Advancing Heart Failure Care: Breakthroughs and Emerging Strategies
Abstract
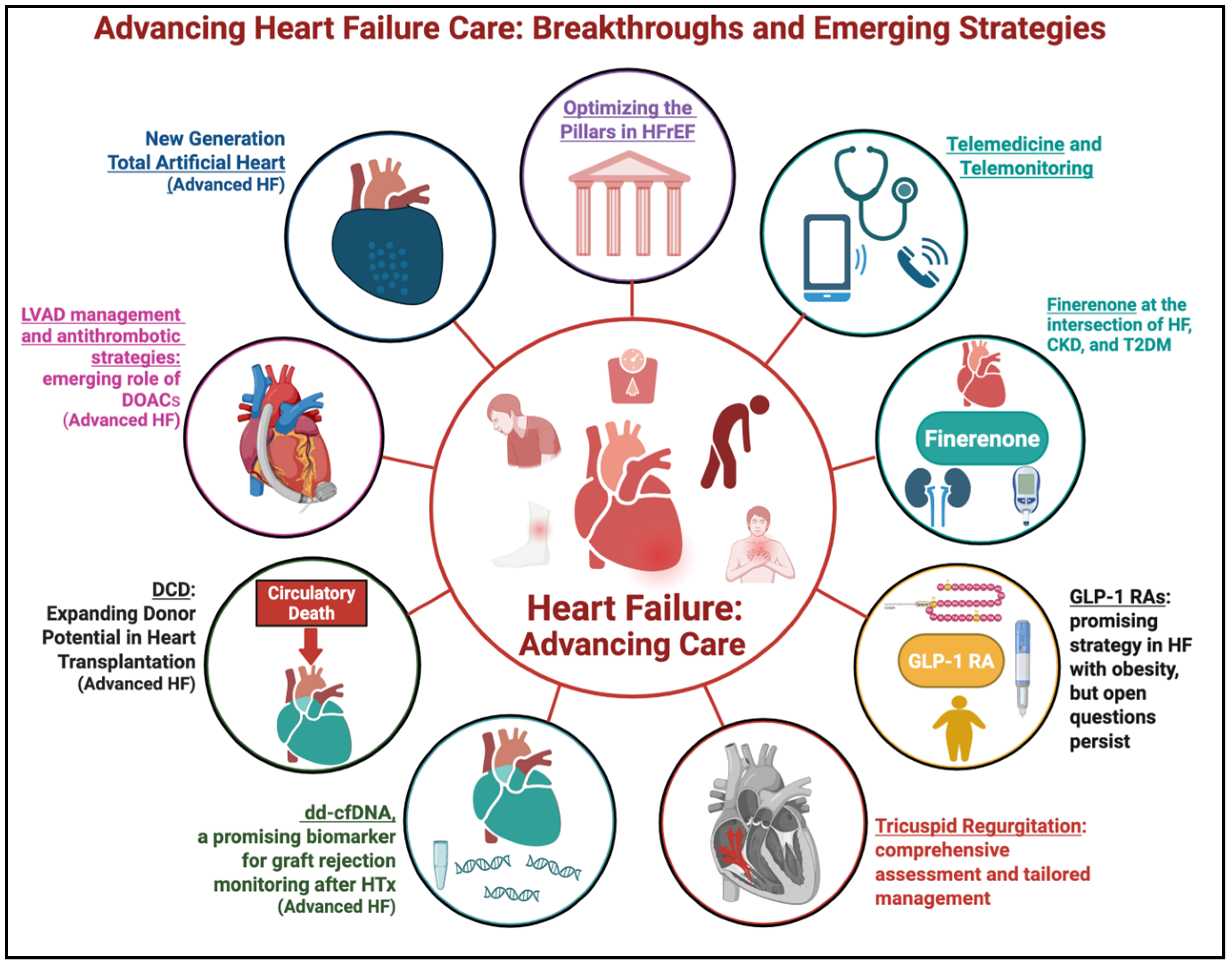
1. Introduction
2. Optimizing the Four Pillars in Heart Failure with Reduced Ejection Fraction: Uptitration, Gaps, and System-Level Strategies
