Correlation Between Severity of Obstructive Sleep Apnea and Dental Arch Form in Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Sample Size and Study Population
2.2. Diagnostic Procedures
2.3. Data Collection and Measurements
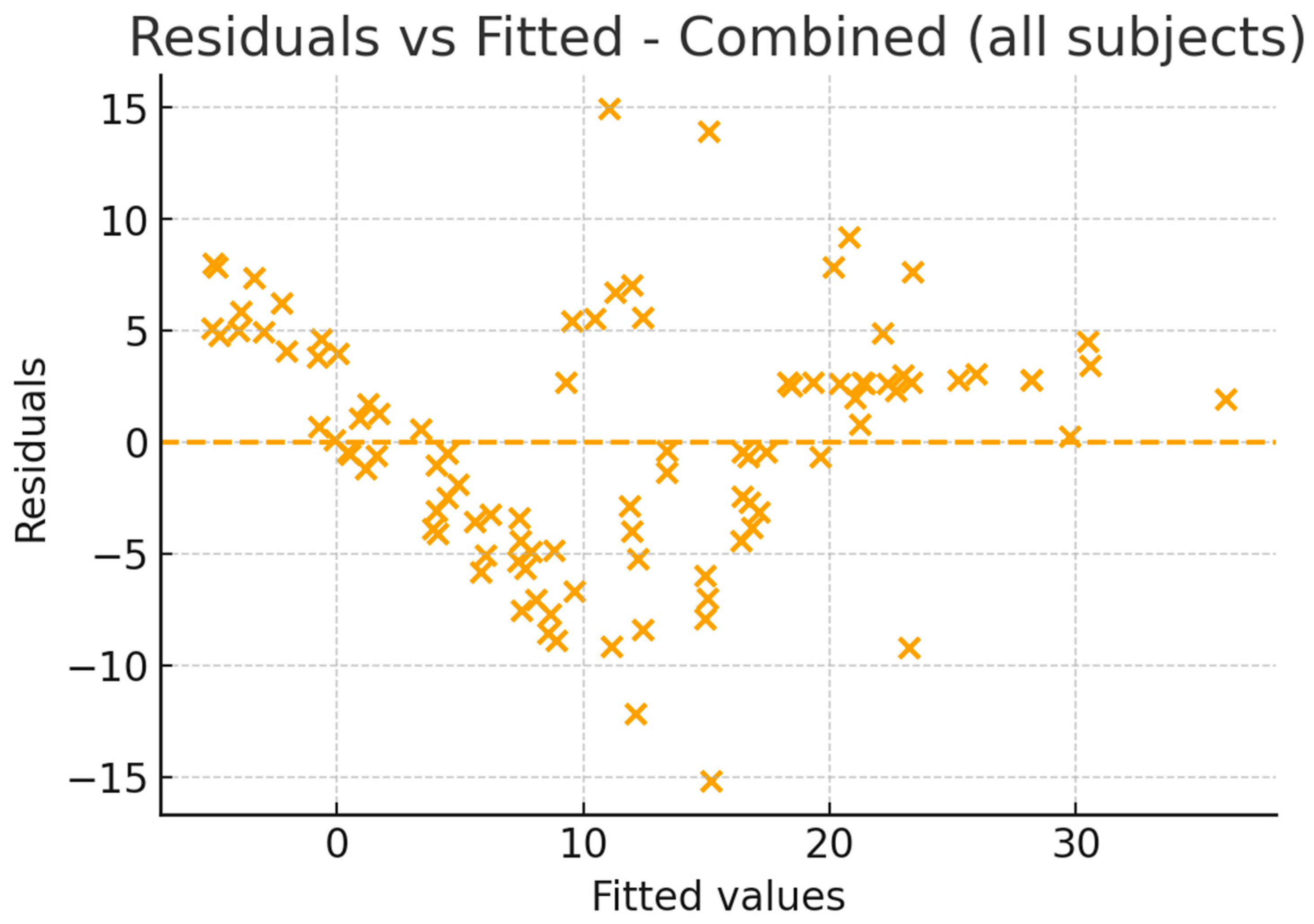
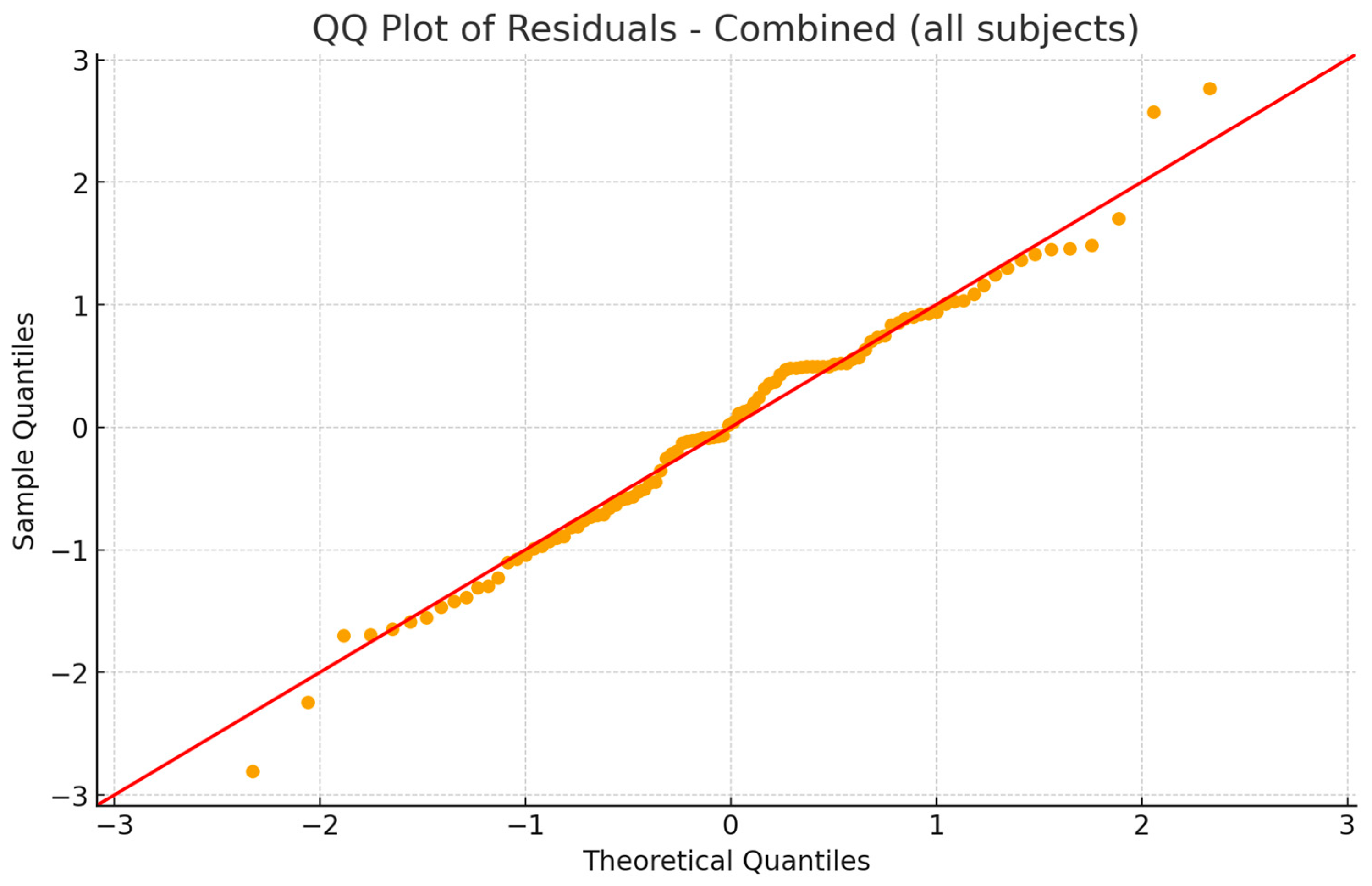
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Talib, T.; Chiang, H.; Pinto, A.; Moreno Hay, I.; Park, R.; Goodman, X.; Essick, G. Risk Factors for OSA Observed During Orofacial Examination: A Review. J. Dent. Sleep Med. 2024, 11. [Google Scholar] [CrossRef]
- Fagundes, N.C.F.; Gianoni-Capenakas, S.; Heo, G.; Flores-Mir, C. Craniofacial features in children with obstructive sleep apnea: A systematic review and meta-analysis. J. Clin. Sleep Med. 2022, 18, 1865–1875. [Google Scholar] [CrossRef] [PubMed]
- Celle, S.; Annweiler, C.; Camicioli, R.; Barthélémy, J.C.; Roche, F.; Beauchet, O. Sleep-related breathing disorders and gait variability: A cross-sectional preliminary study. BMC Pulm. Med. 2014, 14, 140. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H. Implications of Obstructive Sleep-related Breathing Disorder in Dentistry: Focus on Snoring and Obstructive Sleep Apnea. Dent. Res. Oral Health 2022, 5, 74. [Google Scholar] [CrossRef] [PubMed]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef]
