Diagnostic Value of Serum Periostin for Cyst Involution in Children with Multicystic Dysplastic Kidney
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sekine, A.; Hidaka, S.; Moriyama, T.; Shikida, Y.; Shimazu, K.; Ishikawa, E.; Uchiyama, K.; Kataoka, H.; Kawano, H.; Kurashige, M.; et al. Cystic Kidney Diseases That Require a Differential Diagnosis from Autosomal Dominant Polycystic Kidney Disease (ADPKD). J. Clin. Med. 2022, 11, 6528. [Google Scholar] [CrossRef] [PubMed]
- Lanktree, M.B.; Haghighi, A.; Guiard, E.; Iliuta, I.A.; Song, X.; Harris, P.C.; Paterson, A.D.; Pei, Y. Prevalence Estimates of Polycystic Kidney and Liver Disease by Population Sequencing. J. Am. Soc. Nephrol. 2018, 29, 2593–2600. [Google Scholar] [CrossRef]
- Bergmann, C.; Guay-Woodford, L.M.; Harris, P.C.; Horie, S.; Peters, D.J.M.; Torres, V.E. Polycystic kidney disease. Nat. Rev. Dis. Primers 2018, 4, 50. [Google Scholar] [CrossRef]
- Pisani, I.; Giacosa, R.; Giuliotti, S.; Moretto, D.; Regolisti, G.; Cantarelli, C.; Vaglio, A.; Fiaccadori, E.; Manenti, L. Ultrasound to address medullary sponge kidney: A retrospective study. BMC Nephrol. 2020, 21, 430. [Google Scholar] [CrossRef]
- Hains, D.S.; Bates, C.M.; Ingraham, S.; Schwaderer, A.L. Management and etiology of the unilateral multicystic dysplastic kidney: A review. Pediatr. Nephrol. 2009, 24, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.Y.H.; Warady, B.A. Acquired cystic kidney disease: An under-recognized condition in children with end-stage renal disease. Pediatr. Nephrol. 2018, 33, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Feldenberg, L.R.; Siegel, N.J. Clinical course and outcome for children with multicystic dysplastic kidneys. Pediatr. Nephrol. 2000, 14, 1098–1101. [Google Scholar] [CrossRef]
- Rudnik-Schoneborn, S.; John, U.; Deget, F.; Ehrich, J.H.; Misselwitz, J.; Zerres, K. Clinical features of unilateral multicystic renal dysplasia in children. Eur. J. Pediatr. 1998, 157, 666–672. [Google Scholar] [CrossRef]
