Immune Checkpoint-Induced Colitis: A Single-Center Retrospective Cohort Study
Abstract
1. Introduction
- Diagnostic value of biopsies when endoscopic findings are normal: Among patients with endoscopically normal mucosa, how often is histological inflammation present? This is an important gap in current knowledge, as normal endoscopic findings may not reliably exclude ICI-induced colitis and could potentially misguide treatment decisions.
- Impact of ICI regimen: Is combination therapy with anti-CTLA-4 and anti-PD-1/PD-L1 associated with a more steroid-refractory disease course compared with anti-PD-1/PD-L1 monotherapy? This is clinically relevant because the type of ICI regimen may predict the need for escalation to treatment with biologicals.
- Risk of relapse upon rechallenge: What proportion of patients experience recurrent colitis after ICI rechallenge? Although data are limited, this information is crucial for shared decision-making when considering retreatment with ICIs.
2. Materials and Methods
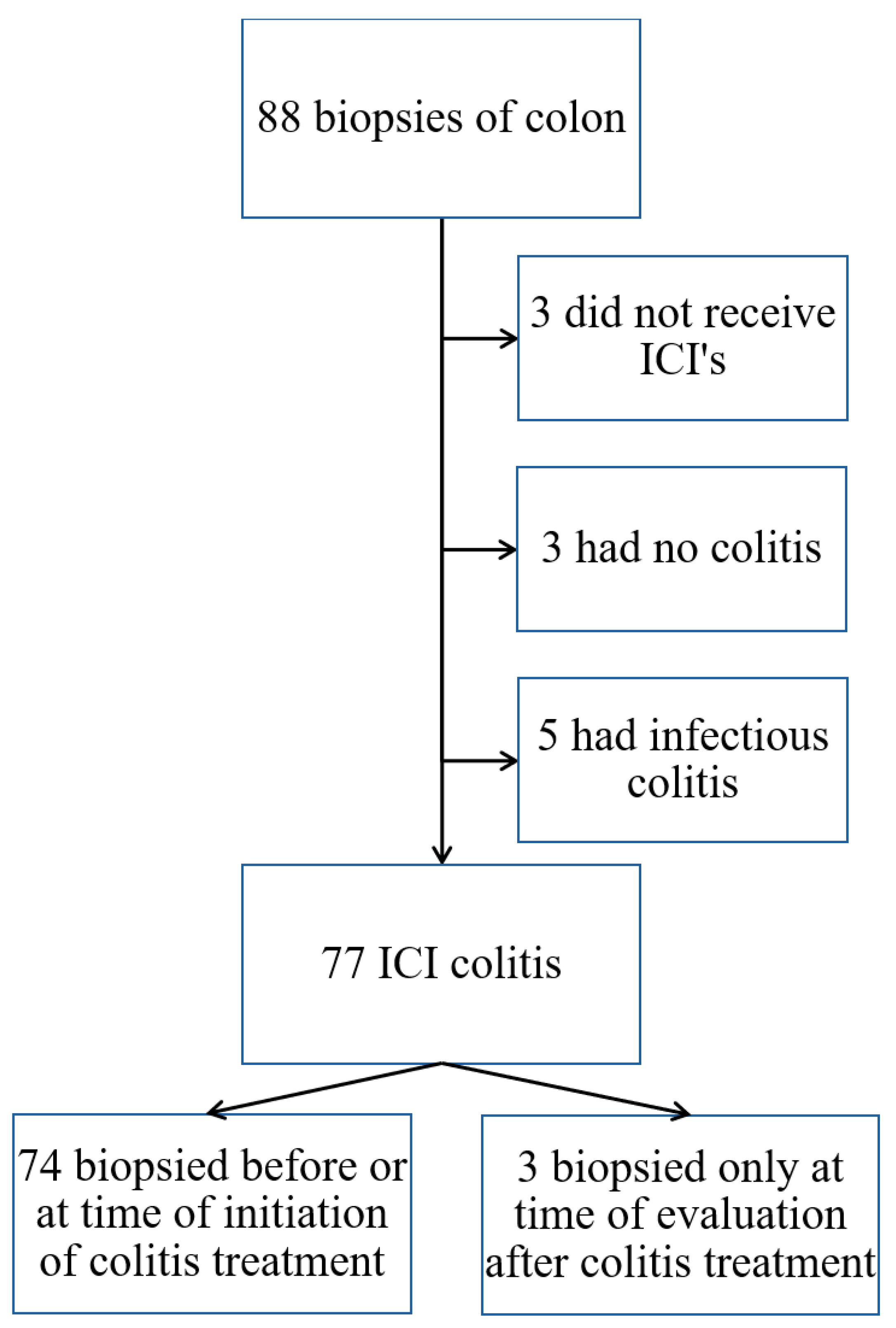
2.1. Study Design and Setting
2.2. Participants
2.3. Data Collection
2.4. Outcomes of Interest and Definitions
2.5. Data Analysis
3. Results
3.1. Patient Characteristics
3.2. Clinical Presentation and Diagnostic Findings
3.3. Treatment of ICI-Induced Colitis
3.3.1. First-Line Treatment
3.3.2. Corticosteroids
3.3.3. Second-Line Biologicals for Steroid-Refractory Disease
3.4. Rechallenge of ICIs
3.5. Complications
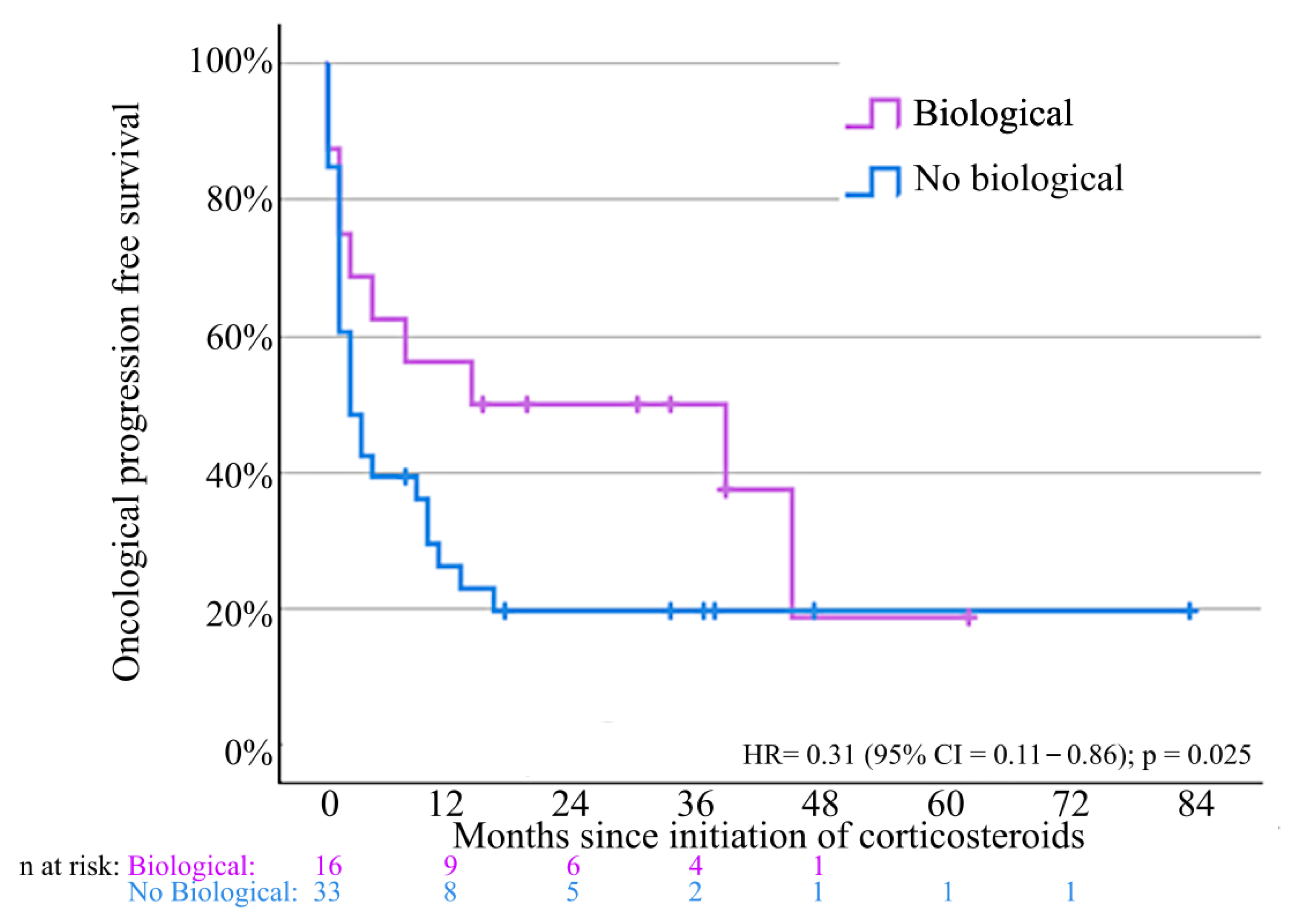
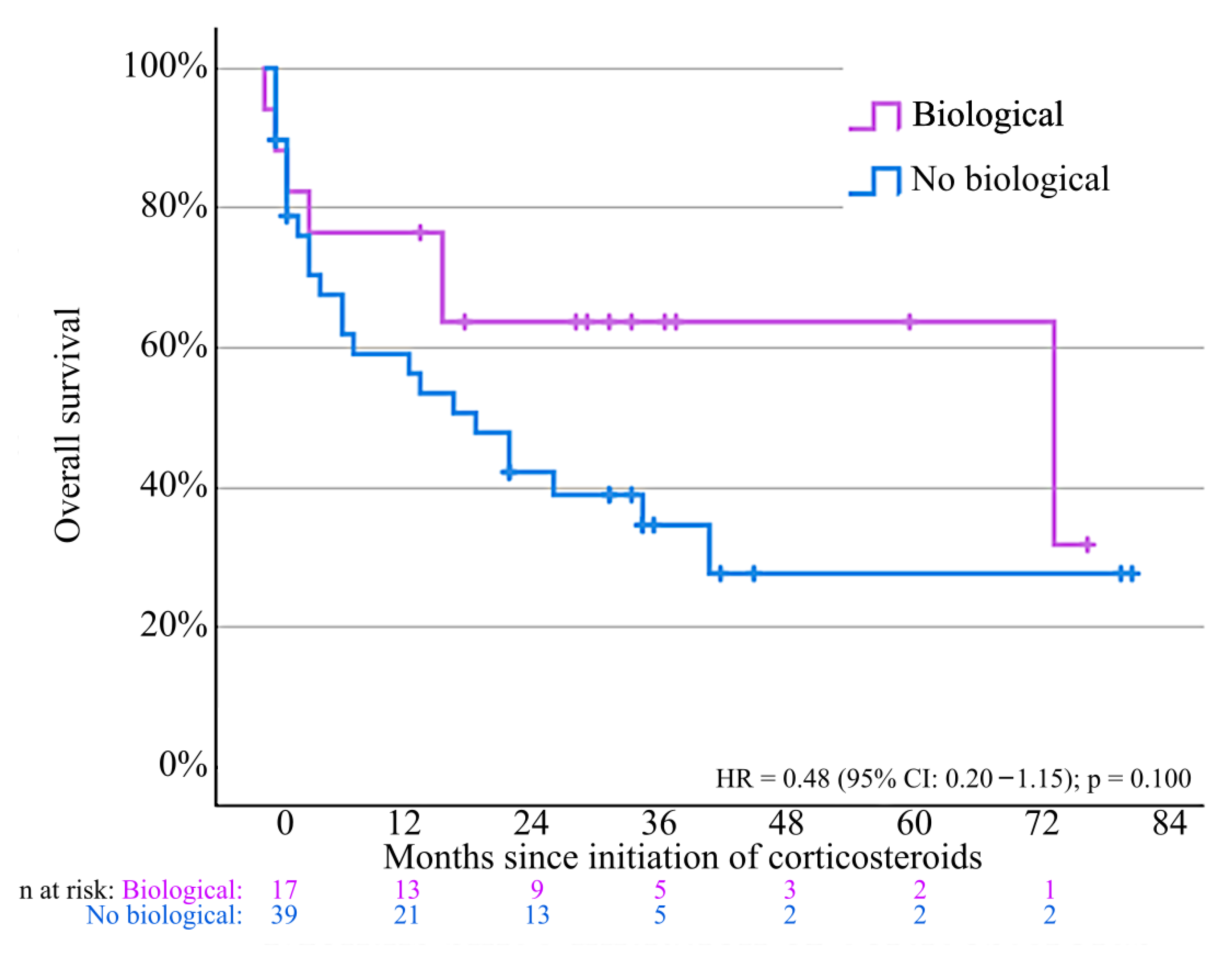
3.6. Oncological Outcomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ICI | Immune checkpoint inhibitor |
| CTLA-4 | Cytotoxic T-lymphocyte antigen 4 |
| LAG-3 | Lymphocyte activating gene 3 |
| PD-1 | Programmed cell death 1 |
| PD-L1 | Programmed cell death ligand 1 |
| GI | Gastro-intestinal |
| irAE | Immune-related adverse event |
| CTCAE | Common Terminology Criteria for Adverse Events |
| TNFα | Tumor necrosis factor alpha |
| IBD | Inflammatory bowel disease |
| CRP | C-reactive protein |
| 5-ASA | 5-aminosalicylic acid |
References
