Linking Intradialytic Blood Volume Dynamics to Extracellular Fluid Status: Toward Personalized Fluid Assessment in Hemodialysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Characteristics and Ethics
2.2. Bioimpedance Spectroscopy (BIS) Measurement Protocol
2.3. Statistical Analysis
3. Results
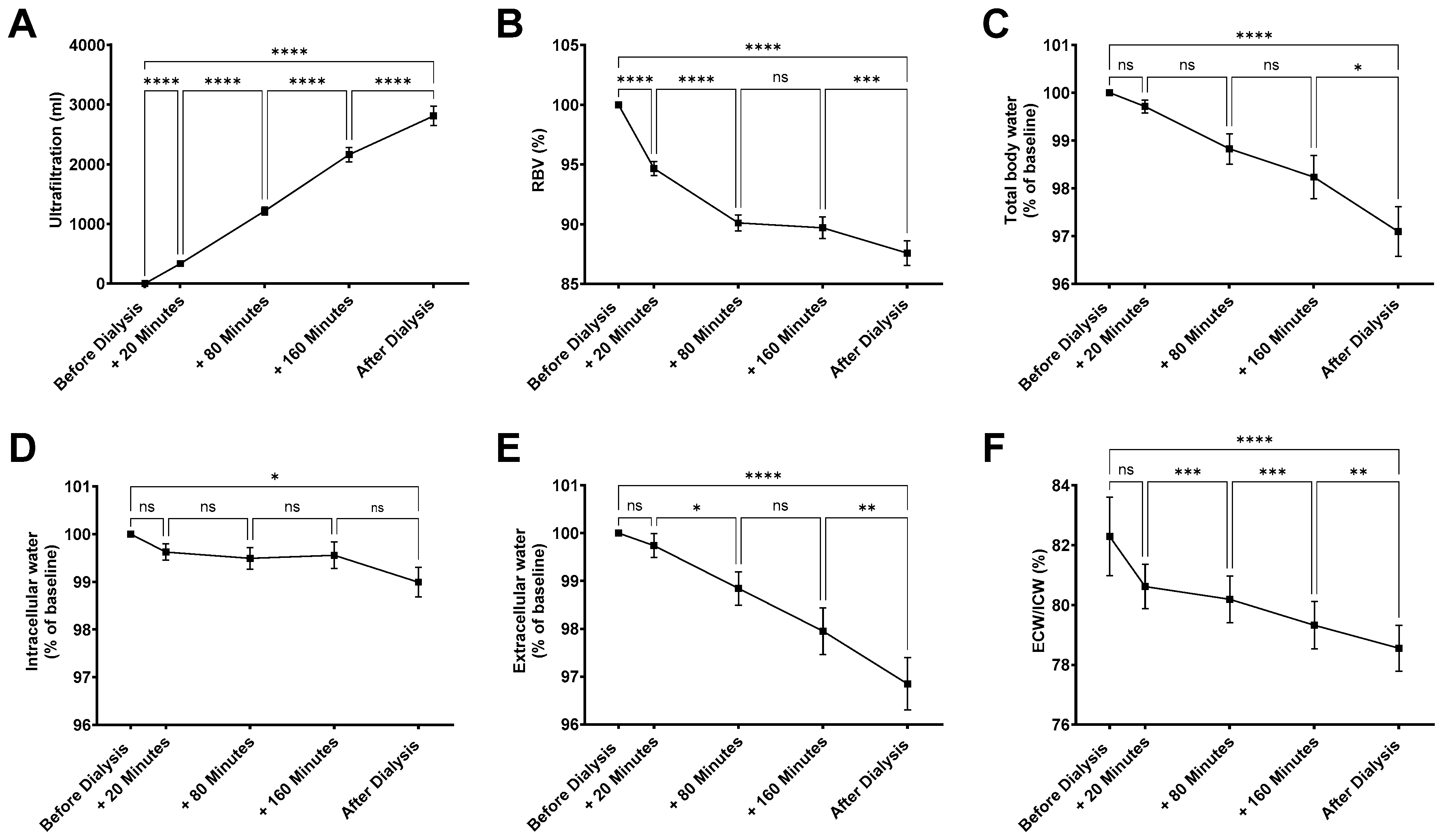
3.1. Hemodialysis-Induced Changes in Body Fluid Compartments
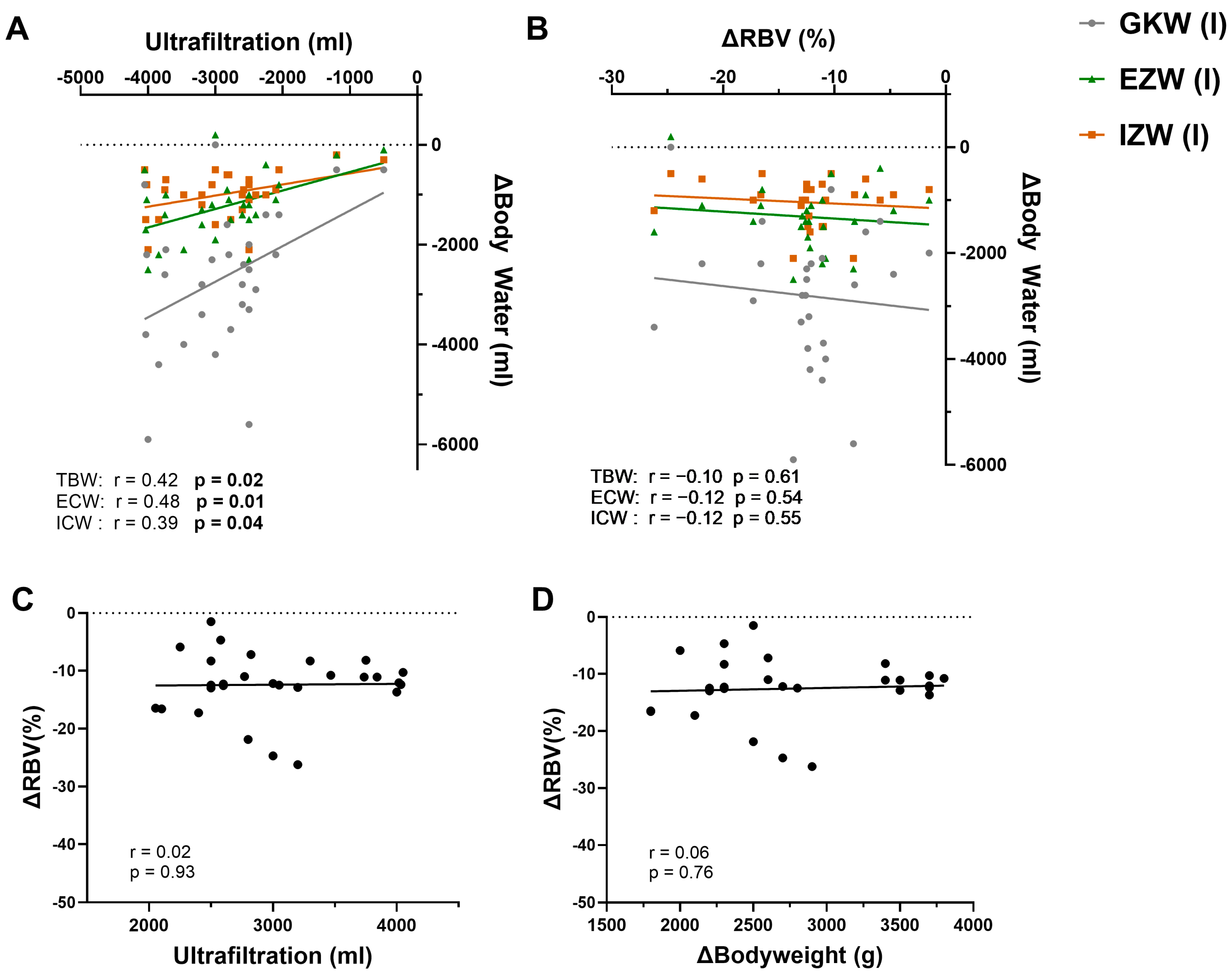
3.2. Correlation of UF with Changes in Body Water
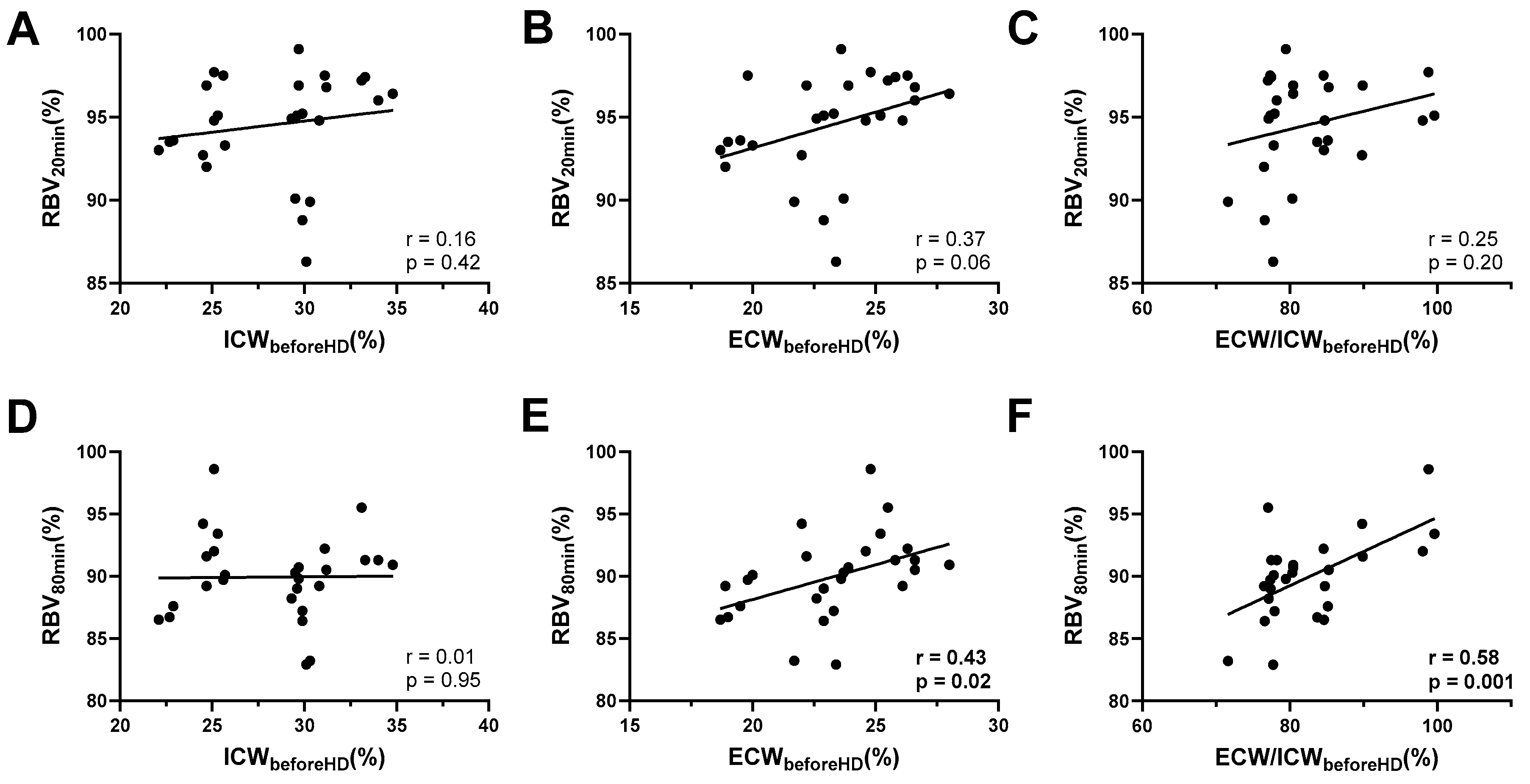
3.3. Early Intradialytic RBV Levels Reflect Pre-Dialysis Volume Status
4. Discussion
4.1. Plausibility of the Observed Signals (RBV, UF, Body Composition)
4.2. Relation to Existing Literature: What Is Known, What Is New
4.3. Alternative Explanations and Constraints
4.4. Implications for a Personalized Fluid-Management Strategy
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peer, D. Precision medicine—Delivering the goods? Cancer Lett. 2014, 352, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Birkelo, B.C.; Koyner, J.L.; Ostermann, M.; Bhatraju, P.K. The Road to Precision Medicine for Acute Kidney Injury. Crit. Care Med. 2024, 52, 1127–1137. [Google Scholar] [CrossRef]
- Hur, I.; Lee, Y.K.; Kalantar-Zadeh, K.; Obi, Y. Individualized Hemodialysis Treatment: A Perspective on Residual Kidney Function and Precision Medicine in Nephrology. Cardiorenal Med. 2019, 9, 69–82. [Google Scholar] [CrossRef]
- Lee, S.; Pham, N.M.; Montez-Rath, M.E.; Bolanos, C.G.; Bonde, S.S.; Meyer, T.W.; Sirich, T.L. Twice Weekly versus Thrice Weekly Hemodialysis—A Pilot Cross-Over Equivalence Trial. Clin. J. Am. Soc. Nephrol. 