Scaffold-Free Bone Regeneration Through Collaboration Between Type IV Collagen and FBXL14
Abstract
1. Introduction
2. Materials and Methods
3. Results
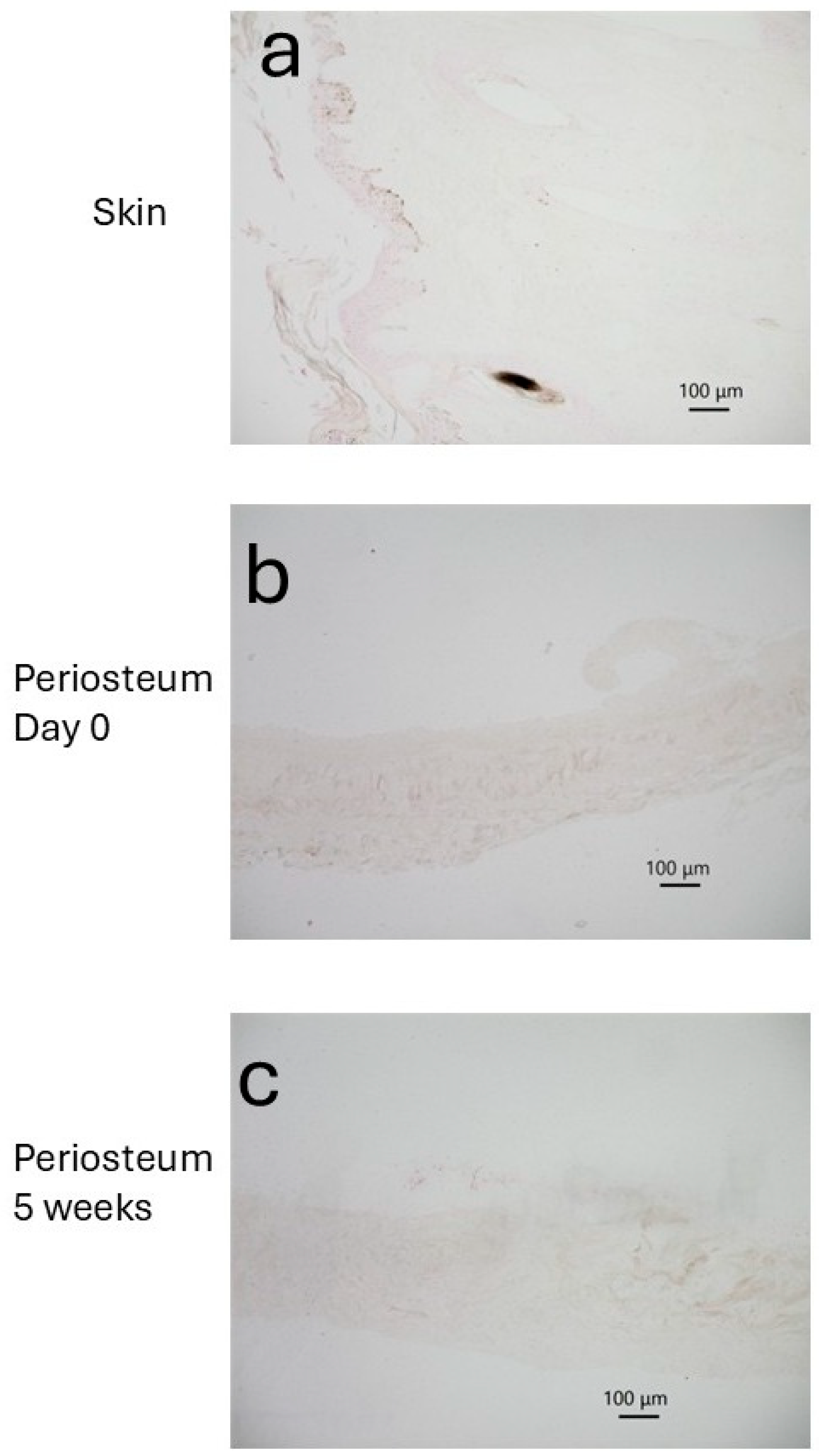
3.1. Skin
3.2. Bone and Periosteum
3.3. Periosteum Explant Culture on Day 0
3.4. Periosteum Explant Culture at 5 Weeks
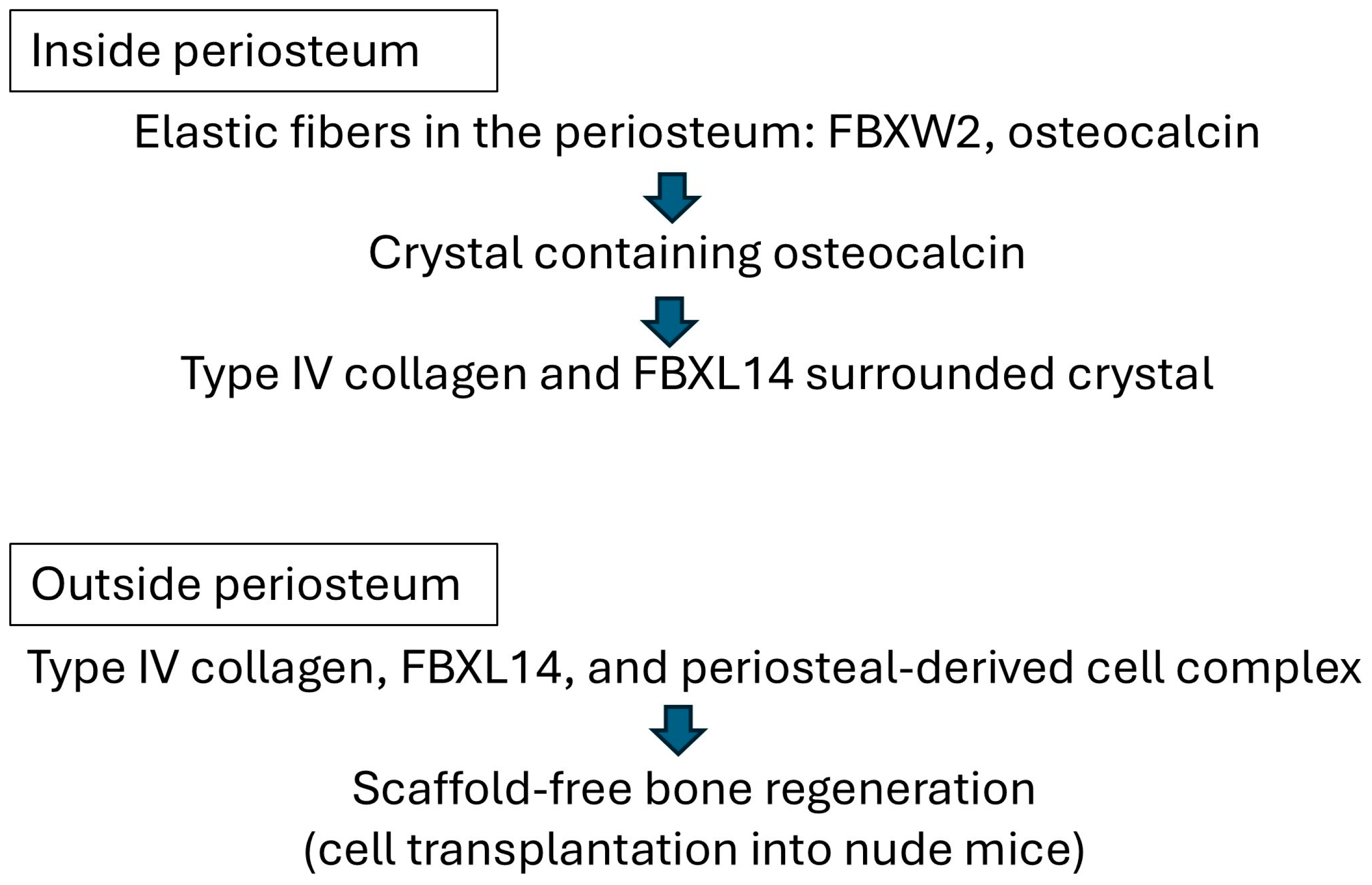
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MS | mass spectrometry |
| ESI-Q-Orbitrap MS | electrospray ionization quadrupole Orbitrap mass spectrometry |
| FBXL14 | F-box/leucine-rich repeat protein 14 |
| ESI-Q-TOF | electrospray ionization quadrupole time-of-flight |
| ECM | extracellular matrix |
| PDC | periosteum-derived cell |
References
- Lin, Z.; Fateh, A.; Salem, D.M.; Intini, G. Periosteum: Biology and applications in craniofacial bone regeneration. J. Dent. Res. 2014, 93, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Deng, C.; Qi, S. Periosteum Containing Implicit Stem Cells: A Progressive Source of Inspiration for Bone Tissue Regeneration. Int. J. Mol. Sci. 2024, 25, 2162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, N.; Yang, M.; Sun, T.; Zhang, J.; Zhao, Y.; Huo, N.; Li, Z. Periosteum and development of the tissue-engineered periosteum for guided bone regeneration. J. Orthop. Transl. 2022, 33, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Li, W.; Li, H.; Zhang, J.; Hou, Z.; Wang, X.; Zhou, E.; Lu, K.; Zhuang, W.; Sang, H. A self-forming bone membrane generated by periosteum-derived stem cell spheroids enhances the repair of bone defects. Acta Biomater. 2025, 193, 185–201. [Google Scholar] [CrossRef]