- Suijkerbuijk, K.P.M.; van Eijs, M.J.M.; van Wijk, F.; Eggermont, A.M.M. Clinical and translational attributes of immune-related adverse events. Nat. Cancer 2024, 5, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Haryal, A.; Townsend, M.J.; Baskaran, V.; Srivoleti, P.; Giobbie-Hurder, A.; Sack, J.S.; Isidro, R.A.; LeBoeuf, N.R.; Buchbinder, E.I.; Hodi, F.S.; et al. Immune checkpoint inhibitor gastritis is often associated with concomitant enterocolitis, which impacts the clinical course. Cancer 2023, 129, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.; Soularue, E.; Marthey, L.; Carbonnel, F. Management of Patients with Immune Checkpoint Inhibitor-Induced Enterocolitis: A Systematic Review. Clin. Gastroenterol. Hepatol. 2020, 18, 1393–1403.e1. [Google Scholar] [CrossRef]
- Larkin, J.; Hodi, F.S.; Wolchok, J.D. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 1270–1271. [Google Scholar] [CrossRef]
- Horvat, T.Z.; Adel, N.G.; Dang, T.-O.; Momtaz, P.; Postow, M.A.; Callahan, M.K.; Carvajal, R.D.; Dickson, M.A.; D’Angelo, S.P.; Woo, K.M.; et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J. Clin. Oncol. 2015, 33, 3193–3198. [Google Scholar] [CrossRef]
- Haanen, J.; Obeid, M.; Spain, L.; Carbonnel, F.; Wang, Y.; Robert, C.; Lyon, A.; Wick, W.; Kostine, M.; Peters, S.; et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1217–1238. [Google Scholar] [CrossRef]
- Odze, R.D.; Goldblum, J.R. Surgical Pathology of the GI Tract, Liver, Biliary Tract and Pancreas, 4th ed.; Elsevier: Philadelphia, PA, USA, 2022. [Google Scholar]
- Desmedt, V.; Jauregui-Amezaga, A.; Fierens, L.; Aspeslagh, S.; Dekervel, J.; Wauters, E.; Peeters, M.; Sabino, J.; Crapé, L.; Somers, M.; et al. Position statement on the management of the immune checkpoint inhibitor-induced colitis via multidisciplinary modified Delphi consensus. Eur. J. Cancer 2023, 187, 36–57. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Abu-Sbeih, H.; Ascierto, P.A.; Brufsky, J.; Cappelli, L.C.; Cortazar, F.B.; Gerber, D.E.; Hamad, L.; Hansen, E.; Johnson, D.B.; et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J. Immunother. Cancer 2021, 9, e002435. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- Daetwyler, E.; Wallrabenstein, T.; König, D.; Cappelli, L.C.; Naidoo, J.; Zippelius, A.; Läubli, H. Corticosteroid-resistant immune-related adverse events: A systematic review. J. Immunother. Cancer 2024, 12, e007409. [Google Scholar] [CrossRef]
