 † |
| 1 | 10 (90.9) | 5 (100.0) | 1 (100.0) | 4 (80.0) | |
| 2 | 1 (9.1) | 0 (0.0) | 0 (0.0) | 1 (20.0) | |
| Median number if events (IQR) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 1.0 (1.0–1.5) | 0.549 * |
| Type of events, n (%) | |||||
| Gastrointestinal | 8 (72.7) | 3 (60.0) | 1 (100.0) | 4 (80.0) | |
| Intracranial | 1 (9.1) | 1 (20.0) | 0 (0.0) | 0 (0.0) | |
| Other | 2 (18.2) | 1 (20.0) | 0 (0.0) | 1 (20.0) | |
| CRNM bleeding | 21 (4.5) a | 6 (4.1) b | 3 (3.6) c | 12 (5.0) d | 0.829 Ω |
| Number of bleeding events, n (%) | 0.353 † | ||||
| 1 | 16 (76.2) | 6 (100.0) | 2 (66.7) | 8 (66.7) | |
| 2 | 3 (14.3) | 0 (0.0) | 0 (0.0) | 3 (25.0) | |
| 3 | 2 (9.5) | 0 (0.0) | 1 (33.3) | 1 (8.3) | |
| Median number of events (IQR) | 1.0 (1.0–1.5) | 1.0 (1.0–1.0) | 1.0 (1.0–X) | 1.0 (1.0–2.0) | 0.287 * |
| Type of events, n (%) | |||||
| Gastrointestinal | 3 (50.0) | 7 (58.3) | |||
| Epistaxis | 0 (0.0) | 2 (66.7) | 1 (8.3) | ||
| Hematuria | 1 (16.7) | 0 (0.0) | 2 (16.7) | ||
| Other | 2 (33.3) | 0 (0.0) | 0 (0.0) | ||
| Minor bleeding | |||||
| Number of bleeding events, n (%) | 26 (5.6) a | 4 (2.7) b | 5 (6.0) c | 17 (7.1) d | 0.180 Ω |
| 1 | 21 (80.8) | 4 (100.0) | 3 (60.0) | 14 (82.4) | |
| 2 | 3 (11.5) | 0 (0.0) | 1 (20.0) | 2 (11.8) | |
| 3–4 | 2 (7.7) | 0 (0.0) | 1 (20.0) | 1 (5.9) | |
| Median number of events (IQR) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 0.291 * |
| Type of events, n (%) | |||||
| Gastrointestinal | 0 (0.0) | 2 (40.0) | 4 (23.5) | ||
| Epistaxis | 1 (25.0) | 0 (0.0) | 2 (11.8) | ||
| Hematuria | 1 (25.0) | 3 (60.0) | 1 (5.9) | ||
| Other | 2 (50.0) | 2 (40.0) | 7 (41.2) | ||
| Cardiovascular death | |||||
| Death due to CV causes, n (%) | 19 (4.1) | 7 (4.8) | 3 (3.6) | 9 (3.8) | 0.871 Ω |
| Reason, n (%) | |||||
| HF | 3 (42.9) | 2 (66.7) | 7 (77.8) | ||
| Stroke | 1 (14.3) | 0 (0.0) | 0 (0.0) | ||
| ICH | 1 (14.3) | 0 (0.0) | 0 (0.0) | ||
| Other | 2 (28.6) | 1 (33.3) | 2 (22.2) | ||
| Thromboembolic events | |||||
| Stroke, n (%) | 7 (1.5) a | 3 (2.0) b | 1 (1.2) c | 3 (1.3) d | 0.876 † |
| Stroke event, n (%) | 0.486 † | ||||
| Ischemic stroke | 3 (42.9) | 2 (66.7) | 1 (100.0) | 0 (0.0) | |
| Hemorrhagic stroke | 1 (14.3) | 0 (0.0) | 0 (0.0) | 1 (33.3) | |
| TIA | 3 (42.9) | 1 (33.3) | 0 (0.0) | 2 (66.7) | |
| Stroke outcome, n (%) | |||||
| Disabling | 2 (28.6) | 1 (33.3) | 0 (0.0) | 1 (33.3) | >0.999 † |
| Fatal | 2 (28.6) | 1 (33.3) | 0 (0.0) | 1 (33.3) | >0.999 † |
| Systemic embolism, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Characteristics | Overall | HFrEF | HFmrEF | HFpEF | p-Value |
| Total number of adverse events | 10 | 2 | 0 | 8 | |
| Treatment-related adverse events, n (%) | 9 (1.8) | 2 (1.3) | 0 (0.0) | 7 (2.7) | |
| Adverse events, n (%) a | |||||
| Anemia | 6 (1.2) | 0 (0.0) | 0 (0.0) | 5 (1.9) | |
| Diarrhea | 2 (0.4) | 1 (0.7) | 0 (0.0) | 0 (0.0) | |
| Exanthema | 1 (0.2) | 0 (0.0) | 0 (0.0) | 1 (0.4) | |
| Acute kidney injury secondary to diarrhea | 1 (0.2) | 1 (0.7) | 0 (0.0) | 0 (0.0) | |
| Abdominal pain | 1 (0.2) | 0 (0.0) | 0 (0.0) | 1 (0.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salguero-Bodes, R.; Padilla Perez, M.; Sánchez, A.A.; Esteban-Fernández, A.; García López, M.; Aparici Feal, M.A.; Santos, J.L.; Gaebelt, H.P.; Arribas, F.; on behalf of the EMAYIC Study Investigators. Edoxaban Safety and Effectiveness in Real-Life Patients with Heart Failure and Atrial Fibrillation: EMAYIC Study. J. Clin. Med. 2025, 14, 7272. https://doi.org/10.3390/jcm14207272
Salguero-Bodes R, Padilla Perez M, Sánchez AA, Esteban-Fernández A, García López M, Aparici Feal MA, Santos JL, Gaebelt HP, Arribas F, on behalf of the EMAYIC Study Investigators. Edoxaban Safety and Effectiveness in Real-Life Patients with Heart Failure and Atrial Fibrillation: EMAYIC Study. Journal of Clinical Medicine. 2025; 14(20):7272. https://doi.org/10.3390/jcm14207272
Chicago/Turabian StyleSalguero-Bodes, Rafael, Miriam Padilla Perez, Arturo Andrés Sánchez, Alberto Esteban-Fernández, Martín García López, Manuel Andrés Aparici Feal, José Luis Santos, Hans Paul Gaebelt, Fernando Arribas, and on behalf of the EMAYIC Study Investigators. 2025. "Edoxaban Safety and Effectiveness in Real-Life Patients with Heart Failure and Atrial Fibrillation: EMAYIC Study" Journal of Clinical Medicine 14, no. 20: 7272. https://doi.org/10.3390/jcm14207272
APA StyleSalguero-Bodes, R., Padilla Perez, M., Sánchez, A. A., Esteban-Fernández, A., García López, M., Aparici Feal, M. A., Santos, J. L., Gaebelt, H. P., Arribas, F., & on behalf of the EMAYIC Study Investigators. (2025). Edoxaban Safety and Effectiveness in Real-Life Patients with Heart Failure and Atrial Fibrillation: EMAYIC Study. Journal of Clinical Medicine, 14(20), 7272. https://doi.org/10.3390/jcm14207272






