Understanding Peritoneal Fluid Estrogen and Progesterone Concentrations Permits Individualization of Medical Treatment of Endometriosis-Associated Pain with Lower Doses, Especially in Adolescents Not Requiring Contraception
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Basics of Medical Treatment with Estrogens or Progestagens
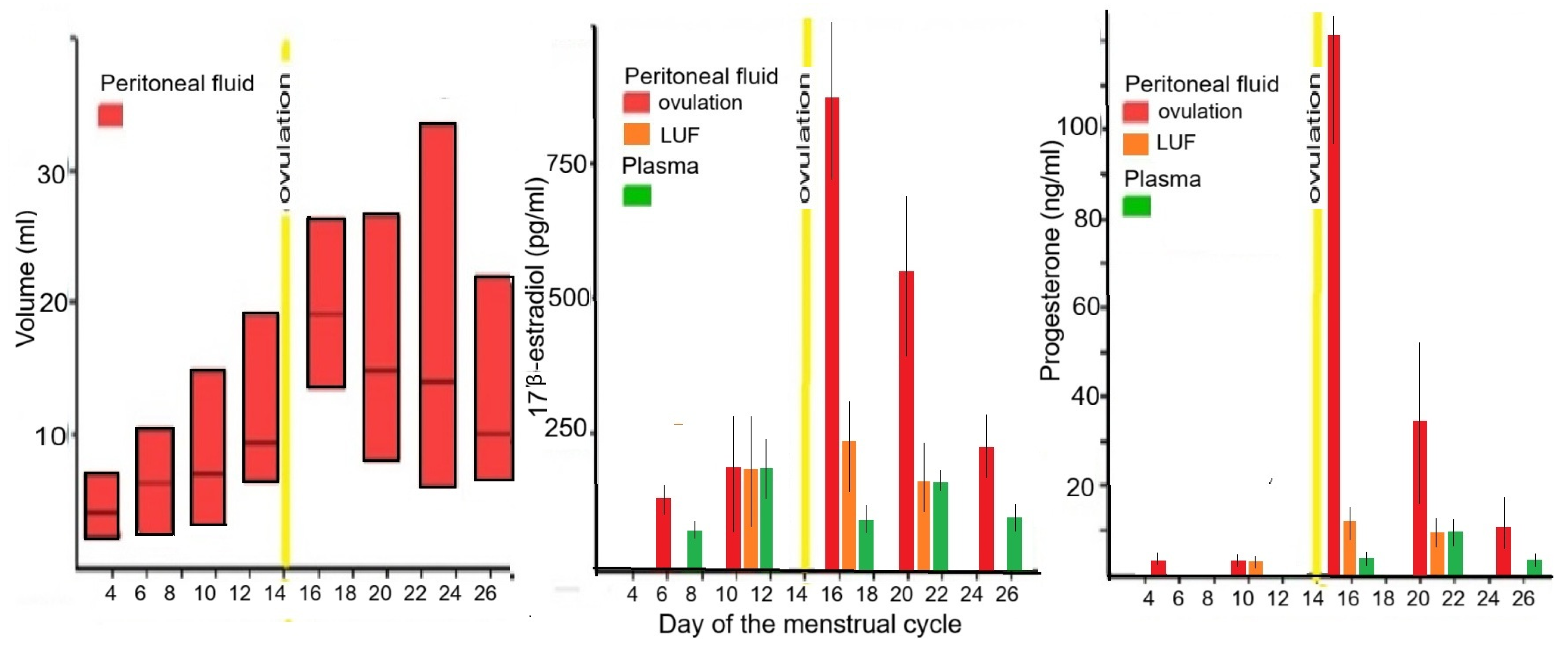
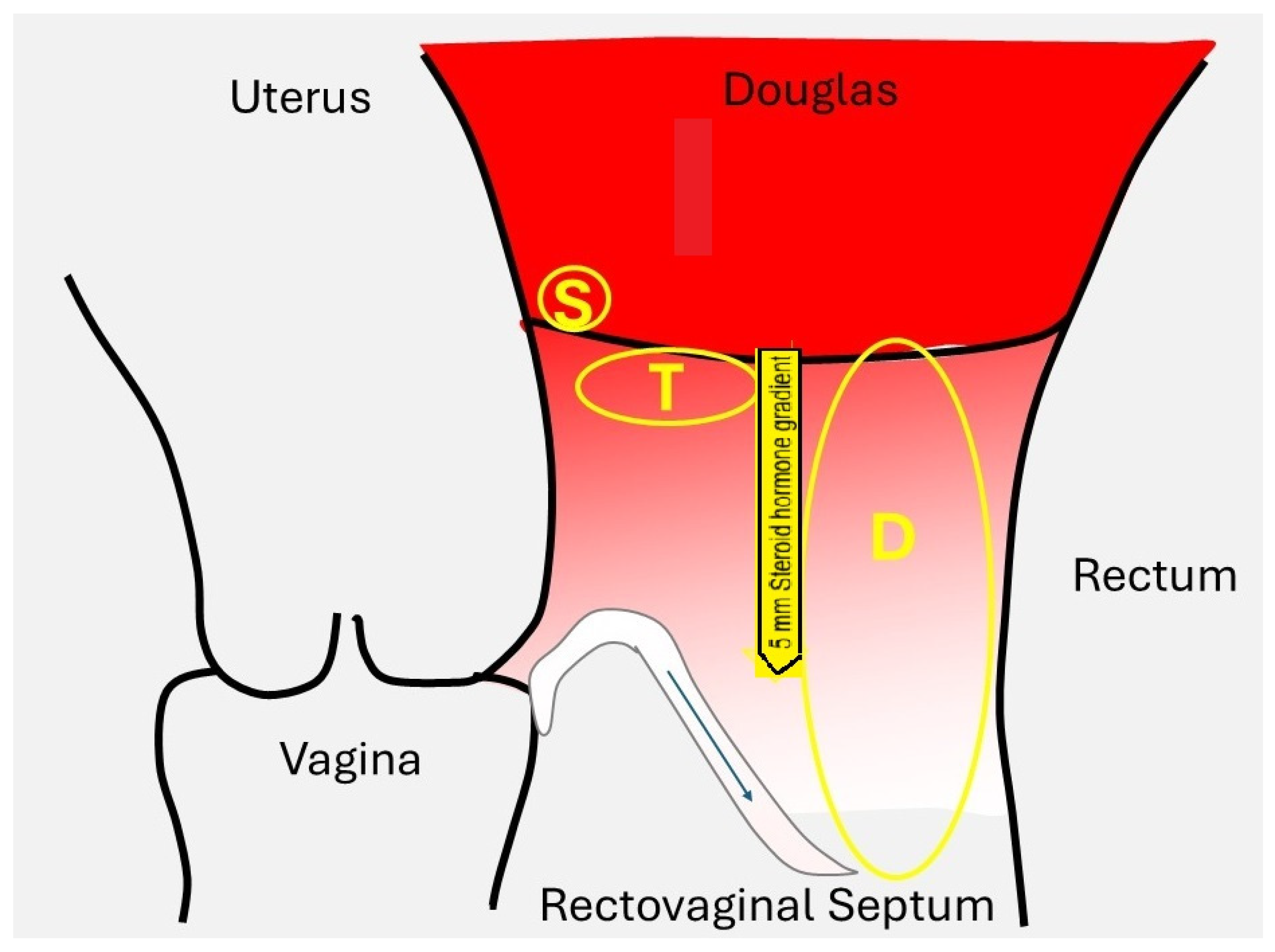
3.1.1. Tissue Concentrations
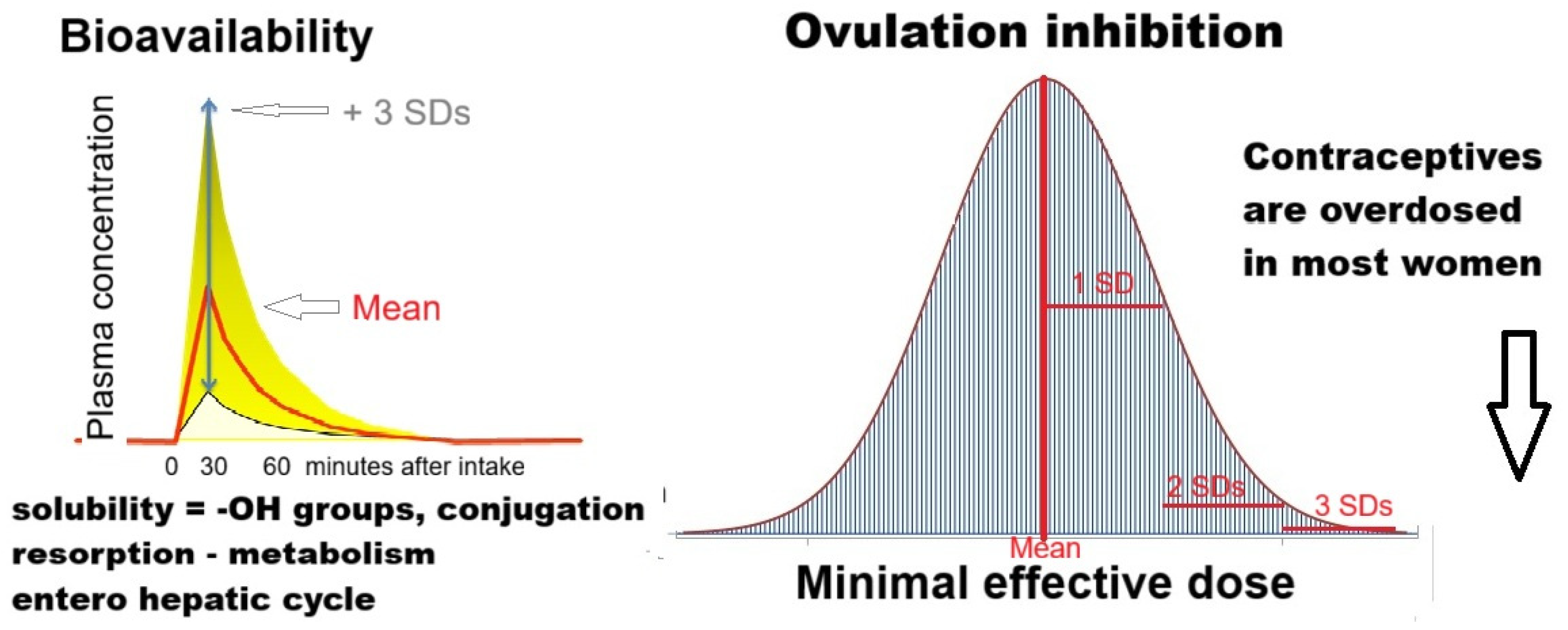
3.1.2. Dose Effect Relationship
3.1.3. Variable Bioavailability
3.1.4. Oral Contraceptives Are Overdosed in Most Women
3.1.5. Conclusions for Steroid Hormone Basics
3.2. Estrogen and Progesterone Concentrations in Endometriosis Lesions
3.3. Hormonal Medical Therapy for Endometriosis
3.4. Individualization of Therapy
3.5. Hormone Replacement Therapy After Menopause and Endometriosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kistner, R.W. The use of newer progestins in the treatment of endometriosis. Am. J. Obs. Gynecol. 1958, 75, 264–278. [Google Scholar] [CrossRef] [PubMed]
- Kistner, R.W. Newer progestins in the treatment of endometriosis. Int. J. Fertil. 1961, 6, 1–14. [Google Scholar] [CrossRef]
- Moghissi, K.S. Pseudopregnancy induced by estrogen-progestogen or progestogens alone in the treatment of endometriosis. Prog. Clin. Biol. Res. 1990, 323, 221–232. [Google Scholar]
- Becker, C.M.; Gattrell, W.T.; Gude, K.; Singh, S.S. Reevaluating response and failure of medical treatment of endometriosis: A systematic review. Fertil. Steril. 2017, 108, 125–136. [Google Scholar] [CrossRef]
- Vercellini, P.; Sergenti, G.; Buggio, L.; Frattaruolo, M.P.; Dridi, D.; Berlanda, N. Advances in the medical management of bowel endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 71, 78–99. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.O.D.; Babayev, E.; Bulun, S.E.; Clark, S.; Garcia-Grau, I.; Gregersen, P.K.; Kilcoyne, A.; Kim, J.J.; Lavender, M.; Marsh, E.E.; et al. Menstruation: Science and society. Am. J. Obs. Gynecol. 2020, 223, 624–664. [Google Scholar] [CrossRef] [PubMed]
- Endrikat, J.; Parke, S.; Trummer, D.; Schmidt, W.; Duijkers, I.; Klipping, C. Ovulation inhibition with four variations of a four-phasic estradiol valerate/dienogest combined oral contraceptive: Results of two prospective, randomized, open-label studies. Contraception 2008, 78, 218–225. [Google Scholar] [CrossRef]
- Kroll, R.; Seidman, L.; Ricciotti, N.; Howard, B.; Weiss, H. A phase 1, multicentre, open-label study to evaluate ovarian follicular activity and hormone levels with an extended-regimen combined oral contraceptive with low-dose ethinyl estradiol supplementation. Eur. J. Contracept. Reprod. Health Care 2015, 20, 249–258. [Google Scholar] [CrossRef]
- Brichant, G.; Laraki, I.; Henry, L.; Munaut, C.; Nisolle, M. New Therapeutics in Endometriosis: A Review of Hormonal, Non-Hormonal, and Non-Coding RNA Treatments. Int. J. Mol. Sci. 2021, 22, 10498. [Google Scholar] [CrossRef]