- Nielsen, D.L.; Juhl, C.B.; Chen, I.M.; Kellermann, L.; Nielsen, O.H. Immune checkpoint Inhibitor-Induced diarrhea and Colitis: Incidence and Management. A systematic review and Meta-analysis. Cancer Treat. Rev. 2022, 109, 102440. [Google Scholar] [CrossRef]
- Zou, F.; Shah, A.Y.; Glitza, I.C.; Richards, D.; Thomas, A.S.; Wang, Y. S0137: Comparative study of vedolizumab and infliximab treatment in patients with immune-mediated diarrhea and colitis. Am. J. Gastroenterol. 2020, 115, S68. [Google Scholar] [CrossRef]
- Wyant, T.; Fedyk, E.; Abhyankar, B. An Overview of the Mechanism of Action of the Monoclonal Antibody Vedolizumab. J. Crohn’s Colitis 2016, 10, 1437–1444. [Google Scholar] [CrossRef]
- Bergqvist, V.; Hertervig, E.; Gedeon, P.; Kopljar, M.; Griph, H.; Kinhult, S.; Carneiro, A.; Marsal, J. Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis. Cancer Immunol. Immunother. 2017, 66, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Abu-Sbeih, H.; Ali, F.S.; Alsaadi, D.; Jennings, J.; Luo, W.; Gong, Z.; Richards, D.M.; Charabaty, A.; Wang, Y. Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor-induced colitis: A multi-center study. J. Immunother. Cancer 2018, 6, 142. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; Faleck, D. P086: Effectiveness of vedolizumab in patients with refractory immunotherapy-related colitis: A case series. Gastroenterology 2020, 158, S119. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- 2018–000340-26; A Phase 1/2a Study of BMS-986253 in Combination with Nivolumab in Advanced Cancers. Bristol-Myers Squibb International Corporation: Princeton, NJ, USA, 2018.
- Lieverse, R.I.Y.; Van Limbergen, E.J.; Oberije, C.J.G.; Troost, E.G.C.; Hadrup, S.R.; Dingemans, A.-M.C.; Hendriks, L.E.L.; Eckert, F.; Hiley, C.; Dooms, C.; et al. Stereotactic ablative body radiotherapy (SABR) combined with immunotherapy (L19-IL2) versus standard of care in stage IV NSCLC patients: ImmunoSABR, a multicentre, randomised controlled open-label phase II trial. BMC Cancer 2020, 20, 557. [Google Scholar] [CrossRef]