- Franklin, K.A.; Lindberg, E. Obstructive sleep apnea is a common disorder in the population—A review on the epidemiology of sleep apnea. J. Thorac. Dis. 2015, 7, 1311. [Google Scholar] [CrossRef]
- Zasadzińska-Stempniak, K.; Zajączkiewicz, H.; Kukwa, A. Clinical Medicine Prevalence of Obstructive Sleep Apnea in the Young Adult Population: A Systematic Review. J. Clin. Med. 2024, 13, 1386. [Google Scholar] [CrossRef]
- Eckert, D.J.; Malhotra, A. Pathophysiology of adult obstructive sleep apnea. Proc. Am. Thorac. Soc. 2008, 5, 144–153. [Google Scholar] [CrossRef]
- Yeghiazarians, Y.; Jneid, H.; Tietjens, J.R.; Redline, S.; Brown, D.L.; El-Sherif, N.; Mehra, R.; Bozkurt, B.; Ndumele, C.E.; Somers, V.K. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 144, E56–E67. [Google Scholar] [CrossRef]
- Lal, C.; Varga, A.W.; Ayappa, I.; Ayas, N.; Beaudin, A.E.; Hoyos, C.; Kushida, C.A.; Kaminska, M.; Mullins, A.; Naismith, S.L.; et al. The Link between Obstructive Sleep Apnea and Neurocognitive Impairment: An Official American Thoracic Society Workshop Report. Ann. Am. Thorac. Soc. 2022, 19, 1245–1256. [Google Scholar] [CrossRef]
- Sutherland, K.; Lee, R.W.W.; Cistulli, P.A. Obesity and craniofacial structure as risk factors for obstructive sleep apnoea: Impact of ethnicity. Respirology 2012, 17, 213–222. [Google Scholar] [CrossRef]