3. Finerenone at the Intersection of Heart Failure, Chronic Kidney Disease, and Type 2 Diabetes Mellitus
4. Exploring the Potential of GLP-1 Receptor Agonists in Heart Failure: Promising Results and Unanswered Questions
5. Tricuspid Regurgitation: From Neglected Lesion to Therapeutic Priority in HF
6. Pushing the Limits: New Frontiers in Advanced Heart Failure
6.1. Donor Derived Cell-Free DNA: The New Gold Standard in Diagnosing Rejection?
6.2. Donation After Circulatory Death in Heart Transplantation: Unlocking New Donor Potential
6.3. Update in Management of LVAD Patients
6.4. New Generation Total Artificial Heart: From Vision to Viable Lifeline
7. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- D’Amario, D.; Rodolico, D.; Delvinioti, A.; Laborante, R.; Iacomini, C.; Masciocchi, C.; Restivo, A.; Ciliberti, G.; Galli, M.; Paglianiti, A.D.; et al. Eligibility for the 4 Pharmacological Pillars in Heart Failure with Reduced Ejection Fraction at Discharge. J. Am. Heart Assoc. 2023, 12, e029071. [Google Scholar] [CrossRef] [PubMed]
- Bauersachs, J. Heart failure drug treatment: The fantastic four. Eur. Heart J. 2021, 42, 681–683. [Google Scholar] [CrossRef]
- Kocabaş, U.; Ergin, I.; Kıvrak, T.; Yılmaz Öztekin, G.M.; Tanık, V.O.; Özdemir, İ.; Avcı Demir, F.; Doğduş, M.; Şen, T.; Altınsoy, M.; et al. Suboptimal guideline-directed medical therapy and prognosis in patients with heart failure and reduced ejection fraction: The SMYRNA Study. Eur. Heart J. 2023, 44 (Suppl. S2), ehad655.863. [Google Scholar] [CrossRef]
- Tedeschi, A.; Palazzini, M.; Trimarchi, G.; Conti, N.; Di Spigno, F.; Gentile, P.; D’Angelo, L.; Garascia, A.; Ammirati, E.; Morici, N.; et al. Heart Failure Management through Telehealth: Expanding Care and Connecting Hearts. J. Clin. Med. 2024, 13, 2592. [Google Scholar] [CrossRef] [PubMed]
- Holst-Hansen, A.; Grimm, D.; Wehland, M. Finerenone in Heart Failure-A Novel Therapeutic Approach. Int. J. Mol. Sci. 2024, 25, 13711. [Google Scholar] [CrossRef]
- Waqas, S.A.; Sohail, M.U.; Saad, M.; Minhas, A.M.K.; Greene, S.J.; Fudim, M.; Fonarow, G.C.; Abramov, D.; Khan, M.S.; Ahmed, R. Efficacy of GLP-1 Receptor Agonists in Patients with Heart Failure and Mildly Reduced or Preserved Ejection Fraction: A Systematic Review and Meta-Analysis. J. Card. Fail. 2025, 31, 1076–1080. [Google Scholar] [CrossRef]
- Sugiura, A.; Tanaka, T.; Kavsur, R.; Öztürk, C.; Vogelhuber, J.; Silaschi, M.; Weber, M.; Zimmer, S.; Nickenig, G. Tricuspid regurgitation: Innovation, current landscape, and future perspective of transcatheter tricuspid valve interventions. J. Cardiol. 2025, 86, 119–126. [Google Scholar] [CrossRef]
- Richmond, M.E.; Zangwill, S.D.; Kindel, S.J.; Deshpande, S.R.; Schroder, J.N.; Bichell, D.P.; Knecht, K.R.; Mahle, W.T.; Wigger, M.A.; Gaglianello, N.A.; et al. Donor fraction cell-free DNA and rejection in adult and pediatric heart transplantation. J. Heart Lung Transplant. 2020, 39, 454–463. [Google Scholar] [CrossRef]
- Kharawala, A.; Nagraj, S.; Seo, J.; Pargaonkar, S.; Uehara, M.; Goldstein, D.J.; Patel, S.R.; Sims, D.B.; Jorde, U.P. Donation After Circulatory Death Heart Transplant: Current State and Future Directions. Circ. Heart Fail. 2024, 17, e011678. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Looby, M.; Dimond, M.; Bagchi, P.; Shah, B.; Isseh, I.; Rollins, A.T.; Abdul-Aziz, A.A.; Kennedy, J.; Tang, D.G.; et al. Evaluation of the Hemocompatibility of the Direct Oral Anticoagulant Apixaban in Left Ventricular Assist Devices: The DOAC LVAD Study. JACC Heart Fail. 2024, 12, 1540–1549. [Google Scholar] [CrossRef]
- Schroder, J.N.; McCartney, S.L.; Jansen, P.; Plichta, R.; Katz, J.N.; Smadja, D.M.; Dewan, K.C.; Milano, C.A. The First Autoregulated Total Artificial Heart Implant in the United States. Ann. Thorac. Surg. Short Rep. 2022, 1, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Mebazaa, A.; Davison, B.; Chioncel, O.; Cohen-Solal, A.; Diaz, R.; Filippatos, G.; Metra, M.; Ponikowski, P.; Sliwa, K.; Voors, A.A.; et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): A multinational, open-label, randomised, trial. Lancet 2022, 400, 1938–1952. [Google Scholar] [CrossRef]
- Savarese, G.; Kishi, T.; Vardeny, O.; Adamsson Eryd, S.; Bodegård, J.; Lund, L.H.; Thuresson, M.; Bozkurt, B. Heart Failure Drug Treatment-Inertia, Titration, and Discontinuation: A Multinational Observational Study (EVOLUTION HF). JACC Heart Fail. 2023, 11, 1–14, Erratum in JACC Heart Fail. 2023, 11, 1773. https://doi.org/10.1016/j.jchf.2023.11.001. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.B.; Vaduganathan, M.; Fonarow, G.C.; Ikeaba, U.; Chiswell, K.; Butler, J.; DeVore, A.D.; Heidenreich, P.A.; Huang, J.C.; Kittleson, M.M.; et al. Contemporary Use of Sodium-Glucose Cotransporter-2 Inhibitor Therapy Among Patients Hospitalized for Heart Failure with Reduced Ejection Fraction in the US: The Get with The Guidelines-Heart Failure Registry. JAMA Cardiol. 2023, 8, 652–661. [Google Scholar] [CrossRef]