- NCT03000257; A Study of Budigalimab (ABBV-181) in Participants with Advanced Solid Tumors. AbbVie: North Chicago, IL, USA, 2016.
- De Jaeghere, E.A.; Tuyaerts, S.; Van Nuffel, A.M.T.; Belmans, A.; Bogaerts, K.; Baiden-Amissah, R.; Lippens, L.; Vuylsteke, P.; Henry, S.; Trinh, X.B.; et al. Pembrolizumab, radiotherapy, and an immunomodulatory five-drug cocktail in pretreated patients with persistent, recurrent, or metastatic cervical or endometrial carcinoma: Results of the phase II PRIMMO study. Cancer Immunol. Immunother. 2023, 72, 475–491. [Google Scholar] [CrossRef]
- Geukes Foppen, M.H.; Rozeman, E.A.; van Wilpe, S.; Postma, C.; Snaebjornsson, P.; van Thienen, J.V.; van Leerdam, M.E.; van den Heuvel, M.; Blank, C.U.; van Dieren, J.; et al. Immune checkpoint inhibition-related colitis: Symptoms, endoscopic features, histology and response to management. ESMO Open 2018, 3, e000278. [Google Scholar] [CrossRef]
- van Not, O.J.; Verheijden, R.J.; Eertwegh, A.J.M.v.D.; Haanen, J.B.A.G.; Aarts, M.J.B.; Berkmortel, F.W.P.J.v.D.; Blank, C.U.; Boers-Sonderen, M.J.; de Groot, J.-W.B.; Hospers, G.A.P.; et al. Association of Immune-Related Adverse Event Management with Survival in Patients with Advanced Melanoma. JAMA Oncol. 2022, 8, 1794–1801. [Google Scholar] [CrossRef]
- Zou, F.; Faleck, D.; Thomas, A.; Harris, J.; Satish, D.; Wang, X.; Charabaty, A.; Ernstoff, M.S.; Oliva, I.C.G.; Hanauer, S.; et al. Efficacy and safety of vedolizumab and infliximab treatment for immune-mediated diarrhea and colitis in patients with cancer: A two-center observational study. J. Immunother. Cancer 2021, 9, e003277. [Google Scholar] [CrossRef]
- NCT04797325; Vedolizumab for Immune Mediated Colitis. University of Copenhagen: Copenhagen, Denmark, 2021.
- M.D.A.C. Center. Infliximab or Vedolizumab in Treating Immune Checkpoint Inhibitor-Related Colitis in Patients with Genitourinary Cancer or Melanoma. NCT04407247. 2020. Available online: https://www.mdanderson.org/patients-family/diagnosis-treatment/clinical-trials/clinical-trials-index/clinical-trials-detail.ID2019-0276.dir.html/1000 (accessed on 16 August 2025).