- Capistrano, A.; Cordeiro, A.; Filho, L.C.; Almeida, V.C.; de Castro e Silva, P.I.; Martinez, S.; Rodrigues de Almeida-Pedrin, R. Facial morphology and obstructive sleep apnea. Dent. Press. J. Orthod. 2015, 20, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wang, Z.; Chang, H.; Zhu, S.; Zhou, Y.; Cao, Z.; Ma, L.; Yuan, Y.; Xie, Y.; Niu, X.; et al. Craniofacial Development Characteristics in Children with Obstructive Sleep Apnea for Establishment and External Validation of the Prediction Model. Nat. Sci. Sleep 2024, 16, 2151–2170. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, N.D.; Algowaifly, M.I.; Almehizia, F.A.; Alraddadi, Z.A.; Al-Sehaibany, F.S.; Almosa, N.A.; Albarakati, S.F.; Bahammam, A.S. The characteristics of dental occlusion in patients with moderate to severe obstructive sleep apnea in Saudi Arabia. Saudi Med. J. 2018, 39, 928. [Google Scholar] [CrossRef]
- Ippolito, D.R.; Parenti, S.I.; Laffranchi, L.; Paganelli, C.; Alessandri-Bonetti, G. Is There a Relationship Between Obstructive Sleep Apnea Severity and Dental Arch Form in Adult Patients? An Observational Retrospective Study. J. Dent. Sleep Med. 2022, 9. [Google Scholar] [CrossRef]
- Ciavarella, D.; Campobasso, A.; Conte, E.; Burlon, G.; Guida, L.; Montaruli, G.; Cassano, M.; Laurenziello, M.; Illuzzi, G.; Tepedino, M. Correlation between dental arch form and OSA severity in adult patients: An observational study. Prog. Orthod. 2023, 24, 19. [Google Scholar] [CrossRef] [PubMed]