- Malgie, J.; Wilde, M.I.; Clephas, P.R.D.; Emans, M.E.; Koudstaal, S.; Schaap, J.; Mosterd, A.; van Ramshorst, J.; Wardeh, A.J.; van Wijk, S.; et al. Contemporary guideline-directed medical therapy in de novo, chronic, and worsening heart failure patients: First data from the TITRATE-HF study. Eur. J. Heart Fail. 2024, 26, 1549–1560. [Google Scholar] [CrossRef]
- Oliva, F.; Orso, F.; Colivicchi, F.; Cipriani, M.G.; Dilenarda, A.; Gabrielli, D.; Gori, M.; Gorini, M.; Iacoviello, M.; Lucci, D.; et al. Medical Treatments in Patients with Ambulatory Heart Failure: First Data From the BRING-UP-3 Heart Failure Study. J. Card. Fail. 2025. ahead of print. [Google Scholar] [CrossRef]
- Artanian, V.; Ross, H.J.; Rac, V.E.; O’Sullivan, M.; Brahmbhatt, D.H.; Seto, E. Impact of Remote Titration Combined with Telemonitoring on the Optimization of Guideline-Directed Medical Therapy for Patients with Heart Failure: Internal Pilot of a Randomized Controlled Trial. JMIR Cardio 2020, 4, e21962. [Google Scholar] [CrossRef]
- Man, J.P.; Koole, M.A.C.; Meregalli, P.G.; Handoko, M.L.; Stienen, S.; de Lange, F.J.; Winter, M.M.; Schijven, M.P.; Kok, W.E.M.; Kuipers, D.I.; et al. Digital consults in heart failure care: A randomized controlled trial. Nat. Med. 2024, 30, 2907–2913. [Google Scholar] [CrossRef]
- Tedeschi, A.; Novara, P.; Matrone, B.A.M.; Cattadori, E.; Lisè, G.; Di Spigno, F.; Breviario, F.; Gerra, L.; Sticozzi, C.; Pisati, M.S.; et al. I primi dati del progetto TeleCuore [The first data of the TeleCuore project]. G. Ital. Cardiol. 2025, 26, 510–518. [Google Scholar] [CrossRef]
- Navar, L.G. Physiology: Hemodynamics, endothelial function, renin-angiotensin-aldosterone system, sympathetic nervous system. J. Am. Soc. Hypertens. 2014, 8, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Rauf, A.; Khan, H.; Abu-Izneid, T. Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed. Pharmacother. 2017, 94, 317–325. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Pitt, B.; Zannad, F. Mineralocorticoid Receptor Antagonists in Heart Failure: An Update. Circ. Heart Fail. 2024, 17, e011629. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Kolkhof, P.; Bakris, G.; Bauersachs, J.; Haller, H.; Wada, T.; Zannad, F. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur. Heart J. 2021, 42, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, S.; Funes Hernandez, M.; Chang, T.I.; Mahaffey, K.W. Cardiovascular and Renal Outcomes with Finerenone, a Selective Mineralocorticoid Receptor Antagonist. Cardiol. Ther. 2022, 11, 337–354. [Google Scholar] [CrossRef]
- Amazit, L.; Le Billan, F.; Kolkhof, P.; Lamribet, K.; Viengchareun, S.; Fay, M.R.; Khan, J.A.; Hillisch, A.; Lombès, M.; Rafestin-Oblin, M.-E.; et al. Finerenone Impedes Aldosterone-dependent Nuclear Import of the Mineralocorticoid Receptor and Prevents Genomic Recruitment of Steroid Receptor Coactivator-1. J. Biol. Chem. 2015, 290, 21876–21889. [Google Scholar] [CrossRef]
- González-Blázquez, R.; Somoza, B.; Gil-Ortega, M.; Martín Ramos, M.; Ramiro-Cortijo, D.; Vega-Martín, E.; Schulz, A.; Ruilope, L.M.; Kolkhof, P.; Kreutz, R.; et al. Finerenone Attenuates Endothelial Dysfunction and Albuminuria in a Chronic Kidney Disease Model by a Reduction in Oxidative Stress. Front. Pharmacol. 2018, 9, 1131. [Google Scholar] [CrossRef]
- Heinig, R.; Lambelet, M.; Nagelschmitz, J.; Alatrach, A.; Halabi, A. Pharmacokinetics of the novel nonsteroidal mineralocorticoid receptor antagonist finerenone (BAY 94-8862) in individuals with mild or moderate hepatic impairment. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Heinig, R.; Gerisch, M.; Engelen, A.; Nagelschmitz, J.; Loewen, S. Pharmacokinetics of the Novel, Selective, Non-steroidal Mineralocorticoid Receptor Antagonist Finerenone in Healthy Volunteers: Results from an Absolute Bioavailability Study and Drug-Drug Interaction Studies In Vitro and In Vivo. Eur. J. Drug Metab. Pharmacokinet. 2018, 43, 715–727. [Google Scholar] [CrossRef]
- Trinchieri, A.; Perletti, G.; Magri, V.; Stamatiou, K.; Trinchieri, M.; Montanari, E. Drug-induced gynecomastia: A systematic review and meta-analysis of randomized clinical trials. Arch. Ital. Urol. Androl. 2021, 93, 489–496. [Google Scholar] [CrossRef]
- Lainscak, M.; Pelliccia, F.; Rosano, G.; Vitale, C.; Schiariti, M.; Greco, C.; Speziale, G.; Gaudio, C. Safety profile of mineralocorticoid receptor antagonists: Spironolactone and eplerenone. Int. J. Cardiol. 2015, 200, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, G.; Anker, S.D.; Böhm, M.; Gheorghiade, M.; Køber, L.; Krum, H.; Maggioni, A.P.; Ponikowski, P.; Voors, A.A.; Zannad, F.; et al. A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur. Heart J. 2016, 37, 2105–2114. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Anker, S.D.; Böhm, M.; Gheorghiade, M.; Køber, L.; Krum, H.; Maggioni, A.P.; Ponikowski, P.; Voors, A.A.; Zannad, F.; et al. Rationale and design of MinerAlocorticoid Receptor antagonist Tolerability Study-Heart Failure (ARTS-HF): A randomized study of finerenone vs. eplerenone in patients who have worsening chronic heart failure with diabetes and/or chronic kidney disease. Eur. J. Heart Fail. 2015, 17, 224–232. [Google Scholar] [CrossRef]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef]