- Ringe, J.; Leinhase, I.; Stich, S.; Loch, A.; Neumann, K.; Haisch, A.; Häupl, T.; Manz, R.; Kaps, C.; Sittinger, M. Human mastoid periosteum-derived stem cells: Promising candidates for skeletal tissue engineering. J. Tissue Eng. Regen. Med. 2008, 2, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Danalache, M.; Kliesch, S.M.; Munz, M.; Naros, A.; Reinert, S.; Alexander, D. Quality Analysis of Minerals Formed by Jaw Periosteal Cells under Different Culture Conditions. Int. J. Mol. Sci. 2019, 20, 4193. [Google Scholar] [CrossRef]
- Uematsu, K.; Ushiki, T.; Ishiguro, H.; Ohashi, R.; Tamura, S.; Watanabe, M.; Fujimoto, Y.; Nagata, M.; Ajioka, Y.; Kawase, T. Osteoclastogenic Potential of Tissue-Engineered Periosteal Sheet: Effects of Culture Media on the Ability to Recruit Osteoclast Precursors. Int. J. Mol. Sci. 2021, 22, 2169. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, H.Y.; Yang, C.Y.; Liu, J.W.; Brey, E.M.; Cheng, M.H. Periosteal Osteogenic Capacity Depends on Tissue Source. Tissue Eng. Part A 2018, 24, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Alblawi, A.; Ranjani, A.S.; Yasmin, H.; Gupta, S.; Bit, A.; Rahimi-Gorji, M. Scaffold-free: A developing technique in field of tissue engineering. Comput. Methods Programs Biomed. 2020, 185, 105148. [Google Scholar] [CrossRef] [PubMed]