- Reis, F.M.; Coutinho, L.M.; Vannuccini, S.; Batteux, F.; Chapron, C.; Petraglia, F. Progesterone receptor ligands for the treatment of endometriosis: The mechanisms behind therapeutic success and failure. Hum. Reprod. Update 2020, 26, 565–585. [Google Scholar] [CrossRef]
- Praetorius, T.H.; Leonova, A.; Lac, V.; Senz, J.; Tessier-Cloutier, B.; Nazeran, T.M.; Köbel, M.; Grube, M.; Kraemer, B.; Yong, P.J.; et al. Molecular analysis suggests oligoclonality and metastasis of endometriosis lesions across anatomically defined subtypes. Fertil. Steril. 2022, 118, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Suda, K.; Nakaoka, H.; Yoshihara, K.; Ishiguro, T.; Adachi, S.; Kase, H.; Motoyama, T.; Inoue, I.; Enomoto, T. Different mutation profiles between epithelium and stroma in endometriosis and normal endometrium. Hum. Reprod. 2019, 34, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Basir, Z.; Kajdacsy-Balla, A.; Strawn, E.; Macias, V.; Montgomery, K.; Guo, S.W. Resolution of clonal origins for endometriotic lesions using laser capture microdissection and the human androgen receptor (HUMARA) assay. Fertil. Steril. 2003, 79 (Suppl. S1), 710–717. [Google Scholar] [CrossRef]
- Jimbo, H.; Hitomi, Y.; Yoshikawa, H.; Yano, T.; Momoeda, M.; Sakamoto, A.; Tsutsumi, O.; Taketani, Y.; Esumi, H. Evidence for monoclonal expansion of epithelial cells in ovarian endometrial cysts. Am. J. Pathol. 1997, 150, 1173–1178. [Google Scholar]
- Tamura, M.; Fukaya, T.; Murakami, I.; Uehara, S.; Yajima, A. Analysis of clonality in human endometriotic cysts based on evaluation of X chromosome inactivation in archival formalin-fixed, paraffin-embedded tissue. Lab. Investig. 1998, 78, 213–218. [Google Scholar]
- Mayr, D.; Amann, G.; Siefert, C.; Diebold, J.; Anderegg, B. Does endometriosis really have premalignant potential? A clonal analysis of laser-microdissected tissue. FASEB J. 2003, 17, 693–695. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.D.; Bulun, S.E. Endometriosis and nuclear receptors. Hum. Reprod. Update 2019, 25, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef]
- Vigano, P.; Ottolina, J.; Bartiromo, L.; Bonavina, G.; Schimberni, M.; Villanacci, R.; Candiani, M. Cellular Components Contributing to Fibrosis in Endometriosis: A Literature Review. J. Minim. Invasive Gynecol. 2020, 27, 287–295. [Google Scholar] [CrossRef]
- Vissers, G.; Giacomozzi, M.; Verdurmen, W.; Peek, R.; Nap, A. The role of fibrosis in endometriosis: A systematic review. Hum. Reprod. Update 2024, 30, 706–750. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Gomel, V.; Martin, D.C. Peritoneal fluid progesterone and progesterone resistance in superficial endometriosis lesions. Hum. Reprod. 2022, 28, 209–219. [Google Scholar] [CrossRef]
- Anglesio, M.S.; Papadopoulos, N.; Ayhan, A.; Nazeran, T.M.; Noe, M.; Horlings, H.M.; Lum, A.; Jones, S.; Senz, J.; Seckin, T.; et al. Cancer-Associated Mutations in Endometriosis without Cancer. N. Engl. J. Med. 2017, 376, 1835–1848. [Google Scholar] [CrossRef]