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 2252–2263. [Google Scholar] [CrossRef]
- Filippatos, G.; Anker, S.D.; Agarwal, R.; Ruilope, L.M.; Rossing, P.; Bakris, G.L.; Tasto, C.; Joseph, A.; Kolkhof, P.; Lage, A.; et al. Finerenone Reduces Risk of Incident Heart Failure in Patients with Chronic Kidney Disease and Type 2 Diabetes: Analyses From the FIGARO-DKD Trial. Circulation 2022, 145, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; McMurray, J.J.V.; Vaduganathan, M.; Claggett, B.; Jhund, P.S.; Desai, A.S.; Henderson, A.D.; Lam, C.S.P.; Pitt, B.; Senni, M.; et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: The FIDELITY pooled analysis. Eur. Heart J. 2022, 43, 474–484. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Vaduganathan, M.; Claggett, B.; Jhund, P.S.; Desai, A.S.; Viswanathan, P. Finerenone in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2024, 391, 1475–1485. [Google Scholar] [CrossRef]
- Green, J.B.; Mottl, A.K.; Bakris, G.; Heerspink, H.J.L.; Mann, J.F.E.; McGill, J.B.; Nangaku, M.; Rossing, P.; Scott, C.; Gay, A.; et al. Design of the COmbinatioN effect of FInerenone anD EmpaglifloziN in participants with chronic kidney disease and type 2 diabetes using a UACR Endpoint study (CONFIDENCE). Nephrol. Dial. Transplant. 2023, 38, 894–903. [Google Scholar] [CrossRef]
- Harrington, J.; Felker, G.M.; Januzzi, J.L.; Lam, C.S.P.; Lingvay, I.; Pagidipati, N.J.; Sattar, N.; Van Spall, H.G.C.; Verma, S.; McGuire, D.K. Worth Their Weight? An Update on New and Emerging Pharmacologic Agents for Obesity and Their Potential Role for Persons with Cardiac Conditions. Curr. Cardiol. Rep. 2024, 26, 61–71. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Saraiva, F.; Sharma, A.; Vasques-Nóvoa, F.; Angélico-Gonçalves, A.; Leite, A.R.; Borges-Canha, M.; Carvalho, D.; Packer, M.; Zannad; et al. Glucagon-like peptide 1 receptor agonists in patients with type 2 diabetes with and without chronic heart failure: A meta-analysis of randomized placebo-controlled outcome trials. Diabetes Obes. Metab. 2023, 25, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- D’Alessio, D. Is GLP-1 a hormone: Whether and When? J. Diabetes Investig. 2016, 7 (Suppl. S1), 50–55. [Google Scholar] [CrossRef]
- Pyke, C.; Heller, R.S.; Kirk, R.K.; Ørskov, C.; Reedtz-Runge, S.; Kaastrup, P.; Hvelplund, A.; Bardram, L.; Calatayud, D.; Knudsen, L.B. GLP-1 receptor localization in monkey and human tissue: Novel distribution revealed with extensively validated monoclonal antibody. Endocrinology 2014, 155, 1280–1290. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20 (Suppl. S1), 5–21. [Google Scholar] [CrossRef]
- Ban, K.; Noyan-Ashraf, M.H.; Hoefer, J.; Bolz, S.S.; Drucker, D.J.; Husain, M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 2008, 117, 2340–2350. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.P.; Pratley, R.E. GLP-1 Analogs and DPP-4 Inhibitors in Type 2 Diabetes Therapy: Review of Head-to-Head Clinical Trials. Front. Endocrinol. 2020, 11, 178. [Google Scholar] [CrossRef]
- Sun, L.; Zheng, Z.M.; Shao, C.S.; Zhang, Z.Y.; Li, M.W.; Wang, L.; Wang, H.; Zhao, G.H.; Wang, P. Rational Design by Structural Biology of Industrializable, Long-Acting Antihyperglycemic GLP-1 Receptor Agonists. Pharmaceuticals 2022, 15, 740. [Google Scholar] [CrossRef]
- Noyan-Ashraf, M.H.; Shikatani, E.A.; Schuiki, I.; Mukovozov, I.; Wu, J.; Li, R.K.; Volchuk, A.; Robinson, L.A.; Billia, F.; Drucker, D.J.; et al. A glucagon-like peptide-1 analog reverses the molecular pathology and cardiac dysfunction of a mouse model of obesity. Circulation 2013, 127, 74–85. [Google Scholar] [CrossRef]
- Bhashyam, S.; Fields, A.V.; Patterson, B.; Testani, J.M.; Chen, L.; Shen, Y.T.; Shannon, R.P. Glucagon-like peptide-1 increases myocardial glucose uptake via p38alpha MAP kinase-mediated, nitric oxide-dependent mechanisms in conscious dogs with dilated cardiomyopathy. Circ. Heart Fail. 2010, 3, 512–521. [Google Scholar] [CrossRef]
- Nikolaidis, L.A.; Elahi, D.; Hentosz, T.; Doverspike, A.; Huerbin, R.; Zourelias, L.; Stolarski, C.; Shen, Y.T.; Shannon, R.P. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation 2004, 110, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Poornima, I.; Brown, S.B.; Bhashyam, S.; Parikh, P.; Bolukoglu, H.; Shannon, R.P. Chronic glucagon-like peptide-1 infusion sustains left ventricular systolic function and prolongs survival in the spontaneously hypertensive, heart failure-prone rat. Circ. Heart Fail. 2008, 1, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Ingelsson, E.; Sundström, J.; Arnlöv, J.; Zethelius, B.; Lind, L. Insulin resistance and risk of congestive heart failure. JAMA 2005, 294, 334–341. [Google Scholar] [CrossRef]