- Ciavarella, D.; Ferrara, D.; Spinoso, G.; Cattaneo, P.; Leo, C.; Russo, L.L.; Burlon, G.; Burlon, C.; Esperouz, F.; Laurenziello, M.; et al. Airway Analysis and Morphometric Assessment of Dental Arches in Obstructive Sleep Apnea Patients. J. Clin. Med. 2025, 14, 296. [Google Scholar] [CrossRef]
- Irlandese, G.; De Stefani, A.; Mezzofranco, L.; Milano, F.; Di Giosia, M.; Bruno, G.; Gracco, A. Dental arch form and interdental widths evaluation in adult Caucasian patients with obstructive sleep apnea syndrome. Cranio 2023, 41, 151–159. [Google Scholar] [CrossRef]
- Wei, Z.; Zhao, T.; Li, Y.; Ngan, P.; Wang, Z.; Hua, F.; He, H. The dentofacial and upper airway morphology of adults with obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med. Rev. 2025, 80, 102065. [Google Scholar] [CrossRef]
- Goyal, M.; Johnson, J. Obstructive Sleep Apnea Diagnosis and Management. Mo. Med. 2017, 114, 120. [Google Scholar]
- Johal, A.; Conaghan, C. Maxillary morphology in obstructive sleep apnea: A cephalometric and model study. Angle Orthod. 2004, 74, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Kecik, D. Three-dimensional analyses of palatal morphology and its relation to upper airway area in obstructive sleep apnea. Angle Orthod. 2017, 87, 300–306. [Google Scholar] [CrossRef]
- Kang, J.H.; Kim, H.J.; Song, S.I. Obstructive sleep apnea and anatomical structures of the nasomaxillary complex in adolescents. PLoS ONE 2022, 17, e0272262. [Google Scholar] [CrossRef]