2024, 19, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Blankestijn, P.J.; Vernooij, R.W.M.; Hockham, C.; Strippoli, G.F.M.; Canaud, B.; Hegbrant, J.; Barth, C.; Covic, A.; Cromm, K.; Cucui, A.; et al. Effect of Hemodiafiltration or Hemodialysis on Mortality in Kidney Failure. N. Engl. J. Med. 2023, 389, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Blagg, C.R. The Early History of Dialysis for Chronic Renal Failure in the United States: A View From Seattle. Am. J. Kidney Dis. 2007, 49, 482–496. [Google Scholar] [CrossRef]
- Loutradis, C.; Sarafidis, P.A.; Ferro, C.J.; Zoccali, C. Volume overload in hemodialysis: Diagnosis, cardiovascular consequences, and management. Nephrol. Dial. Transplant. 2021, 36, 2182–2193. [Google Scholar] [CrossRef]
- Jaeger, J.Q.; Mehta, R.L. Assessment of dry weight in hemodialysis: An overview. J. Am. Soc. Nephrol. 1999, 10, 392–403. [Google Scholar] [CrossRef]
- Flythe, J.E.; Chang, T.I.; Gallagher, M.P.; Lindley, E.; Madero, M.; Sarafidis, P.A.; Unruh, M.L.; Wang, A.Y.; Weiner, D.E.; Cheung, M.; et al. Blood pressure and volume management in dialysis: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2020, 97, 861–876. [Google Scholar] [CrossRef]
- Agarwal, R.; Andersen, M.J.; Pratt, J.H. On the importance of pedal edema in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2008, 3, 153–158. [Google Scholar] [CrossRef]
- Badgett, R.G.; Lucey, C.R.; Mulrow, C.D. Can the clinical examination diagnose left-sided heart failure in adults? JAMA 1997, 277, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.; Abernethy, W.B., 3rd; Simel, D.L. The rational clinical examination. Is this patient hypovolemic? JAMA 1999, 281, 1022–1029. [Google Scholar] [CrossRef]
- Beaubien-Souligny, W.; Kontar, L.; Blum, D.; Bouchard, J.; Denault, A.Y.; Wald, R. Meta-Analysis of Randomized Controlled Trials Using Tool-Assisted Target Weight Adjustments in Chronic Dialysis Patients. Kidney Int. Rep. 2019, 4, 1426–1434. [Google Scholar] [CrossRef]