- Takeoka, Y.; Matsumoto, K.; Taniguchi, D.; Tsuchiya, T.; Machino, R.; Moriyama, M.; Oyama, S.; Tetsuo, T.; Taura, Y.; Takagi, K.; et al. Regeneration of esophagus using a scaffold-free biomimetic structure created with bio-three-dimensional printing. PLoS ONE 2019, 14, e0211339. [Google Scholar] [CrossRef]
- Akiyama, M.; Nonomura, H.; Kamil, S.H.; Ignotz, R.A. Periosteal cell pellet culture system: A new technique for bone engineering. Cell Transplant. 2006, 15, 521–532. [Google Scholar] [CrossRef]
- Akiyama, M.; Nakamura, M. Bone regeneration and neovascularization processes in a pellet culture system for periosteal cells. Cell Transplant. 2009, 18, 443–452. [Google Scholar] [CrossRef]
- Akiyama, M. Characterization of the F-box Proteins FBXW2 and FBXL14 in the Initiation of Bone Regeneration in Transplants given to Nude Mice. Open Biomed. Eng. J. 2018, 12, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.J.; Yun, C.Y.; Lee, J.C.; Kim, J.R.; Park, B.H.; Cho, E.S. Maturation of cortical bone suppresses periosteal osteoprogenitor proliferation in a paracrine manner. J. Mol. Histol. 2016, 47, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Adams, D.; Hagiwara, Y.; Yoshida, R.; Kamimura, M.; Itoi, E.; Rowe, D.W. Identification of a progenitor cell population destined to form fracture fibrocartilage callus in Dickkopf-related protein 3-green fluorescent protein reporter mice. J. Bone Miner. Metab. 2016, 34, 606–614. [Google Scholar] [CrossRef]