- Brichant, G.; Nervo, P.; Albert, A.; Munaut, C.; Foidart, J.M.; Nisolle, M. Heterogeneity of estrogen receptor α and progesterone receptor distribution in lesions of deep infiltrating endometriosis of untreated women or during exposure to various hormonal treatments. Gynecol. Endocrinol. 2018, 34, 651–655. [Google Scholar] [CrossRef]
- Leyendecker, G.; Wildt, L.; Laschke, M.W.; Mall, G. Archimetrosis: The evolution of a disease and its extant presentation: Pathogenesis and pathophysiology of archimetrosis (uterine adenomyosis and endometriosis). Arch. Gynecol. Obs. 2023, 307, 93–112. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Ussia, A.; Wattiez, A.; Adamyan, L.; Martin, D.C.; Gordts, S. The severity and frequency distribution of endometriosis subtypes at different ages: A model to understand the natural history of endometriosis based on single centre/single surgeon data. Facts Views Vis. Obgyn 2021, 13, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Martin, D.C. Deep endometriosis: A consequence of infiltration or retraction or possibly adenomyosis externa? Fertil. Steril. 1992, 58, 924–928. [Google Scholar] [CrossRef]
- Cornillie, F.J.; Oosterlynck, D.; Lauweryns, J.M.; Koninckx, P.R. Deeply infiltrating pelvic endometriosis: Histology and clinical significance. Fertil. Steril. 1990, 53, 978–983. [Google Scholar] [CrossRef]
- Gruenwald, P. Origin of endometriosis from the mesenchyme of the celomic walls. Am. J. Obstet. Gynecol. 1942, 44, 470–474. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Wattiez, A.; Gomel, V.; Martin, D.C. Pathogenesis of endometriosis: The genetic/epigenetic theory. Fertil. Steril. 2019, 111, 327–339. [Google Scholar] [CrossRef]
- Corona, R.; Verguts, J.; Koninckx, R.; Mailova, K.; Binda, M.M.; Koninckx, P.R. Intraperitoneal temperature and desiccation during endoscopic surgery. Intraoperative humidification and cooling of the peritoneal cavity can reduce adhesions. Am. J. Obstet. Gynecol. 2011, 205, 392–397. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Heyns, W.; Verhoeven, G.; Van, B.H.; Lissens, W.D.; De, M.P.; Brosens, I.A. Biochemical characterization of peritoneal fluid in women during the menstrual cycle. J. Clin. Endocrinol. Metab. 1980, 51, 1239–1244. [Google Scholar] [CrossRef]
- Defrere, S.; Lousse, J.C.; Gonzalez-Ramos, R.; Colette, S.; Donnez, J.; Van, L.A. Potential involvement of iron in the pathogenesis of peritoneal endometriosis. Mol. Hum. Reprod 2008, 14, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Ussia, A.; Tahlak, M.; Adamyan, L.; Wattiez, A.; Martin, D.C.; Gomel, V. Infection as a potential cofactor in the genetic-epigenetic pathophysiology of endometriosis: A systematic review. Facts Views Vis. Obgyn 2019, 11, 209–216. [Google Scholar] [PubMed]