- Abu-Sbeih, H.; Ali, F.S.; Naqash, A.R.; Owen, D.H.; Patel, S.; Otterson, G.A.; Kendra, K.; Ricciuti, B.; Chiari, R.; De Giglio, A.; et al. Resumption of Immune Checkpoint Inhibitor Therapy After Immune-Mediated Colitis. J. Clin. Oncol. 2019, 37, 2738–2745. [Google Scholar] [CrossRef]

| Histologic Patterns of Colitis | Definition |
|---|---|
| Active/infectious-type colitis pattern | This pattern often features focal inflammation, though it can also be prominent and diffuse. The focal form is often referred to as focal active colitis, while the diffuse form is known as diffuse acute colitis. In focal active colitis, only a few isolated foci of neutrophilic infiltration may be present. |
| Chronic/IBD-like colitis pattern | This pattern is usually characterized by mild signs of chronicity, such as basal plasmacytosis, without significant architectural distortion. However, in some cases—particularly those resistant to therapy—more pronounced chronic features resembling inflammatory bowel disease (IBD) may be observed. Chronic colitis can be either active or inactive. |
| Lymphocytic colitis pattern | Defined by an increased number of intraepithelial lymphocytes. |
| Collagenous colitis pattern | Characterized by a thickened subepithelial collagen layer. |
| Apoptosis | Increased apoptosis can be observed in the absence of active or significant lymphoplasmacytic inflammation in the lamina propria. As the histological findings do not align with any of the other three patterns, these cases are grouped under the active/infectious-type colitis pattern. Focal active inflammation is absent, although this may be due to sampling error. In some studies, this was classified as the apoptotic/GVHD colitis pattern. However, as our aim is to investigate the relationship between increased apoptosis and specific drug regimens, apoptosis is reported separately and the apoptotic colitis pattern category is discarded. |
| Response/Remission | Definition |
|---|---|
| Clinical response | Decrease in symptom severity according to the treating physician. |
| Clinical remission | Complete resolution of symptoms according to the treating physician. |
| Biochemical response | A decrease in CRP of ≥50% or a decrease in calprotectin of ≥50% if fecal calprotectin was assessed. |