- Oeseburg, H.; de Boer, R.A.; Buikema, H.; van der Harst, P.; van Gilst, W.H.; Silljé, H.H. Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase A. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1407–1414. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Sattar, N.; Rosenstock, J.; Ramasundarahettige, C.; Pratley, R.; Lopes, R.D.; Lam, C.S.P.; Khurmi, N.S.; Heenan, L.; Del Prato, S.; et al. Cardiovascular and Renal Outcomes with Efpeglenatide in Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 896–907. [Google Scholar] [CrossRef]
- Hernandez, A.F.; Green, J.B.; Janmohamed, S.; D’Agostino, R.B.; Sr Granger, C.B.; Jones, N.P.; Leiter, L.A.; Rosenberg, A.E.; Sigmon, K.N.; Somerville, M.C.; et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): A double-blind, randomised placebo-controlled trial. Lancet 2018, 392, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Lee, M.M.Y.; Kristensen, S.L.; Branch, K.R.H.; Del Prato, S.; Khurmi, N.S.; Lam, C.S.P.; Lopes, R.D.; McMurray, J.J.V.; Pratley, R.E.; et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021, 9, 653–662. [Google Scholar] [CrossRef]
- Duhan, S.; Kurpad, K.P.; Keisham, B.; Shtembari, J.; Brener, A.; Kanakadandi, U.B.; Garg, A.; Adoni, N.A.; Mehta, S.S. GLP-1RA versus placebo in patients with heart failure and mildly reduced or preserved ejection fraction: An updated meta-analysis of randomized controlled trials. Int. J. Cardiol. 2025, 438, 133604. [Google Scholar] [CrossRef]
- Holman, R.R.; Bethel, M.A.; Mentz, R.J.; Thompson, V.P.; Lokhnygina, Y.; Buse, J.B.; Chan, J.C.; Choi, J.; Gustavson, S.M.; Iqbal, N.; et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 1228–1239. [Google Scholar] [CrossRef]
- Jorsal, A.; Kistorp, C.; Holmager, P.; Tougaard, R.S.; Nielsen, R.; Hänselmann, A.; Nilsson, B.; Møller, J.E.; Hjort, J.; Rasmussen, J.; et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)-a multicentre, double-blind, randomised, placebo-controlled trial. Eur. J. Heart Fail. 2017, 19, 69–77. [Google Scholar] [CrossRef]
- Quagliariello, V.; Canale, M.L.; Bisceglia, I.; Iovine, M.; Giordano, V.; Giacobbe, I.; Scherillo, M.; Gabrielli, D.; Maurea, C.; Barbato, M.; et al. Glucagon-like Peptide 1 Receptor Agonists in Cardio-Oncology: Pathophysiology of Cardiometabolic Outcomes in Cancer Patients. Int. J. Mol. Sci. 2024, 25, 11299. [Google Scholar] [CrossRef]
- Greco, A.; Canale, M.L.; Quagliariello, V.; Oliva, S.; Tedeschi, A.; Inno, A.; De Biasio, M.; Bisceglia, I.; Tarantini, L.; Maurea, N.; et al. SGLT2 Inhibitors in Cancer Patients: A Comprehensive Review of Clinical, Biochemical, and Therapeutic Implications in Cardio-Oncology. Int. J. Mol. Sci. 2025, 26, 4780. [Google Scholar] [CrossRef] [PubMed]
- Greco, A.; Quagliariello, V.; Rizzo, G.; Tedeschi, A.; Schirinzi, S.; Turco, A.; Galiazzo, M.; De Amicis, M.; Klersy, C.; Ghio, S.; et al. SGLT2i Dapagliflozin in primary prevention of chemotherapy induced cardiotoxicity in breast cancer patients treated with neo-adjuvant anthracycline-based chemotherapy +/− trastuzumab: Rationale and design of the multicenter PROTECT trial. Cardiooncology 2025, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Heitzinger, G.; Pavo, N.; Koschatko, S.; Jantsch, C.; Winter, M.P.; Spinka, G.; Dannenberg, V.; Kastl, S.; Prausmüller, S.; Arfsten, H.; et al. Contemporary insights into the epidemiology, impact and treatment of secondary tricuspid regurgitation across the heart failure spectrum. Eur. J. Heart Fail. 2023, 25, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Benfari, G.; Antoine, C.; Miller, W.L.; Thapa, P.; Topilsky, Y.; Rossi, A.; Michelena, H.I.; Pislaru, S.; Enriquez-Sarano, M. Excess Mortality Associated with Functional Tricuspid Regurgitation Complicating Heart Failure with Reduced Ejection Fraction. Circulation 2019, 140, 196–206. [Google Scholar] [CrossRef]
- Topilsky, Y.; Khanna, A.; Le Tourneau, T.; Park, S.; Michelena, H.; Suri, R.; Mahoney, D.W.; Enriquez-Sarano, M. Clinical context and mechanism of functional tricuspid regurgitation in patients with and without pulmonary hypertension. Circ. Cardiovasc. Imaging 2012, 5, 314–323. [Google Scholar] [CrossRef]
- Lancellotti, P.; Pibarot, P.; Chambers, J.; La Canna, G.; Pepi, M.; Dulgheru, R.; Dweck, M.; Delgado, V.; Garbi, M.; Vannan, M.A.; et al. Multi-modality imaging assessment of native valvular regurgitation: An EACVI and ESC council of valvular heart disease position paper. Eur. Heart J.-Cardiovasc. Imaging 2022, 23, e171–e232. [Google Scholar] [CrossRef]
- Hahn, R.T.; Pinney, S.; Lindenfeld, J. Tricuspid regurgitation management for heart failure. JACC Heart Fail. 2023, 11, 1–12. [Google Scholar] [CrossRef]