- Belk, R.A.; Thomas, D.F.; Mueller, R.F.; Godbole, P.; Markham, A.F.; Weston, M.J. A family study and the natural history of prenatally detected unilateral multicystic dysplastic kidney. J. Urol. 2002, 67, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Cambio, A.J.; Evans, C.P.; Kurzrock, E.A. Non-surgical management of multicystic dysplastic kidney. BJU Int. 2008, 101, 804–808. [Google Scholar] [CrossRef]
- Ylinen, E.; Ahonen, S.; Ala-Houhala, M.; Wikstrom, S. Nephrectomy for multicystic dysplastic kidney: If and when? Urology 2004, 63, 768–771. [Google Scholar] [CrossRef]
- Du, J.; Li, M. Functions of Periostin in Dental Tissues and Its Role in Periodontal Tissue Regeneration. Adv. Exp. Med. Biol. 2019, 1132, 63–72. [Google Scholar] [CrossRef]
- Kruzynska-Frejtag, A.; Machnicki, M.; Rogers, R.; Markwald, R.R.; Conway, S.J. Periostin (an osteoblast-specific factor) is expressed within the embryonic mouse heart during valve formation. Mech. Dev. 2001, 103, 183–188. [Google Scholar] [CrossRef]
- Dorafshan, S.; Razmi, M.; Safaei, S.; Gentilin, E.; Madjd, Z.; Ghods, R. Periostin: Biology and function in cancer. Cancer Cell Int. 2022, 22, 315. [Google Scholar] [CrossRef]
- Alzobaidi, N.; Rehman, S.; Naqvi, M.; Gulati, K.; Ray, A. Periostin: A Potential Biomarker and Therapeutic Target in Pulmonary Diseases. J. Pharm. Pharm. Sci. 2022, 25, 137–148. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Z.; Cui, D.; Ouyang, G. The Multiaspect Functions of Periostin in Tumor Progression. Adv. Exp. Med. Biol. 2019, 1132, 125–136. [Google Scholar] [CrossRef]
- Zhang, Y.; Reif, G.; Wallace, D.P. Extracellular matrix, integrins, and focal adhesion signaling in polycystic kidney disease. Cell Signal. 2020, 72, 109646. [Google Scholar] [CrossRef] [PubMed]
- Prakoura, N.; Chatziantoniou, C. Periostin in kidney diseases. Cell. Mol. Life Sci. 2017, 74, 4315–4320. [Google Scholar] [CrossRef] [PubMed]
- González-González, L.; Alonso, J. Periostin: A Matricellular Protein with Multiple Functions in Cancer Development and Progression. Front. Oncol. 2018, 8, 225. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.; Hu, M.; Berman, Y.; Collins, F.; Grigg, J.; McIver, M.; Jüppner, H.; Alexander, S.I. Multicystic dysplastic kidney and variable phenotype in a family with a novel deletion mutation of PAX2. J. Am. Soc. Nephrol. 2005, 16, 2754–2761. [Google Scholar] [CrossRef]