- Koratala, A.; Argaiz, E.R.; Romero-Gonzalez, G.; Reisinger, N.; Anwar, S.; Beaubien-Souligny, W.; Bhasin-Chhabra, B.; Diniz, H.; Vaca Gallardo, M.; Graterol Torres, F.; et al. Point-of-care ultrasound training in nephrology: A position statement by the International Alliance for POCUS in Nephrology. Clin. Kidney J. 2024, 17, sfae245. [Google Scholar] [CrossRef]
- Al-Saray, M.Z.; Ali, A. Lung Ultrasound and Caval Indices to Assess Volume Status in Maintenance Hemodialysis Patients. POCUS J. 2023, 8, 52–59. [Google Scholar] [CrossRef]
- Beaubien-Souligny, W.; Trott, T.; Neyra, J.A. How to Determine Fluid Management Goals during Continuous Kidney Replacement Therapy in Patients with AKI: Focus on POCUS. Kidney360 2022, 3, 1795–1806. [Google Scholar] [CrossRef]
- Loutradis, C.; Papadopoulos, C.E.; Sachpekidis, V.; Ekart, R.; Krunic, B.; Karpetas, A.; Bikos, A.; Tsouchnikas, I.; Mitsopoulos, E.; Papagianni, A.; et al. Lung Ultrasound-Guided Dry Weight Assessment and Echocardiographic Measures in Hypertensive Hemodialysis Patients: A Randomized Controlled Study. Am. J. Kidney Dis. 2020, 75, 11–20. [Google Scholar] [CrossRef]
- Zheng, D.; Liu, Y.; Li, Y.; Jin, J.; He, Q.; Shen, X. Lung Ultrasound and Bioelectrical Impedance Analysis for Fluid Status Assessing Patients Undergoing Maintenance Hemodialysis. Int. J. Clin. Pract. 2024, 2024, 1232211. [Google Scholar] [CrossRef]
- Horowitz, L.; Karadjian, O.; Braam, B.; Mavrakanas, T.; Weber, C. Bioimpedance-Guided Monitoring of Volume Status in Patients With Kidney Disease: A Systematic Review and Meta-Analysis. Can. J. Kidney Health Dis. 2023, 10, 20543581231185433. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Pan, S.; Yang, N.; Wu, J.; Liu, Y.; He, Q. Effect of bioelectrical impedance technology on the prognosis of dialysis patients: A meta-analysis of randomized controlled trials. Ren. Fail. 2023, 45, 2203247. [Google Scholar] [CrossRef] [PubMed]