| Biochemical remission | Normalization of CRP (to values under the upper limit of normal) or fecal calprotectin < 250 µg/g if fecal calprotectin was assessed. |
| If CRP or fecal calprotectin were below the lower limit of normal at time of diagnosis and treatment assessment, biochemical response was not assessable. | |
| Endoscopic response | Mayo endoscopic subscore-1 level compared to the endoscopy at time of diagnosis. If the Mayo endoscopic subscore at time of diagnosis and evaluation was 0, endoscopic response was not assessable. |
| Endoscopic remission | Mayo endoscopic subscore = 0 upon repeated lower GI endoscopy. |
| ICI-Induced Colitis (n = 77) | |
|---|---|
| Sex | |
| Men | 44 (57.1%) |
| Women | 33 (42.9%) |
| Median age, years | 64 (31–87) |
| Type of tumor | |
| Melanoma | 24 (31.2%) |
| Lung cancer | 16 (20.8%) |
| Renal cell carcinoma | 14 (18.2%) |
| Endometrial cancer | 4 (5.2%) |
| Head and neck cancer | 4 (5.2%) |
| Bladder cancer | 3 (3.9%) |
| Cervic cancer | 3 (3.9%) |
| Breast cancer | 2 (2.6%) |
| Cholangiocarcinoma | 2 (2.6%) |
| Hepatocellular carcinoma | 1 (1.3%) |
| Cutaneous squamous cell carcinoma | 1 (1.3%) |
| Ovarian cancer | 1 (1.3%) |
| Gastric cancer | 1 (1.3%) |
| Malignant pleural mesothelioma | 1 (1.3%) |
| Type of immunotherapy | |
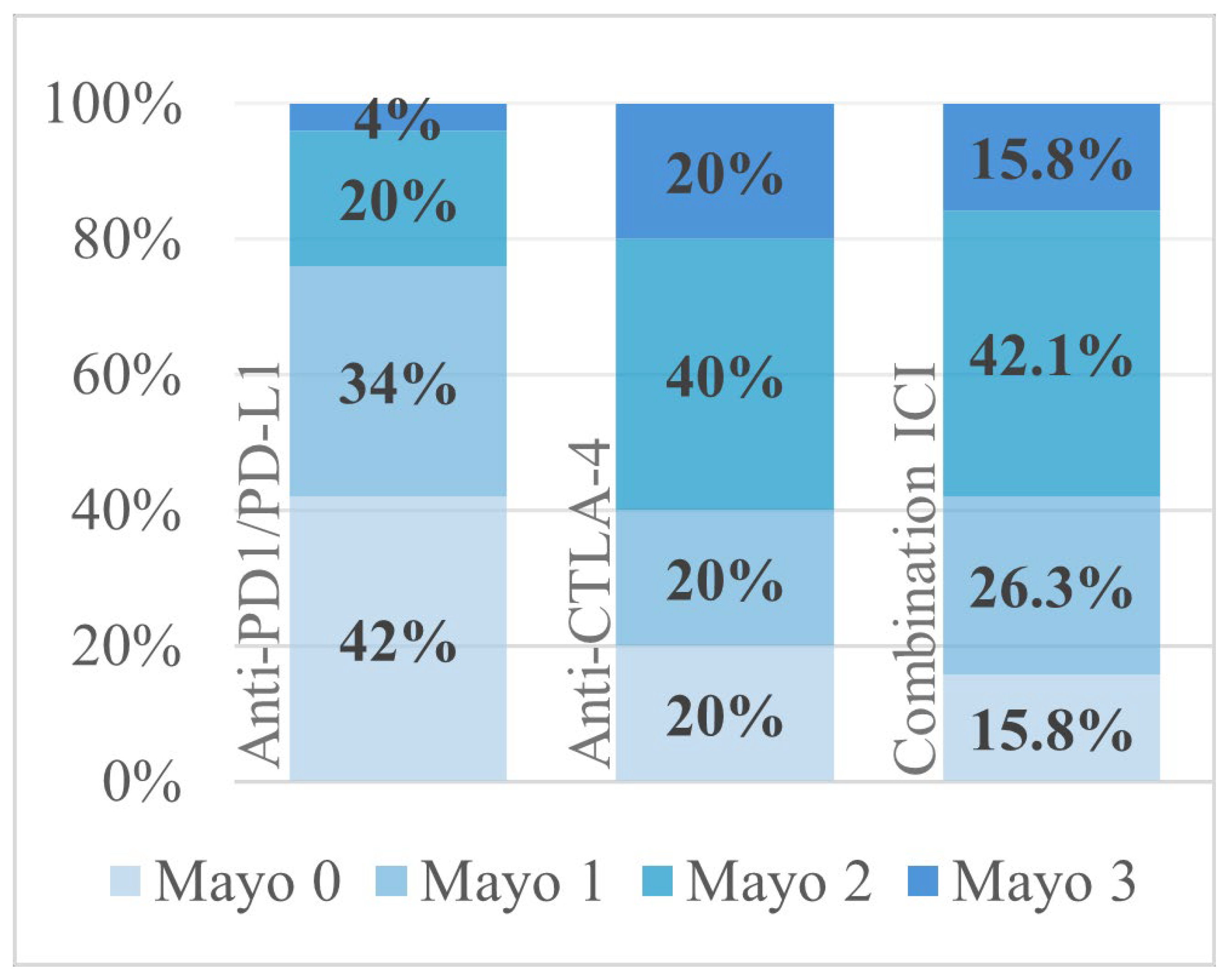
| Anti-PD-1/anti-PD-L1 monotherapy | 28 (36.4%) |
| Anti-CTLA-4 and anti-PD-1/anti-PD-L1 | 18 (23.4%) |
| Anti-PD-1/anti-PD-L1 and chemotherapy | 9 (11.7%) |
| Anti-PD-1/anti-PD-L1 and investigational drugs | 8 (10.4%) |
| Anti-CTLA-4 monotherapy | 7 (9.1%) |
| Anti-PD-1/anti-PD-L1 and anti-VEGF | 2 (2.6%) |
| Anti-PD-1/anti-PD-L1 and TKI | 2 (2.6%) |
| Anti-PD-1/anti-PD-L1 and anti-VEGF and chemotherapy | 1 (1.3%) |
| Anti-CTLA-4 and anti-PD-1/anti-PD-L1 and chemotherapy | 1 (1.3%) |
| Anti-CTLA-4 and anti-PD-1/anti-PD-L1 and investigational drugs | 1 (1.3%) |
| Treatment setting | |
| Neo-adjuvant | 2 (2.6%) |
| Adjuvant | 8 (10.4%) |
| Locally advanced/metastatic | 67 (87%) |
| Evaluation at diagnosis | |
| (1) Clinical: grade of diarrhea (n = 77) | |
| Grade 1 | 14 (18.2%) |
| Grade 2 | 38 (49.4%) |
| Grade 3 | 24 (31.2%) |
| Grade 4 | 1 (1.3%) |