- van der Ven, A.T.; Vivante, A.; Hildebrandt, F. Novel Insights into the Pathogenesis of Monogenic Congenital Anomalies of the Kidney and Urinary Tract. J. Am. Soc. Nephrol. 2018, 29, 36–50. [Google Scholar] [CrossRef]
- Verbitsky, M.; Westland, R.; Perez, A.; Kiryluk, K.; Liu, Q.; Krithivasan, P.; Mitrotti, A.; Fasel, D.A.; Batourina, E.; Sampson, M.G.; et al. The copy number variation landscape of congenital anomalies of the kidney and urinary tract. Nat. Genet. 2019, 51, 117–127. [Google Scholar] [CrossRef]
- Takeshita, S.; Kikuno, R.; Tezuka, K.; Amann, E. Osteoblast-specific factor 2: Cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem. J. 1993, 294, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Conway, S.J.; Izuhara, K.; Kudo, Y.; Litvin, J.; Markwald, R.; Ouyang, G.; Arron, J.R.; Holweg, C.T.; Kudo, A. The role of periostin in tissue remodeling across health and disease. Cell. Mol. Life Sci. 2014, 71, 1279–1288. [Google Scholar] [CrossRef]
- Xue, C.; Mei, C.L. Polycystic Kidney Disease and Renal Fibrosis. Adv. Exp. Med. Biol. 2019, 1165, 81–100. [Google Scholar] [CrossRef]
- Mun, H.; Park, J.H. Inflammation and Fibrosis in ADPKD. Adv. Exp. Med. Biol. 2016, 933, 35–44. [Google Scholar] [CrossRef]
- Wallace, D.P.; Quante, M.T.; Reif, G.A.; Nivens, E.; Ahmed, F.; Hempson, S.J.; Blanco, G. Periostin induces proliferation of human autosomal dominant polycystic kidney cells through alphaV-integrin receptor. Am. J. Physiol. Ren. Physiol. 2008, 295, F1463–F1471. [Google Scholar] [CrossRef]
- Rottenberg, G.T.; Gordon, I.; De Bruyn, R. The natural history of the multicystic dysplastic kidney in children. Br. J. Radiol. 1997, 70, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Sukthankar, S.; Watson, A.R.; on behalf of the Trent & Anglia Nephrourology Group. Unilateral multicystic dysplastic kidney disease: Defining the natural history. Acta Paediatr. 2000, 89, 811–813. [Google Scholar] [CrossRef]