- Dreyfus, J.; Ghalem, N.; Garbarz, E.; Cimadevilla, C.; Nataf, P.; Vahanian, A.; Caranhac, G.; Messika-Zeitoun, D. Timing of Referral of Patients with Severe Isolated Tricuspid Valve Regurgitation to Surgeons (from a French Nationwide Database). Am. J. Cardiol. 2018, 122, 323–326. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F., 3rd; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar] [CrossRef]
- Praz, F.; Muraru, D.; Kreidel, F.; Lurz, P.; Hahn, R.T.; Delgado, V.; Senni, M.; von Bardeleben, R.S.; Nickenig, G.; Hausleiter, J.; et al. Transcatheter treatment for tricuspid valve disease. EuroIntervention 2021, 17, 791–808. [Google Scholar] [CrossRef]
- Sorajja, P.; Whisenant, B.; Hamid, N.; Naik, H.; Makkar, R.; Tadros, P.; Price, M.J.; Singh, G.; Fam, N.; Kar, S.; et al. Transcatheter Repair for Patients with Tricuspid Regurgitation. N. Engl. J. Med. 2023, 388, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Makkar, R.R.; Whisenant, B.K.; Hamid, N.; Naik, H.; Tadros, P.; Price, M.J.; Singh, G.; Schwartz, J.G.; Kapadia, S., for the TRILUMINATE Pivotal Investigators. Two-year outcomes of transcatheter edge-to-edge repair for severe tricuspid regurgitation: The TRILUMINATE pivotal randomized controlled trial. Circulation 2025, 151, 1036–1048. [Google Scholar] [CrossRef] [PubMed]
- Xi, R.; Mumtaz, M.A.; Xu, D.; Zeng, Q. Tricuspid Regurgitation Complicating Heart Failure: A Novel Clinical Entity. Rev. Cardiovasc. Med. 2024, 25, 330. [Google Scholar] [CrossRef] [PubMed]
- Estévez-Loureiro, R.; Sánchez-Recalde, A.; Amat-Santos, I.J.; Cruz-González, I.; Baz, J.A.; Pascual, I.; Mascherbauer, J.; Abdul-Jawad Altisent, O.; Nombela-Franco, L.; Pan, M.; et al. 6-Month Outcomes of the TricValve System in Patients with Tricuspid Regurgitation: The TRICUS EURO Study. JACC Cardiovasc. Interv. 2022, 15, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Heitzinger, G.; Dreyfus, J.; Dannenberg, V.; Topilsky, Y.; Benfari, G.; Marsan, N.A.; Taramasso, M.; Russo, G.; Bohbot, Y.; Iliadis, C.; et al. Left ventricular ejection fraction and benefit of tricuspid valve interventions—Insights from the international TRIGISTRY. Eur. J. Heart Fail. 2025. ahead of print. [Google Scholar] [CrossRef]
- Garascia, A.; Palazzini, M.; Tedeschi, A.; Sacco, A.; Oliva, F.; Gentile, P. Advanced heart failure: From definitions to therapeutic options. Eur. Heart J. Suppl. 2023, 25 (Suppl. C), C283–C291. [Google Scholar] [CrossRef]
- Gentile, P.; Masciocco, G.; Palazzini, M.; Tedeschi, A.; Ruzzenenti, G.; Conti, N.; D’Angelo, L.; Foti, G.; Perna, E.; Verde, A.; et al. Intravenous continuous home inotropic therapy in advanced heart failure: Insights from an observational retrospective study. Eur. J. Intern. Med. 2023, 116, 65–71. [Google Scholar] [CrossRef]
- Velleca, A.; Shullo, M.A.; Dhital, K.; Azeka, E.; Colvin, M.; DePasquale, E.; Farrero, M.; García-Guereta, L.; Jamero, G.; Khush, K.; et al. The International Society for Heart and Lung Transplantation (ISHLT) guidelines for the care of heart transplant recipients. J. Heart Lung Transplant. 2023, 42, e1–e141. [Google Scholar] [CrossRef]
- Agbor-Enoh, S.; Shah, P.; Tunc, I.; Hsu, S.; Russell, S.; Feller, E.; Shah, K.; Rodrigo, M.E.; Najjar, S.S.; Kong, H.; et al. Cell-Free DNA to Detect Heart Allograft Acute Rejection. Circulation 2021, 143, 1184–1197. [Google Scholar] [CrossRef]
- Kittleson, M.M.; Garg, S. Solid Gold, or Liquid Gold?: Towards a New Diagnostic Standard for Heart Transplant Rejection. Circulation 2021, 143, 1198–1201, Erratum in Circulation 2021, 143, e1029. https://doi.org/10.1161/CIR.0000000000000989. [Google Scholar] [CrossRef]
- Dharmavaram, N.; Hess, T.; Jaeger, H.; Smith, J.; Hermsen, J.; Murray, D.; Dhingra, R. National Trends in Heart Donor Usage Rates: Are We Efficiently Transplanting More Hearts? J. Am. Heart Assoc. 2021, 10, e019655. [Google Scholar] [CrossRef] [PubMed]
- Schroder, J.N.; Patel, C.B.; DeVore, A.D.; Bryner, B.S.; Casalinova, S.; Shah, A.; Smith, J.W.; Fiedler, A.G.; Daneshmand, M.; Silvestry, S.; et al. Transplantation Outcomes with Donor Hearts after Circulatory Death. N. Engl. J. Med. 2023, 388, 2121–2131. [Google Scholar] [CrossRef]
- Mehra, M.R.; Goldstein, D.J.; Cleveland, J.C.; Cowger, J.A.; Hall, S.; Salerno, C.T.; Naka, Y.; Horstmanshof, D.; Chuang, J.; Wang, A.; et al. Five-Year Outcomes in Patients with Fully Magnetically Levitated vs Axial-Flow Left Ventricular Assist Devices in the MOMENTUM 3 Randomized Trial. JAMA 2022, 328, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Dual, S.A.; Cowger, J.; Roche, E.; Nayak, A. The Future of Durable Mechanical Circulatory Support: Emerging Technological Innovations and Considerations to Enable Evolution of the Field. J. Card. Fail. 2024, 30, 596–609. [Google Scholar] [CrossRef] [PubMed]
- Hollis, I.B.; Jennings, D.L.; Krim, S.; Ton, V.K.; Ducharme, A.; Cowger, J.; Looby, M.; Eulert-Green, J.J.; Bansal, N.; Horn, E.; et al. An ISHLT consensus statement on strategies to prevent and manage hemocompatibility related adverse events in patients with a durable, continuous-flow ventricular assist device. J. Heart Lung Transplant. 2024, 43, 1199–1234. [Google Scholar] [CrossRef]