- Scanlon, V.; Walia, B.; Yu, J.; Hansen, M.; Drissi, H.; Maye, P.; Sanjay, A. Loss of Cbl-PI3K interaction modulates the periosteal response to fracture by enhancing osteogenic commitment and differentiation. Bone 2017, 95, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M. Identification of UACA, EXOSC9, and ΤΜX2 in bovine periosteal cells by mass spectrometry and immunohistochemistry. Anal. Bioanal. Chem. 2014, 406, 5805–5813. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M. Roles of Two F-Box Proteins: FBXL14 in the Periosteum and FBXW2 at Elastic Fibers. Osteology 2023, 3, 1–10. [Google Scholar] [CrossRef]
- Díaz, V.M.; de Herreros, A.G. F-box proteins: Keeping the epithelial-to-mesenchymal transition (EMT) in check. Semin. Cancer Biol. 2016, 36, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Chen, Y.; Fang, C.; Shi, H.; Luo, F. FBXL14 inhibits foam cell formation and atherosclerosis plaque progression by activating the NRF2 signal axis through ubiquitination of DUSP6. J. Recept. Signal Transduct. Res. 2025, 45, 107–117. [Google Scholar] [CrossRef]
- Lu, L.; Tian, L. Postmenopausal osteoporosis coexisting with sarcopenia: The role and mechanisms of estrogen. J. Endocrinol. 2023, 259, e230116. [Google Scholar] [CrossRef]
- Fusaro, M.; Cianciolo, G.; Brandi, M.L.; Ferrari, S.; Nickolas, T.L.; Tripepi, G.; Plebani, M.; Zaninotto, M.; Iervasi, G.; La Manna, G.; et al. Vitamin K and Osteoporosis. Nutrients 2020, 12, 3625. [Google Scholar] [CrossRef]
- Chi, P.J.; Hung, S.Y.; Hsiao, F.T.; Liou, H.H.; Tsai, J.P. Serum osteocalcin concentration as an independent biomarker of osteoporosis in patients with chronic kidney disease. Clin. Nephrol. 2022, 98, 1–9. [Google Scholar] [CrossRef]