- Oishi, S.; Mekaru, K.; Tanaka, S.E.; Arai, W.; Ashikawa, K.; Sakuraba, Y.; Nishioka, M.; Nakamura, R.; Miyagi, M.; Akamine, K.; et al. Microbiome analysis in women with endometriosis: Does a microbiome exist in peritoneal fluid and ovarian cystic fluid? Reprod. Med. Biol. 2022, 21, e12441. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Gomel, V.; Ussia, A.; Adamyan, L. Role of the peritoneal cavity in the prevention of postoperative adhesions, pain, and fatigue. Fertil. Steril. 2016, 106, 998–1010. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Renaer, M.; Brosens, I.A. Origin of peritoneal fluid in women: An ovarian exudation product. Br. J. Obstet. Gynaecol. 1980, 87, 177–183. [Google Scholar] [CrossRef]
- Donnez, J.; Langerock, S.; Thomas, K. Peritoneal fluid volume and 17 beta-estradiol and progesterone concentrations in ovulatory, anovulatory, and postmenopausal women. Obs. Gynecol. 1982, 59, 687–692. [Google Scholar]
- Bouckaert, P.X.J.M.; Evers, J.L.H.; Doesburg, W.H.; Schellekens, L.A.; Brombacher, P.H.; Rolland, R. Patterns of changes in proteins in the peritoneal fluid of women during the periovulatory phase of the menstrual cycle. Reproduction 1986, 77, 329–336. [Google Scholar] [CrossRef]
- Goldsman, M.P.; Pedram, A.; Dominguez, C.E.; Ciuffardi, I.; Levin, E.; Asch, R.H. Increased capillary permeability induced by human follicular fluid: A hypothesis for an ovarian origin of the hyperstimulation syndrome. Fertil. Steril. 1995, 63, 268–272. [Google Scholar] [CrossRef]
- Scheenjes, E.; te Velde, E.R.; Kremer, J. Inspection of the ovaries and steroids in serum and peritoneal fluid at various time intervals after ovulation in fertile women: Implications for the luteinized unruptured follicle syndrome. Fertil. Steril. 1990, 54, 38–41. [Google Scholar] [CrossRef]
- Devroey, P.; Naaktgeboren, N.; Traey, E.; Wisanto, A.; Van Steirteghem, A.C. Follicular rupture changes the endocrine profile of peritoneal fluid. Acta Eur. Fertil. 1985, 16, 237–240. [Google Scholar]
- Koninckx, P.R.; Brosens, I.A. Clinical significance of the luteinized unruptured follicle syndrome as a cause of infertility. Eur. J. Obstet. Gynecol. Reprod. Biol. 1982, 13, 355–368. [Google Scholar] [CrossRef]
- Koninckx, P.R.; De Moor, P.; Brosens, I.A. Diagnosis of the luteinized unruptured follicle syndrome by steroid hormone assays on peritoneal fluid. Br. J. Obs. Gynaecol. 1980, 87, 929–934. [Google Scholar] [CrossRef]
- Rosner, W. Free estradiol and sex hormone-binding globulin. Steroids 2015, 99, 113–116. [Google Scholar] [CrossRef]
- Oren, I.; Fleishman, S.J.; Kessel, A.; Ben-Tal, N. Free Diffusion of Steroid Hormones Across Biomembranes: A Simplex Search with Implicit Solvent Model Calculations. Biophys. J. 2004, 87, 768–779. [Google Scholar] [CrossRef]
- Lee, Y.; Gorski, J. Estrogen-induced transcription of the progesterone receptor gene does not parallel estrogen receptor occupancy. Proc. Natl. Acad. Sci. USA 1996, 93, 15180–15184. [Google Scholar] [CrossRef]
- Chow, C.C.; Ong, K.M.; Dougherty, E.J.; Simons, S.S., Jr. Inferring mechanisms from dose-response curves. Methods Enzym. 2011, 487, 465–483. [Google Scholar] [CrossRef]