- Mehra, M.R.; Netuka, I.; Uriel, N.; Katz, J.N.; Pagani, F.D.; Jorde, U.P.; Gustafsson, F.; Connors, J.M.; Ivak, P.; Cowger, J.; et al. Aspirin and Hemocompatibility Events with a Left Ventricular Assist Device in Advanced Heart Failure: The ARIES-HM3 Randomized Clinical Trial. JAMA 2023, 330, 2171–2181. [Google Scholar] [CrossRef]
- Gustafsson, F.; Uriel, N.; Netuka, I.; Katz, J.N.; Pagani, F.D.; Connors, J.M.; Jorde, U.P.; Zimpfer, D.; Pya, Y. Aspirin and Hemocompatibility After LVAD Implantation in Patients with Atherosclerotic Vascular Disease: A Secondary Analysis From the ARIES-HM3 Randomized Clinical Trial. JAMA Cardiol. 2025, 10, 235–242, Erratum in JAMA Cardiol. 2025, 10, 635. https://doi.org/10.1001/jamacardio.2025.1212. [Google Scholar] [CrossRef]
- Netuka, I.; Tucanova, Z.; Ivak, P.; Gregor, S.; Kolesar, D.M.; Marek, T.; Melenovsky, V.; Binova, J.; Dorazilova, Z.; Hegarova, M.; et al. A Prospective Randomized Trial of Direct Oral Anticoagulant Therapy with a Fully Magnetically Levitated LVAD: The DOT-HM3 Study. Circulation 2024, 150, 509–511. [Google Scholar] [CrossRef]
- Carpentier, A.; Latrémouille, C.; Cholley, B.; Smadja, D.M.; Roussel, J.C.; Boissier, E.; Trochu, J.N.; Gueffet, J.P.; Treillot, M.; Bizouarn, P.; et al. First clinical use of a bioprosthetic total artificial heart: Report of two cases. Lancet 2015, 386, 1556–1563. [Google Scholar] [CrossRef]
- Pya, Y.; Bekbossynova, M.; Medressova, A.; Latremouille, C.; Jansen, P.; Tauyekelova, A.; Kaliyev, R.; Jetybayeva, S.; Ziegler, L.A.; Rajapreyar, I.; et al. Precise monitoring of transpulmonic resistance in bridge-to-transplant patients supported by the Aeson total artificial heart. J. Heart Lung Transplant. 2025, 44, 1161–1164. [Google Scholar] [CrossRef]
- Netuka, I.; Pya, Y.; Bekbossynova, M.; Ivak, P.; Konarik, M.; Gustafsson, F.; Smadja, D.M.; Jansen, P.; Latrémouille, C. Initial bridge to transplant experience with a bioprosthetic autoregulated artificial heart. J. Heart Lung Transplant. 2020, 39, 1491–1493. [Google Scholar] [CrossRef] [PubMed]
- Bavendiek, U.; Großhennig, A.; Schwab, J.; Berliner, D.; Rieth, A.; Maier, L.S.; Gaspar, T.; Thomas, N.H.; Liu, X.; Schallhorn, S.; et al. Digitoxin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 2025, 393, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Fioretti, F.; McMullan, C.J.; Anstrom, K.J.; Barash, I.; Bonaca, M.P.; Borentain, M.; Corda, S.; Teixeira, P.P.; Ezekowitz, J.A.; et al. Vericiguat and mortality in heart failure and reduced ejection fraction: The VICTOR trial. Eur. Heart J. 2025, ehaf655. [Google Scholar] [CrossRef] [PubMed]
| Trial (Year) | Design | n/Population | Control Group/Setting | Main Findings |
|---|---|---|---|---|
| EVOLUTION-HF (2024) [15] | Retrospective analysis (real-world, multi-country) | ~600,000 HF patients across multiple countries | Observational (secondary data) | Delayed initiation of dapagliflozin and sacubitril/valsartan; frequent discontinuation of ACEis/ARB/BB/MRA; higher persistence with SGLT2is |
| GWTG-HF (USA, >100k) [16] | National registry, quality improvement program (non-randomized) | Hospitalized HF patients | Registry-based comparison | Low post-discharge initiation of SGLT2is (<20%) in eligible patients; suboptimal GDMT uptitration |
| TITRATE-HF (NL, 2023) [17] | Prospective national registry | 4288 patients with de novo, chronic, or worsening HFrEF | Routine clinical practice | Quadruple therapy in 44% of chronic/worsening HFrEF; only 1% reached target doses; specialized HF clinics improved implementation |
| BRING-UP-3 HF (Italy, 2024) [18] | National implementation science initiative | 3830 ambulatory HF patients (58.4% HFrEF) | Real-world, multicenter program | Quadruple therapy in 65% of HFrEF; >90% BBs and RASi/ARNI use; >80% SGLT2is and MRA use; SGLT2is uptake also high in HFmrEF (72%) and HFpEF (50%) |
| Trial (Year) | Design | n | Inclusion Criteria | Control Group | Primary Endpoint | Main Findings |
|---|---|---|---|---|---|---|
| ARTS (2013) [33] | Double-blinded | 457 | HFrEF (≤40%) and mild-moderate CKD | Placebo and Spironolactone | BNP, amino-terminal proBNP, albuminuria, hyperkalemia | Similar natriuretic peptide and albuminuria reduction Lower incidence of hyperkalemia (5.3% vs. 12.7%, p = 0.048) |
| ARTS-HF (2016) [34] | Double-blinded | 1066 | HFrEF and CKD and/or T2DM | Eplerenone | % of patients with a decrease of >30% in NT-proBNP | Similar NT-proBNP reduction at different Finerenone dosages |
| FIDELIO-DKD (2020) [35] | Double-blinded | 5734 | CKD and T2DM (~8% with HF) | Placebo | Composite of AKI, CKD progression and death from renal causes | Lower risk of composite outcome (HR 0.82, 95% CI 0.73–0.93) |
| FIGARO-DKD (2021) [36] | Double-blinded | 7437 | CKD and T2DM (~8% with HF) | Placebo | Composite of CV death, MI, stroke, HF hospitalization | Lower risk of composite outcome (HR 0.87, 95% CI 0.76–0.98) Benefit driven primarily by a lower incidence of hospitalization for HF (HR 0.71, 95% CI 0.56–0.90) |