- Martiniakova, M.; Biro, R.; Kovacova, V.; Babikova, M.; Zemanova, N.; Mondockova, V.; Omelka, R. Current knowledge of bone-derived factor osteocalcin: Its role in the management and treatment of diabetes mellitus, osteoporosis, osteopetrosis and inflammatory joint diseases. J. Mol. Med. 2024, 102, 435–452. [Google Scholar] [CrossRef]
- Zoch, M.L.; Clemens, T.L.; Riddle, R.C. New insights into the biology of osteocalcin. Bone 2016, 82, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M. FBXW2 localizes with osteocalcin in bovine periosteum on culture dishes as visualized by double immunostaining. Heliyon 2018, 4, e00782. [Google Scholar] [CrossRef]
- Akiyama, M. Role of FBXW2 in explant cultures of bovine periosteum-derived cells. BMC Res. Notes 2021, 14, 410. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M. Dataset for proteomic analysis of secreted proteins from bovine periosteum and blood vessels with explant culture. J. Proteome Data Methods 2024, 6, 23. [Google Scholar] [CrossRef]
- Chen, P.; Cescon, M.; Bonaldo, P. The Role of Collagens in Peripheral Nerve Myelination and Function. Mol. Neurobiol. 2015, 52, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Harvey, S.J.; Thorner, P.S. Type IV collagen: A network for development, differentiation, and disease. Adv. Dev. Biol. 2005, 15, 1–64. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Y.; Chen, J.; Peng, J. The role of peripheral nerve ECM components in the tissue engineering nerve construction. Rev. Neurosci. 2013, 24, 443–453. [Google Scholar] [CrossRef]