- Calabrese, E.J. Estrogen and related compounds: Biphasic dose responses. Crit. Rev. Toxicol. 2001, 31, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Sobstyl, A.; Chalupnik, A.; Mertowska, P.; Grywalska, E. How Do Microorganisms Influence the Development of Endometriosis? Participation of Genital, Intestinal and Oral Microbiota in Metabolic Regulation and Immunopathogenesis of Endometriosis. Int. J. Mol. Sci. 2023, 24, 10920. [Google Scholar] [CrossRef] [PubMed]
- Brenner, P.F.; Goebelsmann, U.; Stanczyk, F.Z.; Mishell, D.R., Jr. Serum levels of ethinylestradiol following its ingestion alone or in oral contraceptive formulations. Contraception 1980, 22, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.; Nygren, K.G. Ethinyl estradiol in peripheral plasma after oral administration of 30 microgram and 50 microgram to women. Contraception 1978, 18, 469–475. [Google Scholar] [CrossRef]
- Mahajan, D.K.; Billiar, R.B.; Jassani, M.; Little, A.B. Ethinyl estradiol administration and plasma steroid concentrations in ovariectomized women. Am. J. Obs. Gynecol. 1978, 130, 398–402. [Google Scholar] [CrossRef]
- Ouellet*, D.; Hsu, A.; Qian, J.; Locke, C.S.; Eason, C.J.; Cavanaugh, J.H.; Leonard, J.M.; Granneman, G.R. Effect of ritonavir on the pharmacokinetics of ethinyl oestradiol in healthy female volunteers. Br. J. Clin. Pharmacol. 1998, 46, 111–116. [Google Scholar] [CrossRef]
- Carol, W.; Klinger, G.; Jäger, R.; Kasch, R.; Brandstädt, A. Pharmacokinetics of ethinylestradiol and levonorgestrel after administration of two oral contraceptive preparations. Exp. Clin. Endocrinol. 1992, 99, 12–17. [Google Scholar] [CrossRef]
- Giatti, S.; Diviccaro, S.; Falvo, E.; Garcia-Segura, L.M.; Melcangi, R.C. Physiopathological role of the enzymatic complex 5α-reductase and 3α/β-hydroxysteroid oxidoreductase in the generation of progesterone and testosterone neuroactive metabolites. Front. Neuroendocrinol. 2020, 57, 100836. [Google Scholar] [CrossRef] [PubMed]
- Lyons, R.A.; Saridogan, E.; Djahanbakhch, O. The effect of ovarian follicular fluid and peritoneal fluid on Fallopian tube ciliary beat frequency. Hum. Reprod. 2006, 21, 52–56. [Google Scholar] [CrossRef]
- Bordanaba-Florit, G.; Liempd, S.V.; Cabrera, D.; Royo, F.; Falcón-Pérez, J.M. Simultaneous Quantification of Steroid Hormones Using hrLC-MS in Endocrine Tissues of Male Rats and Human Samples. Metabolites 2022, 12, 714. [Google Scholar] [CrossRef]
- Hunter, R.H.; Cook, B.; Poyser, N.L. Regulation of oviduct function in pigs by local transfer of ovarian steroids and prostaglandins: A mechanism to influence sperm transport. Eur. J. Obs. Gynecol. Reprod. Biol. 1983, 14, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Einer-Jensen, N.; Hunter, R. Counter-current transfer in reproductive biology. Reproduction 2005, 129, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Kunz, G. Sonographic evidence for the involvement of the utero-ovarian counter-current system in the ovarian control of directed uterine sperm transport. Hum. Reprod. Update 1998, 4, 667–672. [Google Scholar] [CrossRef]