| FIDELITY (2022) [37] | Pooled analysis | 13,026 (pooled) | CKD and T2DM (~8% with HF) | Placebo | Composite CV outcome and composite kidney outcome | Lower risk of composite CV outcome (HR 0.86, 95% CI 0.78–0.95) Lower risk of composite kidney outcome (HR 0.77, 95% CI 0.67–0.88) |
| FINEHEART-HF (2024) [38] | Double-blinded | 6001 | HFmrEF and HFpEF (LVEF ≥40%) | Placebo | Composite of worsening HF or CV death | Lower risk of composite outcome (HR 0.84, 95% CI 0.74–0.95) Lower risk of worsening HF (HR 0.82, 95% CI 0.71–0.94) Similar CV death (HR 0.93, 95% CI 0.78–1.1) |
| Trial (Year) | Design | n | Inclusion Criteria | Control Group | Primary Endpoint | Main Findings |
|---|---|---|---|---|---|---|
| AMPLITUDE-O (2021) [55] | Double-blinded | 4076 | T2D and either a history of CV disease or CKD (≈18% history of HF) | Placebo | First MACE (CV death, myocardial infarction, or stroke) | Risk of outcome was lower among those who received weekly subcutaneous injections of Efpeglenatide at a dose of 4 or 6 mg (HR 0.73, 95% CI 0.58–0.92) |
| Harmony Outcomes (2018) [56] | Double-blinded | 9463 | T2D and either a history of CV disease or CKD (≈20% history of HF) | Placebo | First MACE (CV death, myocardial infarction, or stroke) | Albiglutide was superior to placebo in lowering the primary outcome (HR 0.78, 95% CI 0.68–0.90) |
| EXSCEL (2017) [59] | Double-blinded | 14,752 | T2D, with or without history of CV disease (≈19% history of HF) | Placebo | First MACE (CV death, myocardial infarction, or stroke) | Subcutaneous injections of extended-release Exenatide at a dose of 2 mg once weekly not superior to placebo with respect to efficacy (HR 0.91, 95% CI 0.83–1.00) |
| LIVE (2017) [60] | Double-blinded | 241 | HF (LVEF ≤45%), with or without history T2D | Placebo | Change in LVEF | Liraglutide 1.8 mg once daily did not affect left ventricular systolic function |
| Domain | Donation After Brain Death | Donation After Circulatory Death |
|---|---|---|
| Definition | Organ procurement following confirmed irreversible cessation of all brain function | Organ procurement after cessation of circulatory and respiratory activity post withdrawal of support |
| Donor Selection Criteria | Neurologically deceased individuals meeting legal and clinical brain death criteria | Patients with irreversible brain injury but not fulfilling brain death criteria |
| Hemodynamic Stability | Maintained via mechanical ventilation and vasopressors | Rapid deterioration post support withdrawal; variable ischemic time |
| Organ Preservation | Immediate cold perfusion under controlled conditions | Ex vivo (e.g., Organ Care System) or regional in situ normothermic perfusion |
| Warm Ischemia Time | Negligible | Variable (2–30 min); critical for graft function |
| Graft Function | Low rates of primary graft dysfunction (PGD) | Slightly higher PGD; improving with technique optimization |
| Infrastructure | Standard; well-established across centers | Requires specialized infrastructure and rapid-response logistics |
| Ethical Considerations | Clear protocols and legal frameworks | Requires societal trust, transparent consent, and ethical oversight |
| Clinical Outcomes | Excellent short- and long-term outcomes | Non-inferior to DBD in selected populations; long-term data still accruing |
| Advantages | Predictable logistics; high success rate | Expands donor pool; reduces waiting list times |
| Limitations | Limited donor availability tied to brain death | Warm ischemia risk; resource-intensive implementation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tedeschi, A.; Barocelli, F.; Gerra, L.; Breviario, F.; Palazzini, M.; Conti, N.; Ferraro, S.; Bolognesi, M.G.; Di Spigno, F.; Gentile, P.; et al. Advancing Heart Failure Care: Breakthroughs and Emerging Strategies. J. Clin. Med. 2025, 14, 7253. https://doi.org/10.3390/jcm14207253
Tedeschi A, Barocelli F, Gerra L, Breviario F, Palazzini M, Conti N, Ferraro S, Bolognesi MG, Di Spigno F, Gentile P, et al. Advancing Heart Failure Care: Breakthroughs and Emerging Strategies. Journal of Clinical Medicine. 2025; 14(20):7253. https://doi.org/10.3390/jcm14207253
Chicago/Turabian StyleTedeschi, Andrea, Federico Barocelli, Luigi Gerra, Federico Breviario, Matteo Palazzini, Nicolina Conti, Stefano Ferraro, Maria Giulia Bolognesi, Francesco Di Spigno, Piero Gentile, and et al. 2025. "Advancing Heart Failure Care: Breakthroughs and Emerging Strategies" Journal of Clinical Medicine 14, no. 20: 7253. https://doi.org/10.3390/jcm14207253
APA StyleTedeschi, A., Barocelli, F., Gerra, L., Breviario, F., Palazzini, M., Conti, N., Ferraro, S., Bolognesi, M. G., Di Spigno, F., Gentile, P., Garascia, A., Ammirati, E., Magnani, G., Niccoli, G., Morici, N., & Aschieri, D. (2025). Advancing Heart Failure Care: Breakthroughs and Emerging Strategies. Journal of Clinical Medicine, 14(20), 7253. https://doi.org/10.3390/jcm14207253