- Urabe, N.; Naito, I.; Saito, K.; Yonezawa, T.; Sado, Y.; Yoshioka, H.; Kusachi, S.; Tsuji, T.; Ohtsuka, A.; Taguchi, T.; et al. Basement membrane type IV collagen molecules in the choroid plexus, pia mater and capillaries in the mouse brain. Arch. Histol. Cytol. 2002, 65, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Boudko, S.P.; Danylevych, N.; Hudson, B.G.; Pedchenko, V.K. Basement membrane collagen IV: Isolation of functional domains. Methods Cell Biol. 2018, 143, 171–185. [Google Scholar] [CrossRef]
- Kashtan, C.E.; Ding, J.; Garosi, G.; Heidet, L.; Massella, L.; Nakanishi, K.; Nozu, K.; Renieri, A.; Rheault, M.; Wang, F.; et al. Alport syndrome: A unified classification of genetic disorders of collagen IV α345: A position paper of the Alport Syndrome Classification Working Group. Kidney Int. 2018, 93, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Meehan, D.T.; Delimont, D.; Dufek, B.; Zallocchi, M.; Phillips, G.; Gratton, M.A.; Cosgrove, D. Endothelin-1 mediated induction of extracellular matrix genes in strial marginal cells underlies strial pathology in Alport mice. Hear. Res. 2016, 341, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Wuergezhen, D.; Gindroz, E.; Morita, R.; Hashimoto, K.; Abe, T.; Kiyonari, H.; Fujiwara, H. An eGFP-Col4a2 mouse model reveals basement membrane dynamics underlying hair follicle morphogenesis. J. Cell Biol. 2025, 224, e202404003. [Google Scholar] [CrossRef]