- Zervomanolakis, I.; Ott, H.W.; Müller, J.; Seeber, B.E.; Friess, S.C.; Mattle, V.; Virgolini, I.; Heute, D.; Wildt, L. Uterine mechanisms of ipsilateral directed spermatozoa transport: Evidence for a contribution of the utero-ovarian countercurrent system. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 144, S45–S49. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Oosterlynck, D.; D’Hooghe, T.; Meuleman, C. Deeply infiltrating endometriosis is a disease whereas mild endometriosis could be considered a non-disease. Ann. N. Y. Acad. Sci. 1994, 734, 333–341. [Google Scholar] [CrossRef]
- de Almeida Asencio, F.; Ribeiro, H.A.; Ribeiro, P.A.; Malzoni, M.; Adamyan, L.; Ussia, A.; Gomel, V.; Martin, D.C.; Koninckx, P.R. Symptomatic endometriosis developing several years after menopause in the absence of increased circulating estrogen concentrations: A systematic review and seven case reports. Gynecol. Surg. 2019, 16, 3. [Google Scholar] [CrossRef]
- Selak, V.; Farquhar, C.; Prentice, A.; Singla, A. Danazol for pelvic pain associated with endometriosis. Cochrane Database. Syst. Rev. 2007, CD000068. [Google Scholar] [CrossRef]
- Evers, J.L. The second-look laparoscopy for evaluation of the result of medical treatment of endometriosis should not be performed during ovarian suppression. Fertil. Steril. 1987, 47, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Andres Mde, P.; Lopes, L.A.; Baracat, E.C.; Podgaec, S. Dienogest in the treatment of endometriosis: Systematic review. Arch. Gynecol. Obs. 2015, 292, 523–529. [Google Scholar] [CrossRef]
- Brown, J.; Kives, S.; Akhtar, M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database Syst. Rev. 2012, 3. [Google Scholar] [CrossRef]
- Dragoman, M.V.; Gaffield, M.E. The safety of subcutaneously administered depot medroxyprogesterone acetate (104 mg/0.65 mL): A systematic review. Contraception 2016, 94, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Crawford, T.J.; Datta, S.; Prentice, A. Oral contraceptives for pain associated with endometriosis. Cochrane Database Syst. Rev. 2018, 5. [Google Scholar] [CrossRef]
- Veth, V.B.; van de Kar, M.M.; Duffy, J.M.; van Wely, M.; Mijatovic, V.; Maas, J.W. Gonadotropin-releasing hormone analogues for endometriosis. Cochrane Database Syst. Rev. 2023, 6, Cd014788. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Ma, Y.; Ye, M.; Chen, L.; Liu, F.; Hou, Q. Efficacy and safety of oral gonadotropin-releasing hormone antagonists in moderate-to-severe endometriosis-associated pain: A systematic review and network meta-analysis. Arch. Gynecol. Obs. 2023, 308, 1047–1056. [Google Scholar] [CrossRef]
- Hofmann, B.; Reinecke, I.; Schuett, B.; Merz, M.; Zurth, C. Pharmacokinetic overview of ethinyl estradiol dose and bioavailability using two transdermal contraceptive systems and a standard combined oral contraceptive. Int. J. Clin. Pharmacol. Ther. 2014, 52, 1059–1070. [Google Scholar] [CrossRef]
- Stanczyk, F.Z.; Archer, D.F.; Bhavnani, B.R. Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: Pharmacokinetics, pharmacodynamics and risk assessment. Contraception 2013, 87, 706–727. [Google Scholar] [CrossRef]