- Cistulli, P.A.; Richards, G.N.; Palmisano, R.G.; Unger, G.; Berthon-Jones, M.; Sullivan, C.E. Influence of maxillary constriction on nasal resistance and sleep apnea severity in patients with Marfan’s syndrome. Chest 1996, 110, 1184–1188. [Google Scholar] [CrossRef]
- Seto, B.H.; Gotsopoulos, H.; Sims, M.R.; Cistulli, P.A. Maxillary morphology in obstructive sleep apnoea syndrome. Eur. J. Orthod. 2001, 23, 703–714. [Google Scholar] [CrossRef]
- Nainan, O.; Jayan, B.; Mitra, R.; Ghosh, S.; Chopra, S.S.; Mukherjee, M. Commanding Officer, Military Dental Centre. Classif. Spec.-Periodontol. 2017, 19, 30–37. [Google Scholar] [CrossRef]
- Nada, R.M.; Van Loon, B.; Schols, J.G.J.H.; Maal, T.J.J.; de Koning, M.J.; Mostafa, Y.A.; Kuijpers-Jagtman, A.M. Volumetric changes of the nose and nasal airway 2 years after tooth-borne and bone-borne surgically assisted rapid maxillary expansion. Eur. J. Oral Sci. 2013, 121, 450–456. [Google Scholar] [CrossRef]
- Fastuca, R.; Lorusso, P.; Lagravère, M.O.; Michelotti, A.; Portelli, M.; Zecca, P.A.; D’Antò, V.; Militi, A.; Nucera, R.; Caprioglio, A. Digital evaluation of nasal changes induced by rapid maxillary expansion with different anchorage and appliance design. BMC Oral Health 2017, 17, 113. [Google Scholar] [CrossRef]
- Chang, Y.; Koenig, L.J.; Pruszynski, J.E.; Bradley, T.G.; Bosio, J.A.; Liu, D. Dimensional changes of upper airway after rapid maxillary expansion: A prospective cone-beam computed tomography study. Am. J. Orthod. Dentofac. Orthop. 