- Jawa, N.A.; Rosenblum, N.D.; Radhakrishnan, S.; Pearl, R.J.; Levin, L.; Matsuda-Abedini, M. Reducing Unnecessary Imaging in Children with Multicystic Dysplastic Kidney or Solitary Kidney. Pediatrics 2021, 148, e2020035550. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Sivananthan, D.; Nataraja, R.M.; Johnstone, L.; Webb, N.; Lopez, P.J. Evidence-based treatment of multicystic dysplastic kidney: A systematic review. J. Pediatr. Urol. 2018, 14, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Nakai, H.; Asanuma, H.; Shishido, S.; Kitahara, S.; Yasuda, K. Changing concepts in urological management of the congenital anomalies of kidney and urinary tract, CAKUT. Pediatr. Int. 2003, 45, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.T.; Sebastião, Y.V.; McLeod, D.J. Trends in surgical management of multicystic dysplastic kidney at USA children’s hospitals. J. Pediatr. Urol. 2019, 15, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Huettinger, M.; Bogner, G.; Fischer, T.; Tovilo, K.; Fazelnia, C. Clinical outcome of children with prenatally diagnosed isolated unilateral multicystic dysplastic kidney. Eur. J. Obstet. Gynecol. Reprod. Biol. 2025, 312, 114112. [Google Scholar] [CrossRef]
- Kopač, M.; Kordič, R. Associated Anomalies and Complications of Multicystic Dysplastic Kidney. Pediatr. Rep. 2022, 14, 375–379. [Google Scholar] [CrossRef]
- Turkyilmaz, G.; Cetin, B.; Erturk, E.; Sivrikoz, T.; Kalelioglu, I.; Has, R.; Yuksel, A.; Oktar, T.; Ziylan, O. Prenatal diagnosis and outcome of unilateral multicystic kidney. J. Obstet. Gynaecol. 2021, 41, 1071–1075. [Google Scholar] [CrossRef]

| Group A N = 34 (53.1%) | Group B N = 10 (15.6%) | Group C N = 20 (31.3%) | p Value | |
|---|---|---|---|---|
| Age (months) | 36 (9) | 38 (6) | 34 (7) | NS |
| Boys/Girls | 21/13 | 5/5 | 10/10 | NS |
| Height (cm) | 95 (7) | 90 (4) | 90 (6) | NS |
| Weight (kg) | 15 (4) | 14 (5) | 15 (6) | NS |
| BP (mmH) according to age | Normal | Normal | Normal | NS |