- van den Heuvel, M.W.; van Bragt, A.J.; Alnabawy, A.K.; Kaptein, M.C. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: The vaginal ring, the transdermal patch and an oral contraceptive. Contraception 2005, 72, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Peitsidis, P.; Tsikouras, P.; Laganà, A.S.; Laios, A.; Gkegkes, I.D.; Iavazzo, C. A Systematic Review of Systematic Reviews on the Use of Aromatase Inhibitors for the Treatment of Endometriosis: The Evidence to Date. Drug Des. Dev. Ther. 2023, 17, 1329–1346. [Google Scholar] [CrossRef] [PubMed]
- Perrone, U.; Evangelisti, G.; Laganà, A.S.; Bogliolo, S.; Ceccaroni, M.; Izzotti, A.; Gustavino, C.; Ferrero, S.; Barra, F. A review of phase II and III drugs for the treatment and management of endometriosis. Expert. Opin. Emerg. Drugs 2023, 28, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Cantineau, A.E.; Rutten, A.G.; Cohlen, B.J. Agents for ovarian stimulation for intrauterine insemination (IUI) in ovulatory women with infertility. Cochrane Database Syst. Rev. 2021, 11. [Google Scholar] [CrossRef]
- Giudice, L.C.; As-Sanie, S.; Arjona Ferreira, J.C.; Becker, C.M.; Abrao, M.S.; Lessey, B.A.; Dynowski, K.; Wilk, K.; Li, Y.; Mathur, V.; et al. A Plain Language Summary to learn about relugolix combination therapy for the treatment of pain associated with endometriosis. Pain Manag. 2023, 13, 631–640. [Google Scholar] [CrossRef]
- Giudice, L.C.; As-Sanie, S.; Arjona Ferreira, J.C.; Becker, C.M.; Abrao, M.S.; Lessey, B.A.; Brown, E.; Dynowski, K.; Wilk, K.; Li, Y.; et al. Once daily oral relugolix combination therapy versus placebo in patients with endometriosis-associated pain: Two replicate phase 3, randomised, double-blind, studies (SPIRIT 1 and 2). Lancet 2022, 399, 2267–2279. [Google Scholar] [CrossRef]
- Geng, T.; Sun, Y.; Cheng, L.; Cao, Y.; Zhang, M.; Hong, Z.; Ma, L.; Zhang, Y. Downregulation of LHCGR Attenuates COX-2 Expression and Induces Luteinized Unruptured Follicle Syndrome in Endometriosis. Front. Endocrinol. 2022, 13, 853563. [Google Scholar] [CrossRef]
- Vallée, A.; Saridogan, E.; Petraglia, F.; Keckstein, J.; Polyzos, N.; Wyns, C.; Gianaroli, L.; Tarlatzis, B.; Ayoubi, J.M.; Feki, A. Horizons in Endometriosis: Proceedings of the Montreux Reproductive Summit, 14–15 July 2023. Facts Views Vis. ObGyn 2024, 16, 1. [Google Scholar] [CrossRef]
- Zanello, M.; Borghese, G.; Manzara, F.; Degli Esposti, E.; Moro, E.; Raimondo, D.; Abdullahi, L.O.; Arena, A.; Terzano, P.; Meriggiola, M.C.; et al. Hormonal Replacement Therapy in Menopausal Women with History of Endometriosis: A Review of Literature. Medicina 2019, 55, 477. [Google Scholar] [CrossRef] [PubMed]
- Amer, S.; Bazmi, S. HRT in Women Undergoing Pelvic Clearance for Endometriosis—A Case Report and a National Survey. J. Clin. Med. 2023, 12, 336. [Google Scholar] [CrossRef]
- Andrieu, T.; du Toit, T.; Vogt, B.; Mueller, M.D.; Groessl, M. Parallel targeted and non-targeted quantitative analysis of steroids in human serum and peritoneal fluid by liquid chromatography high-resolution mass spectrometry. Anal. Bioanal. Chem. 2022, 414, 7461–7472. [Google Scholar] [CrossRef]