2013, 143, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Vinha, P.P.; Faria, A.C.; Xavier, S.P.; Christino, M.; De Mello-Filho, F.V. Enlargement of the Pharynx Resulting from Surgically Assisted Rapid Maxillary Expansion. J. Oral Maxillofac. Surg. 2016, 74, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Villa, M.P.; Rizzoli, A.; Rabasco, J.; Vitelli, O.; Pietropaoli, N.; Cecili, M.; Marino, A.; Malagola, C. Rapid maxillary expansion outcomes in treatment of obstructive sleep apnea in children. Sleep Med. 2015, 16, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Fastuca, R.; Perinetti, G.; Zecca, P.A.; Nucera, R.; Caprioglio, A. Airway compartments volume and oxygen saturation changes after rapid maxillary expansion: A longitudinal correlation study. Angle Orthod. 2015, 85, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Akay, M.C.; Aras, I.; Günbay, T.; Aras, A. Does transpalatal distraction affect pharyngeal airway dimensions and related soft tissues? J. Oral Maxillofac. Surg. 2014, 72, 1559–1564. [Google Scholar] [CrossRef] [PubMed]

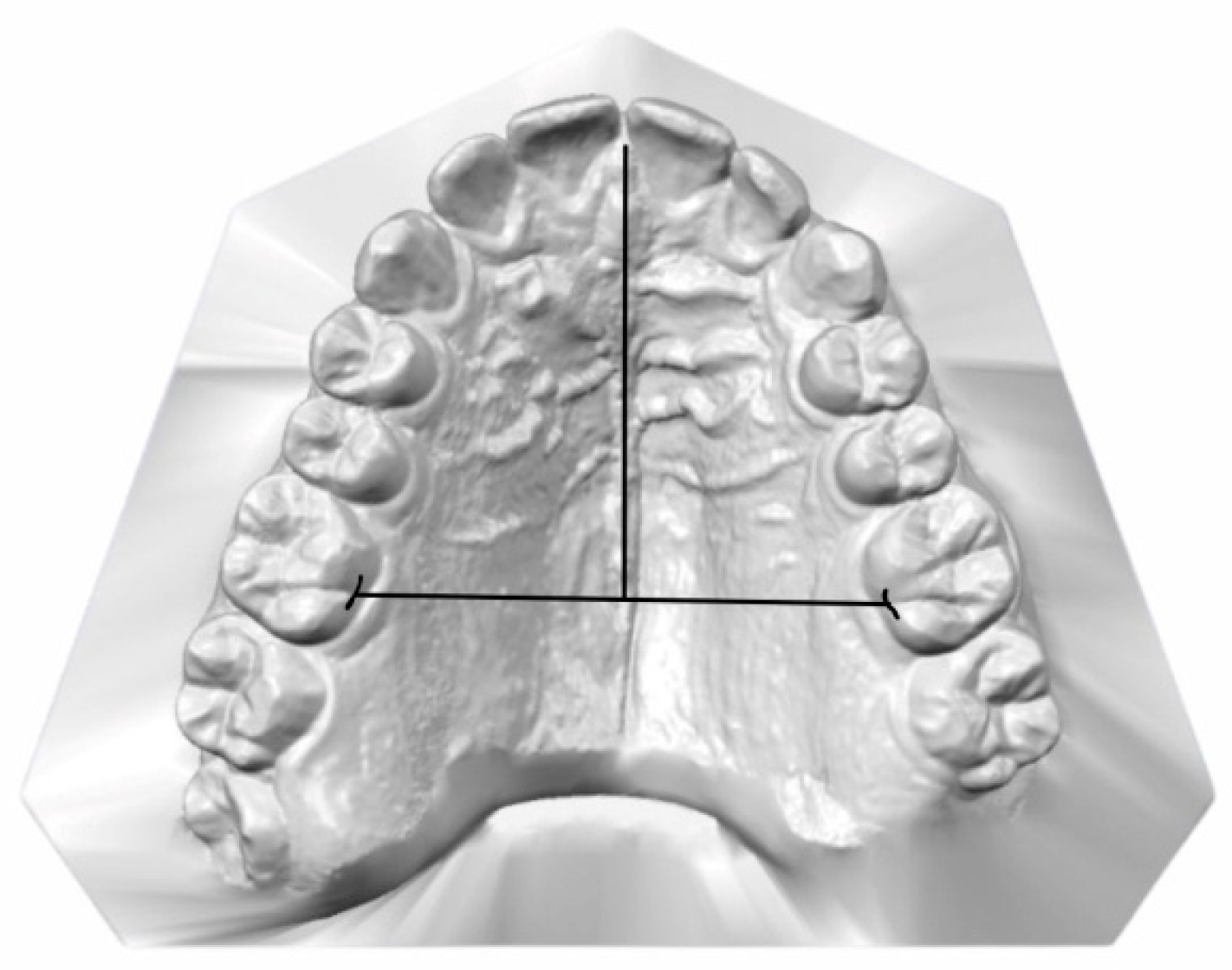
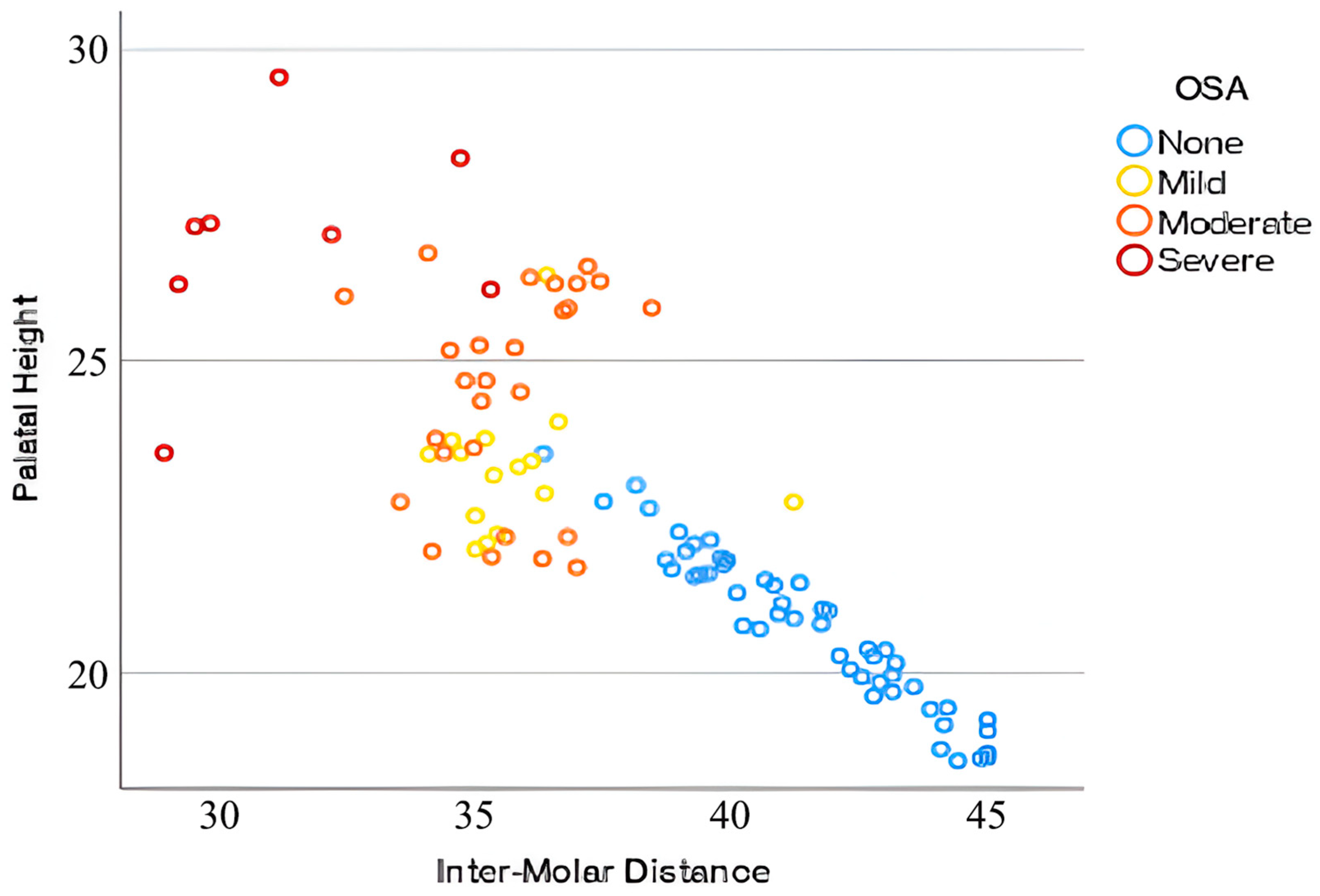
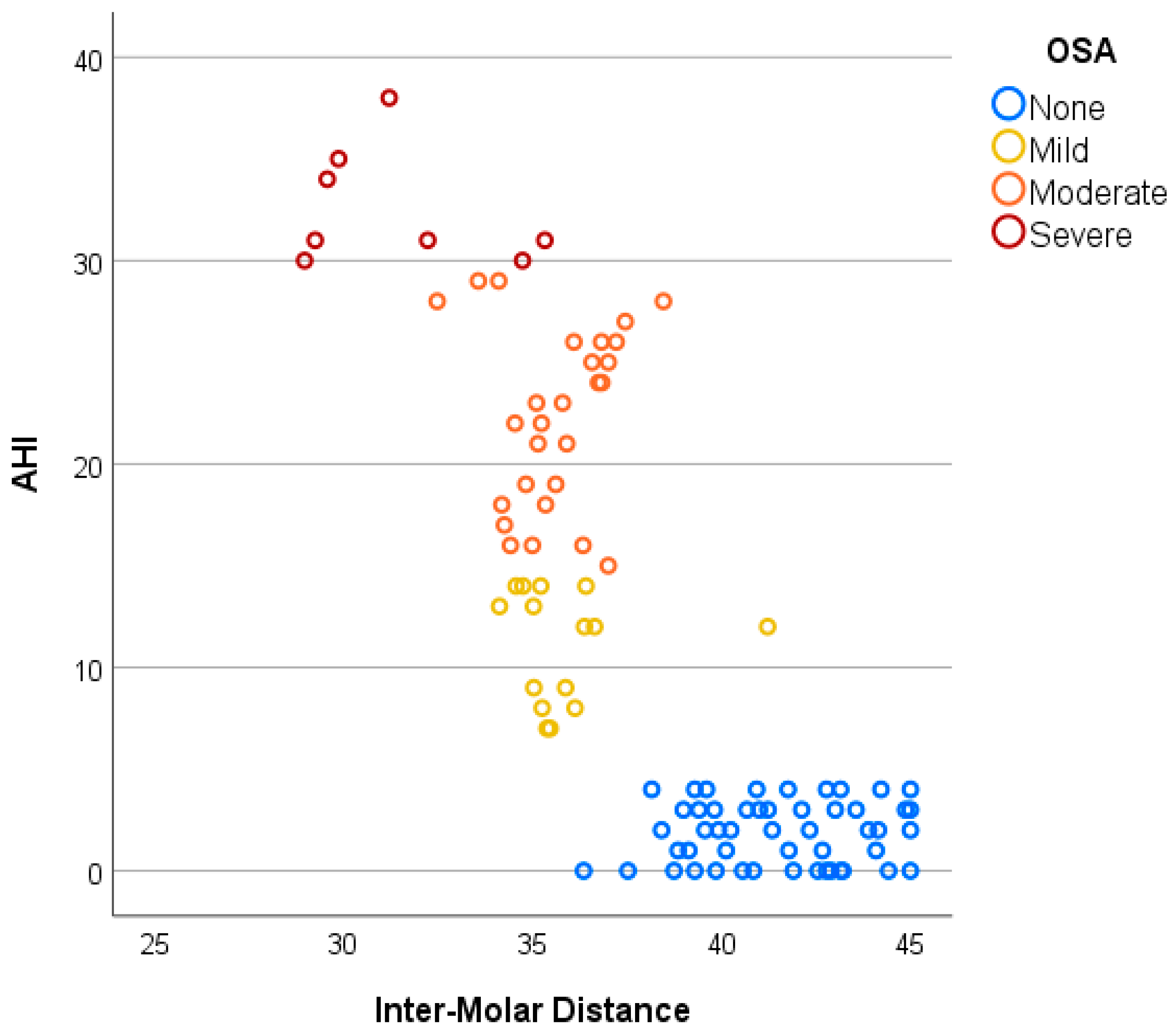
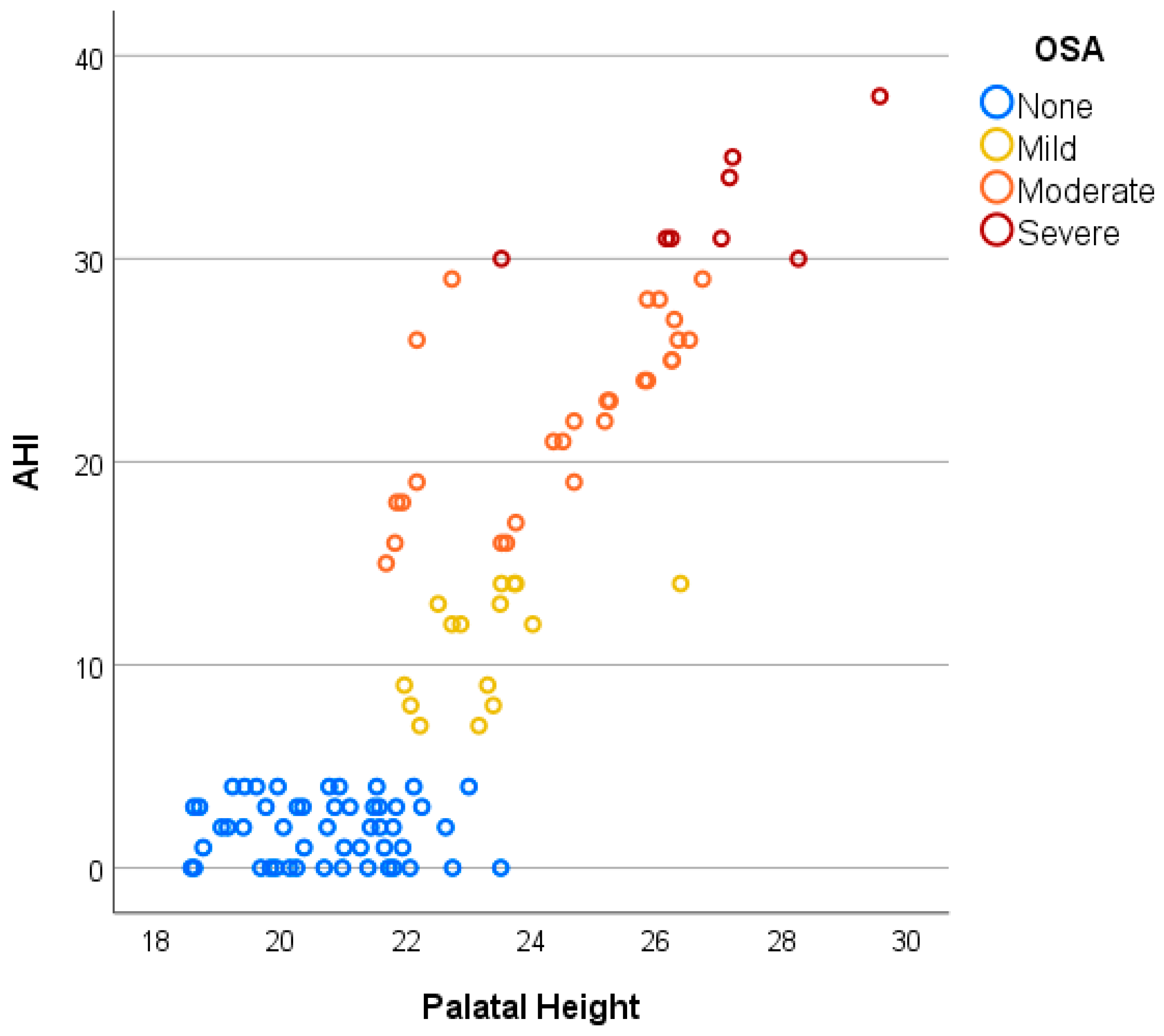


| Groups | Total | OSA Severity | F | M | Age (Years) Mean (SD) | IMD (mm) Mean (SD) | PH (mm) Mean (SD) |
|---|---|---|---|---|---|---|---|
| Control | 50 | 22 | 28 | 41.14 (3.12) | 41.50 (2.37) | 20.72 (1.25) | |