| CRP (mg/dL) | <0.5 | <0.5 | <0.5 | NS |
| White blood cells (×109/L) | 7.86 | 8.28 | 6.34 | NS |
| Urea (mg/dL) | 23 | 16 | 15 | NS |
| Creatinine (mg/dL) | 0.31 | 0.39 | 0.41 | NS |
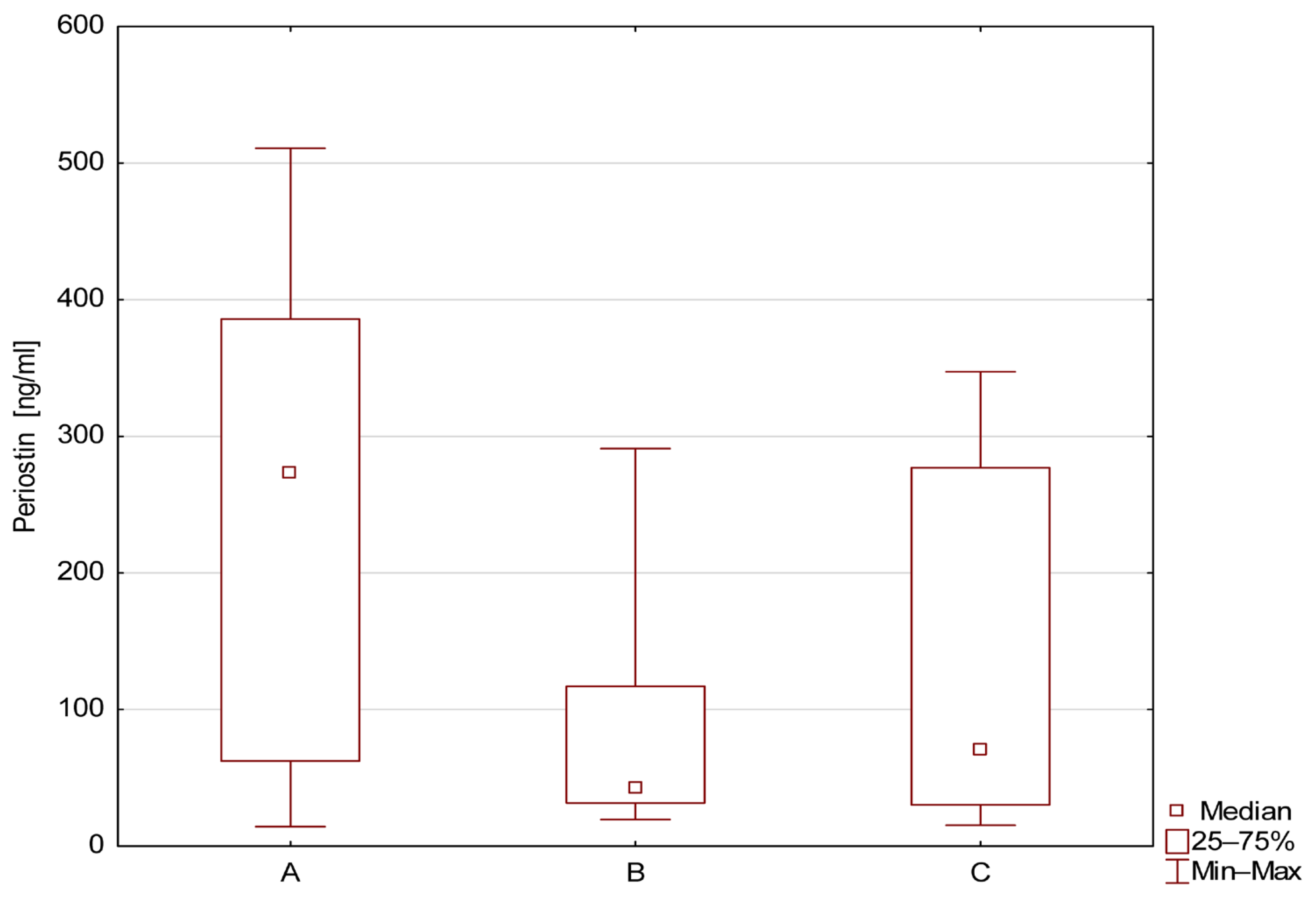
| Number of Patients | Mean ± SD | Median | Min | Max | QR1 | QR3 | |
|---|---|---|---|---|---|---|---|
| A | 34 | 239.1 ± 168.1 | 273.3 | 14.3 | 510.9 | 62.4 | 385.7 |
| B | 10 | 77.7 ± 82.8 | 42.4 | 19.5 | 290.9 | 31.7 | 117.0 |
| C | 20 | 141.1 ± 129 | 70.8 | 15.3 | 347.3 | 30.3 | 276.9 |
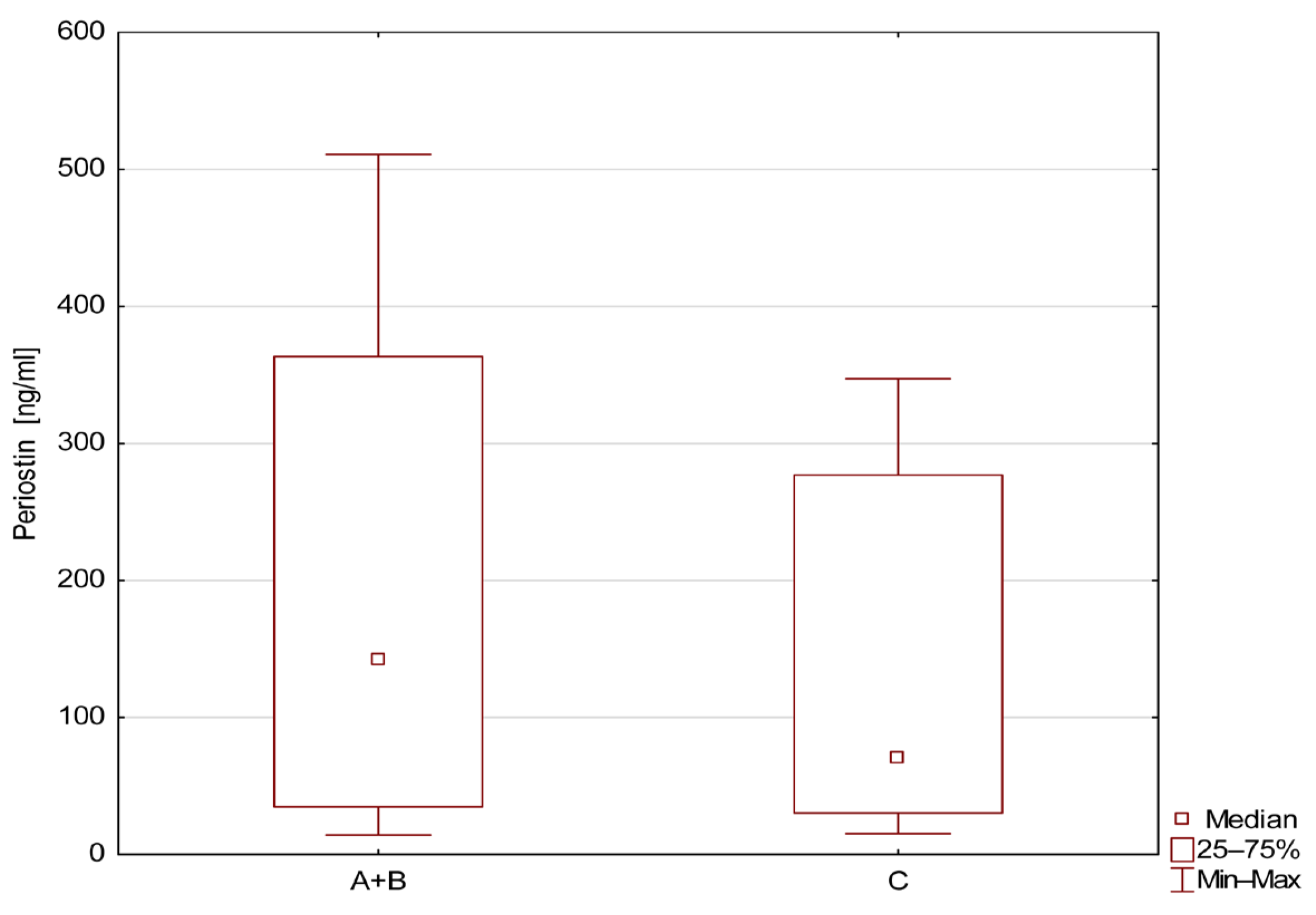
| Group | AUC | 95%CI Upper–Lower | SE | z | Sensitivity % | Specificity % | p |
|---|---|---|---|---|---|---|---|
| A vs. C | 0.7 | 0.527–0.813 | 0.073 | 2.342 | 76.5 | 50 | <0.05 |
| B vs. C | 0.608 | 0.405–0.810 | 0.103 | 1.040 | - | - | 0.298 |
| A vs. B | 0.765 | 0.618–0.912 | 0.075 | 3.530 | 67.6 | 88.9 | <0.05 |
| A+B vs. C | 0.609 | 0.467–0.750 | 0.072 | 1.504 | - | - | 0.132 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szmigielska, A.; Kotuła, I.; Demkow, U.; Szmigielska, M.A.; Tutka, A. Diagnostic Value of Serum Periostin for Cyst Involution in Children with Multicystic Dysplastic Kidney. J. Clin. Med. 2025, 14, 7264. https://doi.org/10.3390/jcm14207264
Szmigielska A, Kotuła I, Demkow U, Szmigielska MA, Tutka A. Diagnostic Value of Serum Periostin for Cyst Involution in Children with Multicystic Dysplastic Kidney. Journal of Clinical Medicine. 2025; 14(20):7264. https://doi.org/10.3390/jcm14207264
Chicago/Turabian StyleSzmigielska, Agnieszka, Iwona Kotuła, Urszula Demkow, Maria A. Szmigielska, and Agnieszka Tutka. 2025. "Diagnostic Value of Serum Periostin for Cyst Involution in Children with Multicystic Dysplastic Kidney" Journal of Clinical Medicine 14, no. 20: 7264. https://doi.org/10.3390/jcm14207264
APA StyleSzmigielska, A., Kotuła, I., Demkow, U., Szmigielska, M. A., & Tutka, A. (2025). Diagnostic Value of Serum Periostin for Cyst Involution in Children with Multicystic Dysplastic Kidney. Journal of Clinical Medicine, 14(20), 7264. https://doi.org/10.3390/jcm14207264