- Onofriescu, M.; Mardare, N.G.; Segall, L.; Voroneanu, L.; Cuşai, C.; Hogaş, S.; Ardeleanu, S.; Nistor, I.; Prisadă, O.V.; Sascău, R.; et al. Randomized trial of bioelectrical impedance analysis versus clinical criteria for guiding ultrafiltration in hemodialysis patients: Effects on blood pressure, hydration status, and arterial stiffness. Int. Urol. Nephrol. 2012, 44, 583–591. [Google Scholar] [CrossRef]
- Chen, H.S.; Lee, K.C.; Cheng, C.T.; Hou, C.C.; Liou, H.H.; Lin, C.J.; Lim, P.S. Application of Bioimpedance Spectroscopy in Asian Dialysis Patients (ABISAD): Serial follow-up and dry weight evaluation. Clin. Kidney J. 2013, 6, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Johner, C.; Chamney, P.W.; Schneditz, D.; Krämer, M. Evaluation of an ultrasonic blood volume monitor. Nephrol. Dial. Transplant. 1998, 13, 2098–2103. [Google Scholar] [CrossRef] [PubMed]
- Dasselaar, J.J.; Huisman, R.M.; PE, D.E.J.; Franssen, C.F. Relative blood volume measurements during hemodialysis: Comparisons between three noninvasive devices. Hemodial. Int. 2007, 11, 448–455. [Google Scholar] [CrossRef]
- Leung, K.C.; Quinn, R.R.; Ravani, P.; MacRae, J.M. Ultrafiltration biofeedback guided by blood volume monitoring to reduce intradialytic hypotensive episodes in hemodialysis: Study protocol for a randomized controlled trial. Trials 2014, 15, 483. [Google Scholar] [CrossRef]
- Dasselaar, J.J.; Huisman, R.M.; de Jong, P.E.; Franssen, C.F. Measurement of relative blood volume changes during haemodialysis: Merits and limitations. Nephrol. Dial. Transplant. 2005, 20, 2043–2049. [Google Scholar] [CrossRef]
- Dasselaar, J.J.; van der Sande, F.M.; Franssen, C.F. Critical evaluation of blood volume measurements during hemodialysis. Blood Purif. 2012, 33, 177–182. [Google Scholar] [CrossRef]