| OSA | 50 | 24 | 26 | 40.77 (3.23) | 35.01 (2.29) | 24.50 (1.93) | |
| Mild | 8 | 7 | 35.83 (1.66) | 23.74 (1.07) | |||
| Moderate | 15 | 12 | 35.62 (1.37) | 24.48 (1.71) | |||
| Severe | 1 | 7 | 31.39 (2.49) | 26.89 (1.75) |
| Model | R | R2 | Adjusted R2 | Std. Error of Estimate |
|---|---|---|---|---|
| 1 | 0.871 | 0.759 | 0.754 | 5.489 |
| Source | Sum of Squares | df | Mean Square | F | Sig. |
|---|---|---|---|---|---|
| Regression | 9192.87 | 2 | 4596.44 | 152.57 | <0.001 |
| Residual | 2922.29 | 97 | 30.13 | ||
| Total | 12115.16 | 99 |
| Predictor | B | Std. Error | β (Beta) | t | p-Value | 95% CI (Lower–Upper) |
|---|---|---|---|---|---|---|
| (Constant) | −23.433 | 17.564 | — | −1.334 | 0.185 | −58.29 to 11.43 |
| IMD | −0.755 | 0.248 | −0.271 | −3.049 | 0.003 | −1.25 to −0.26 |
| PH | 2.810 | 0.393 | 0.634 | 7.145 | <0.001 | 2.03 to 3.59 |
| Model | R | R2 | Adjusted R2 | Std. Error of Estimate |
|---|---|---|---|---|
| 2 | 0.872 | 0.760 | 0.750 | 5.535 |
| Source | Sum of Squares | df | Mean Square | F | Sig. |
|---|---|---|---|---|---|
| Regression | 9204.21 | 4 | 2301.05 | 75.10 | <0.001 |
| Residual | 2910.95 | 95 | 30.64 | ||
| Total | 12,115.16 | 99 |
| Predictor | B | Std. Error | β (Beta) | t | p-Value | 95% CI (Lower–Upper) |
|---|---|---|---|---|---|---|
| (Constant) | −20.752 | 19.177 | — | −1.082 | 0.282 | −58.82 to 17.32 |
| IMD | −0.745 | 0.250 | −0.267 | −2.977 | 0.004 | −1.24 to −0.25 |