- Ping, Z.; Wen, Z.; Jinhua, L.; Jinghe, L. Research on central sensitization of endometriosis-associated pain: A systematic review of the literature. J. Pain Res. 2019, 12, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Cetera, G.E.; Facchin, F.; Viganò, P.; Merli, C.E.M.; Frassineti, A.; Fiorini, J.; Somigliana, E.; Vercellini, P. “SO FAR AWAY” How Doctors Can Contribute to Making Endometriosis Hell on Earth. A Call for Humanistic Medicine and Empathetic Practice for Genuine Person-Centered Care. A Narrative Review. Int. J. Womens Health 2024, 16, 273–287. [Google Scholar] [CrossRef]
- Dewald, C.L.A.; Becker, L.S.; Meyer, B.C. Interventional Therapy of Pelvic Venous Disorders (PeVD). Rofo 2024, 196, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, D.; Mohammed, S.R.; Caesar, K.; Dindyal, S. Nutcracker syndrome: A case-based review. Ann. R. Coll. Surg. Engl. 2023, 106, 396–400. [Google Scholar] [CrossRef]
- Ozsvath, K.; Raffetto, J.D.; Lindner, E.; Murphy, E.H. Venous compression syndromes in females: A descriptive review. Semin. Vasc. Surg. 2023, 36, 550–559. [Google Scholar] [CrossRef]
- Mijatovic, V.; Vercellini, P. Towards comprehensive management of symptomatic endometriosis: Beyond the dichotomy of medical versus surgical treatment. Hum. Reprod. 2024, 39, 464–477. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koninckx, P.R.; Ussia, A.; Adamyan, L.; Wattiez, A.; Vigano, P. Understanding Peritoneal Fluid Estrogen and Progesterone Concentrations Permits Individualization of Medical Treatment of Endometriosis-Associated Pain with Lower Doses, Especially in Adolescents Not Requiring Contraception. J. Clin. Med. 2025, 14, 7196. https://doi.org/10.3390/jcm14207196
Koninckx PR, Ussia A, Adamyan L, Wattiez A, Vigano P. Understanding Peritoneal Fluid Estrogen and Progesterone Concentrations Permits Individualization of Medical Treatment of Endometriosis-Associated Pain with Lower Doses, Especially in Adolescents Not Requiring Contraception. Journal of Clinical Medicine. 2025; 14(20):7196. https://doi.org/10.3390/jcm14207196
Chicago/Turabian StyleKoninckx, Philippe R., Anastasia Ussia, Leila Adamyan, Arnaud Wattiez, and Paola Vigano. 2025. "Understanding Peritoneal Fluid Estrogen and Progesterone Concentrations Permits Individualization of Medical Treatment of Endometriosis-Associated Pain with Lower Doses, Especially in Adolescents Not Requiring Contraception" Journal of Clinical Medicine 14, no. 20: 7196. https://doi.org/10.3390/jcm14207196
APA StyleKoninckx, P. R., Ussia, A., Adamyan, L., Wattiez, A., & Vigano, P. (2025). Understanding Peritoneal Fluid Estrogen and Progesterone Concentrations Permits Individualization of Medical Treatment of Endometriosis-Associated Pain with Lower Doses, Especially in Adolescents Not Requiring Contraception. Journal of Clinical Medicine, 14(20), 7196. https://doi.org/10.3390/jcm14207196