- Preciado, P.; Zhang, H.; Thijssen, S.; Kooman, J.P.; van der Sande, F.M.; Kotanko, P. All-cause mortality in relation to changes in relative blood volume during hemodialysis. Nephrol. Dial. Transplant. 2019, 34, 1401–1408. [Google Scholar] [CrossRef]
- Davies, S.J.; Davenport, A. The role of bioimpedance and biomarkers in helping to aid clinical decision-making of volume assessments in dialysis patients. Kidney Int. 2014, 86, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.J.; Coyle, D.; Lindley, E.J.; Keane, D.; Belcher, J.; Caskey, F.J.; Dasgupta, I.; Davenport, A.; Farrington, K.; Mitra, S.; et al. Bio-impedance spectroscopy added to a fluid management protocol does not improve preservation of residual kidney function in incident hemodialysis patients in a randomized controlled trial. Kidney Int. 2023, 104, 587–598. [Google Scholar] [CrossRef]
- Hur, E.; Usta, M.; Toz, H.; Asci, G.; Wabel, P.; Kahvecioglu, S.; Kayikcioglu, M.; Demirci, M.S.; Ozkahya, M.; Duman, S.; et al. Effect of fluid management guided by bioimpedance spectroscopy on cardiovascular parameters in hemodialysis patients: A randomized controlled trial. Am. J. Kidney Dis. 2013, 61, 957–965. [Google Scholar] [CrossRef]
- Onofriescu, M.; Hogas, S.; Voroneanu, L.; Apetrii, M.; Nistor, I.; Kanbay, M.; Covic, A.C. Bioimpedance-guided fluid management in maintenance hemodialysis: A pilot randomized controlled trial. Am. J. Kidney Dis. 2014, 64, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Bansal, A.; Perez, L.; Stenson, E.K.; Kendrick, J. Use of Relative Blood Volume Monitoring to Reduce Intradialytic Hypotension in Hospitalized Patients Receiving Dialysis. Kidney Int. Rep. 2022, 7, 2105–2107. [Google Scholar] [CrossRef] [PubMed]