| PH | 2.825 | 0.398 | 0.637 | 7.102 | <0.001 | 2.04 to 3.62 |
| Age | −0.077 | 0.190 | −0.020 | −0.405 | 0.687 | −0.45 to 0.30 |
| Sex | −0.497 | 1.114 | −0.022 | −0.446 | 0.657 | −2.71 to 1.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahony, D.; Harding, S.; Chowdhury, C.R.; Jamilian, A.; Fetrati, A.; Bhattarai, N.; Borbély, P.; Kárpáti, K. Correlation Between Severity of Obstructive Sleep Apnea and Dental Arch Form in Adults. J. Clin. Med. 2025, 14, 7183. https://doi.org/10.3390/jcm14207183
Mahony D, Harding S, Chowdhury CR, Jamilian A, Fetrati A, Bhattarai N, Borbély P, Kárpáti K. Correlation Between Severity of Obstructive Sleep Apnea and Dental Arch Form in Adults. Journal of Clinical Medicine. 2025; 14(20):7183. https://doi.org/10.3390/jcm14207183
Chicago/Turabian StyleMahony, Derek, Stewart Harding, Chitta Ranjan Chowdhury, Abdolreza Jamilian, Asal Fetrati, Niroj Bhattarai, Peter Borbély, and Krisztina Kárpáti. 2025. "Correlation Between Severity of Obstructive Sleep Apnea and Dental Arch Form in Adults" Journal of Clinical Medicine 14, no. 20: 7183. https://doi.org/10.3390/jcm14207183
APA StyleMahony, D., Harding, S., Chowdhury, C. R., Jamilian, A., Fetrati, A., Bhattarai, N., Borbély, P., & Kárpáti, K. (2025). Correlation Between Severity of Obstructive Sleep Apnea and Dental Arch Form in Adults. Journal of Clinical Medicine, 14(20), 7183. https://doi.org/10.3390/jcm14207183