| (2) Biochemical | |
| Calprotectin µg/g (n = 29), median (IQR) | 323 (115–1055) |
| CRP mg/L (n = 74), median (IQR) | 28.9 (7.68–78.6) |
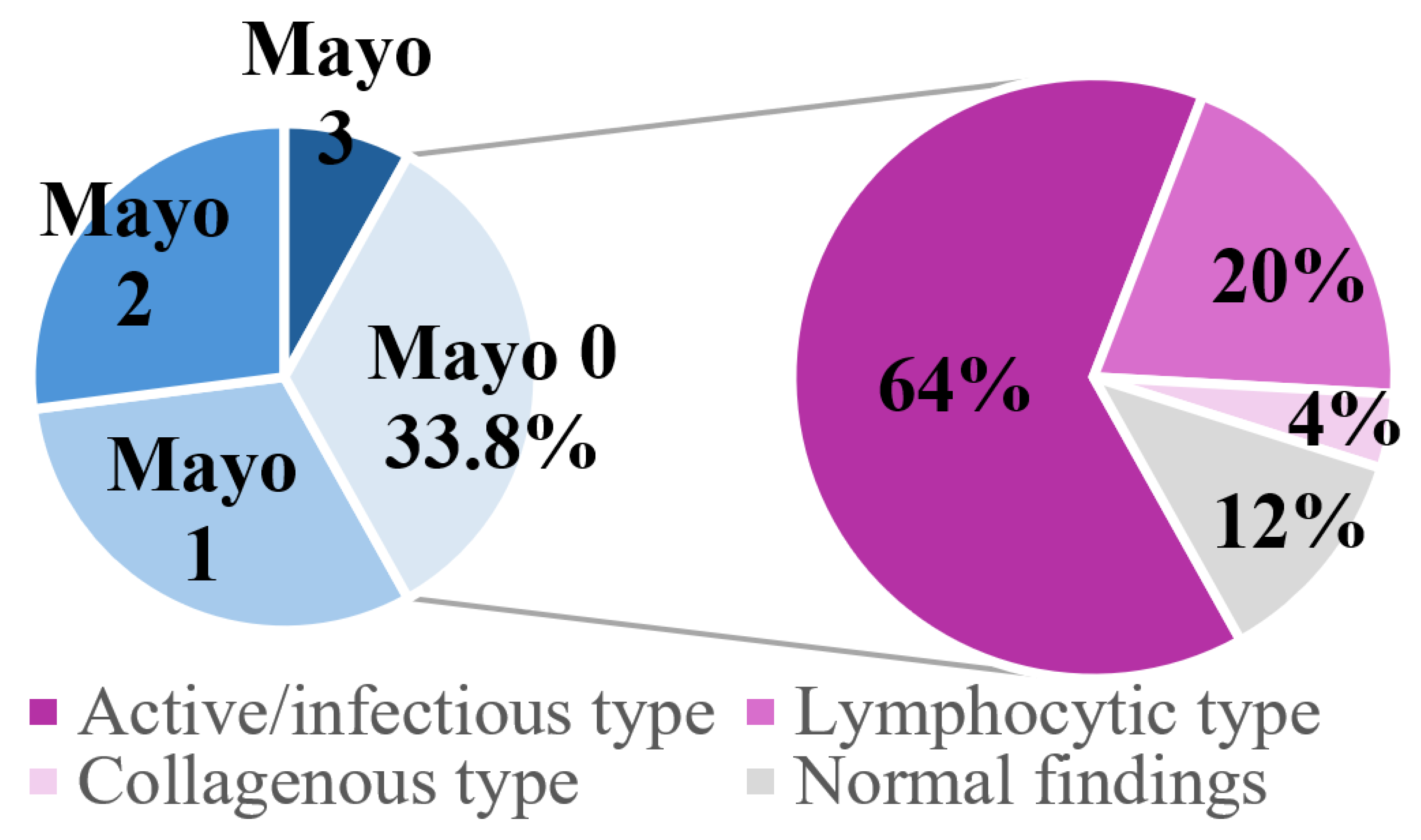
| (3) Endoscopic findings (n = 74) | |
| Mayo 0 | 25 (33.8%) |
| Mayo 1 | 22 (29.7%) |
| Mayo 2 | 21 (28.4%) |
| Mayo 3 | 6 (8.1%) |
| (4) Histopathological findings (n = 74) | |
| Active/infectious-type colitis | 48 (64.9%) |
| Chronic/IBD-type colitis | 12 (16.2%) |
| Lymphocytic-type colitis | 7 (9.5%) |
| Collagenous-type colitis | 3 (4.1%) |
| Normal | 4 (5.4%) |
| Presence of apoptosis | 35 (47.3%) |
| 1st Line Treatment | n (%) | 2nd Line Treatment | n (%) | 3rd Line Treatment | n (%) |
|---|---|---|---|---|---|
| Cs * | 53 (68.8) | Cs | 1 (1.3) | Infliximab | 1 (1.3) |
| Cs + 5-ASA | 1 (1.3) | Infliximab | 7 (9.1) | Vedolizumab | 4 (5.2%) |
| Cs + oral budesonide | 1 (1.3) | Vedolizumab | 8 (10.4) | No treatment needed | 72 (93.5) |
| Cs + rectal budesonide | 2 (2.6) | Vedolizumab + 5-ASA | 1 (1.3) | ||
| Cs + 5-ASA + oral budesonide | 1 (1.3) | Vedolizumab + rectal budesonide | 1 (1.3) | ||
| 5-ASA | 1 (1.3) | 5-ASA | 1 (1.3) | ||
| Oral budesonide | 1 (1.3) | 5-ASA + beclomethasone | 1 (1.3) | ||
| Beclomethasone | 6 (7.8) | Oral budesonide | 2 (2.6) | ||
| No treatment needed | 11 (14.3) | Rectal budesonide | 1 (1.3) | ||
| Rectal budesonide + beclomethasone | 1 (1.3) | ||||
| No treatment needed | 53 (68.8) |
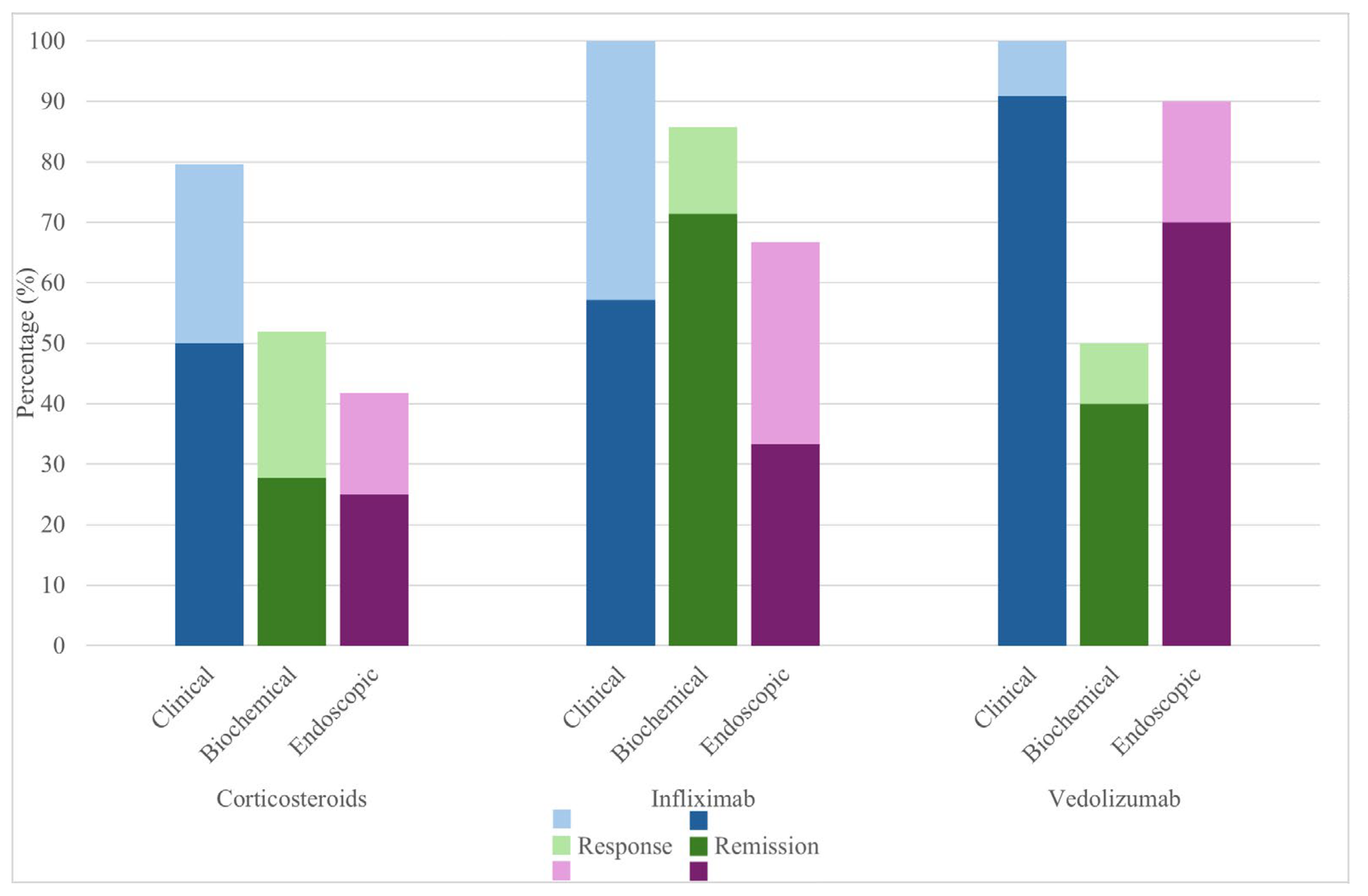
| Response/Remission | Clinical | Biochemical | Endoscopic |
|---|---|---|---|
| Systemic corticosteroids (overall) * | Response 79.6% (43/54) Remission 50% (27/54) | Response 51.9% (28/54) Remission 27.8% (15/54) | Response 41.7% (10/24) Remission 25% (6/24) |
| Infliximab | Response 100% (7/7) Remission 57.1% (4/7) | Response 85.7% (6/7) Remission 71.4% (5/7) | Response 66.7% (2/3) Remission 33.3% (1/3) |
| Vedolizumab | Response 100% (11/11) Remission 90.9% (10/11) | Response 50% (5/10) Remission 40% (4/10) | Response 90% (9/10) Remission 70% (7/10) |
| Vedolizumab and infliximab | n = 1, response (follow-up in another hospital) | Response | No response |
| Response/ Remission | Time to Clinical Response/ Remission | Time to biochemical Response/ Remission | Time to endoscopic Response/ Remission |