- Dasselaar, J.J.; Lub-de Hooge, M.N.; Pruim, J.; Nijnuis, H.; Wiersum, A.; de Jong, P.E.; Huisman, R.M.; Franssen, C.F. Relative blood volume changes underestimate total blood volume changes during hemodialysis. Clin. J. Am. Soc. Nephrol. 2007, 2, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Raimann, J.G.; Zhu, F.; Wang, J.; Thijssen, S.; Kuhlmann, M.K.; Kotanko, P.; Levin, N.W.; Kaysen, G.A. Comparison of fluid volume estimates in chronic hemodialysis patients by bioimpedance, direct isotopic, and dilution methods. Kidney Int. 2014, 85, 898–908. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russwurm, M.; Braun, M.; Menne, J.; Ploeger, L.; Miran, M.; Max, F.; Dahmen, L.; Hoyer, J.; Wild, J. Linking Intradialytic Blood Volume Dynamics to Extracellular Fluid Status: Toward Personalized Fluid Assessment in Hemodialysis. J. Clin. Med. 2025, 14, 7188. https://doi.org/10.3390/jcm14207188
Russwurm M, Braun M, Menne J, Ploeger L, Miran M, Max F, Dahmen L, Hoyer J, Wild J. Linking Intradialytic Blood Volume Dynamics to Extracellular Fluid Status: Toward Personalized Fluid Assessment in Hemodialysis. Journal of Clinical Medicine. 2025; 14(20):7188. https://doi.org/10.3390/jcm14207188
Chicago/Turabian StyleRusswurm, Martin, Marvin Braun, Julia Menne, Lara Ploeger, Marc Miran, Fabian Max, Lotte Dahmen, Joachim Hoyer, and Johannes Wild. 2025. "Linking Intradialytic Blood Volume Dynamics to Extracellular Fluid Status: Toward Personalized Fluid Assessment in Hemodialysis" Journal of Clinical Medicine 14, no. 20: 7188. https://doi.org/10.3390/jcm14207188
APA StyleRusswurm, M., Braun, M., Menne, J., Ploeger, L., Miran, M., Max, F., Dahmen, L., Hoyer, J., & Wild, J. (2025). Linking Intradialytic Blood Volume Dynamics to Extracellular Fluid Status: Toward Personalized Fluid Assessment in Hemodialysis. Journal of Clinical Medicine, 14(20), 7188. https://doi.org/10.3390/jcm14207188