|---|---|---|---|
| Systemic corticosteroids | Response: n = 43, median: 13 days Remission: n = 27, median: 17.5 days | Response: n = 28, median: 13 days Remission: n = 15, median: 14 days | Response: n = 10, median: 54 days Remission: n = 6, median: 65 days |
| Infliximab | Response: n = 7, median: 7 days | Response: n = 6, median: 9.5 days | Response: n = 2, median: 71 days |
| Remission: n = 4, median: 12 days | Remission: n = 5, median: 14 days | Remission: n = 1, mean: 61 days | |
| Vedolizumab | Response: n = 11, median: 19 days | Response: n = 5, median: 53 days | Response: n = 9, median: 52 days |
| Remission: n = 10, median: 50.5 days | Remission: n = 4, median: 52.5 days | Remission: n = 7, median: 53 days | |
| Vedolizumab and infliximab | Response: n = 1, median: 20 days | Response: n = 1, median: 8 days | No response |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Holder, B.; Vereecke, J.; Ureel, N.; Lobatón, T.; Hoorens, A.; Lievens, A.; Truyens, M.; Rottey, S.; Vermaelen, K.; D’Cruz, V.; et al. Immune Checkpoint-Induced Colitis: A Single-Center Retrospective Cohort Study. J. Clin. Med. 2025, 14, 7219. https://doi.org/10.3390/jcm14207219
Van Holder B, Vereecke J, Ureel N, Lobatón T, Hoorens A, Lievens A, Truyens M, Rottey S, Vermaelen K, D’Cruz V, et al. Immune Checkpoint-Induced Colitis: A Single-Center Retrospective Cohort Study. Journal of Clinical Medicine. 2025; 14(20):7219. https://doi.org/10.3390/jcm14207219
Chicago/Turabian StyleVan Holder, Bengt, Julie Vereecke, Nathan Ureel, Triana Lobatón, Anne Hoorens, Amber Lievens, Marie Truyens, Sylvie Rottey, Karim Vermaelen, Venita D’Cruz, and et al. 2025. "Immune Checkpoint-Induced Colitis: A Single-Center Retrospective Cohort Study" Journal of Clinical Medicine 14, no. 20: 7219. https://doi.org/10.3390/jcm14207219
APA StyleVan Holder, B., Vereecke, J., Ureel, N., Lobatón, T., Hoorens, A., Lievens, A., Truyens, M., Rottey, S., Vermaelen, K., D’Cruz, V., Geldof, J., Jacobs, C., Naert, E., De Mulder, E., & Saerens, M. (2025). Immune Checkpoint-Induced Colitis: A Single-Center Retrospective Cohort Study. Journal of Clinical Medicine, 14(20), 7219. https://doi.org/10.3390/jcm14207219