- Thai, J.; Kyloh, M.; Travis, L.; Spencer, N.J.; Ivanusic, J.J. Identifying spinal afferent (sensory) nerve endings that innervate the marrow cavity and periosteum using anterograde tracing. J. Comp. Neurol. 2020, 528, 1903–1916. [Google Scholar] [CrossRef]
- Fetsch, J.F.; Laskin, W.B.; Miettinen, M. Nerve sheath myxoma: A clinicopathologic and immunohistochemical analysis of 57 morphologically distinctive, S-100 protein- and GFAP-positive, myxoid peripheral nerve sheath tumors with a predilection for the extremities and a high local recurrence rate. Am. J. Surg. Pathol. 2005, 29, 1615–1624. [Google Scholar] [CrossRef]
- Akiyama, M. Elastic Fibers and F-Box and WD-40 Domain-Containing Protein 2 in Bovine Periosteum and Blood Vessels. Biomimetics 2022, 8, 7. [Google Scholar] [CrossRef]
- Wang, J.S.; Mazur, C.M.; Wein, M.N. Sclerostin and Osteocalcin: Candidate Bone-Produced Hormones. Front. Endocrinol. 2021, 12, 584147. [Google Scholar] [CrossRef]
- Tavakol, M.; Liu, J.; Hoff, S.E.; Zhu, C.; Heinz, H. Osteocalcin: Promoter or Inhibitor of Hydroxyapatite Growth? Langmuir 2024, 40, 1747–1760. [Google Scholar] [CrossRef] [PubMed]
- Licini, C.; Vitale-Brovarone, C.; Mattioli-Belmonte, M. Collagen and non-collagenous proteins molecular crosstalk in the pathophysiology of osteoporosis. Cytokine Growth Factor. Rev. 2019, 49, 59–69. [Google Scholar] [CrossRef] [PubMed]
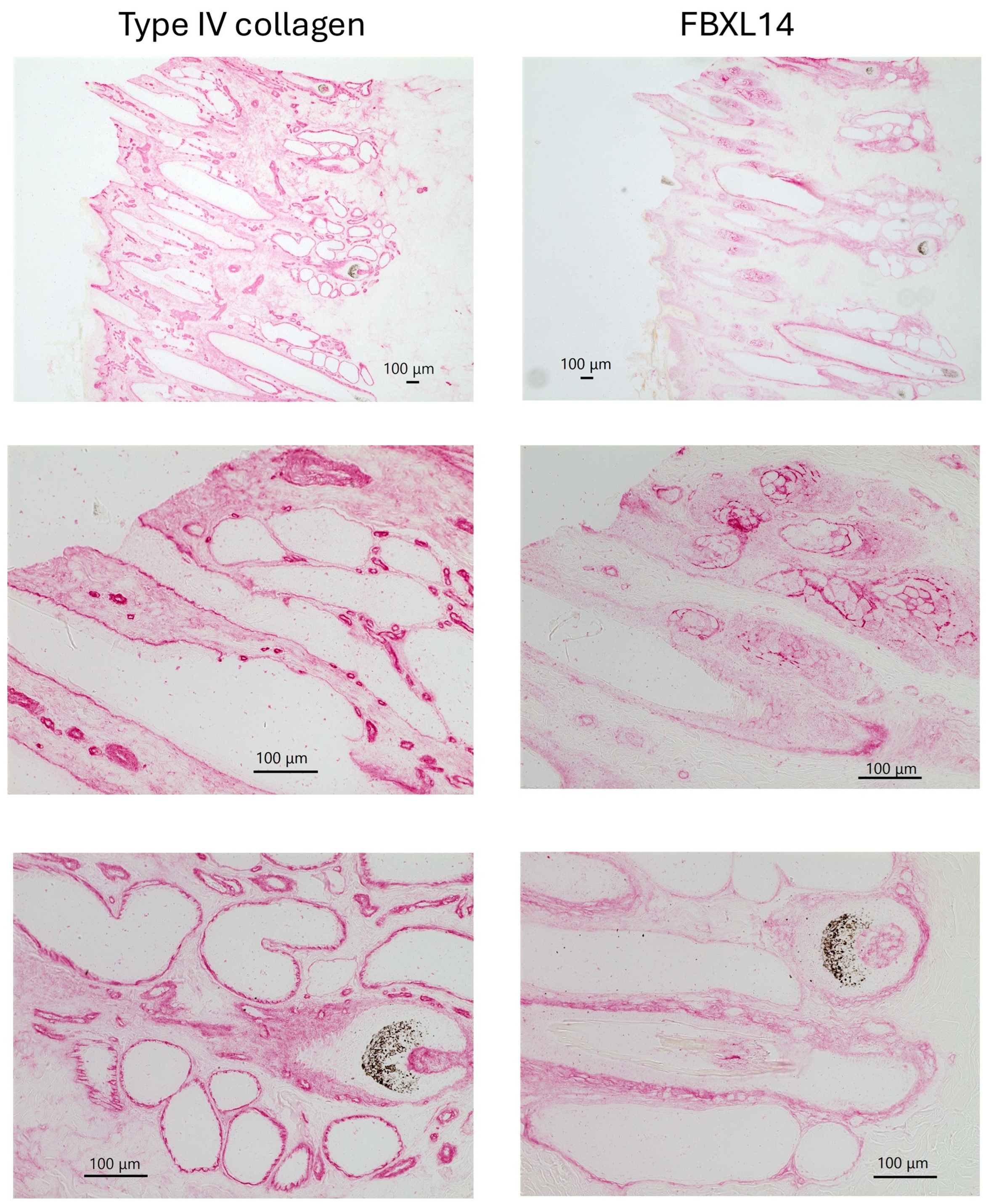

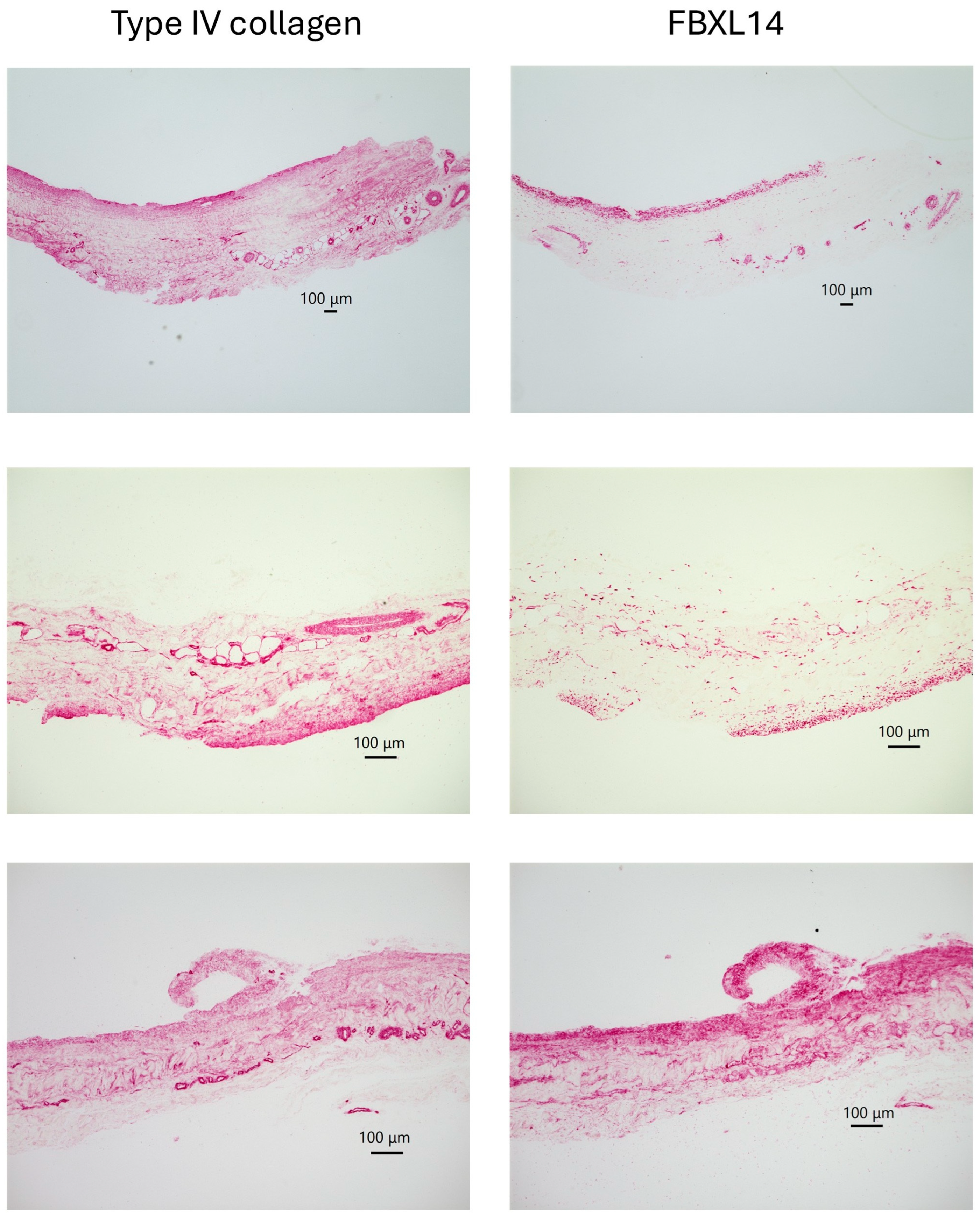
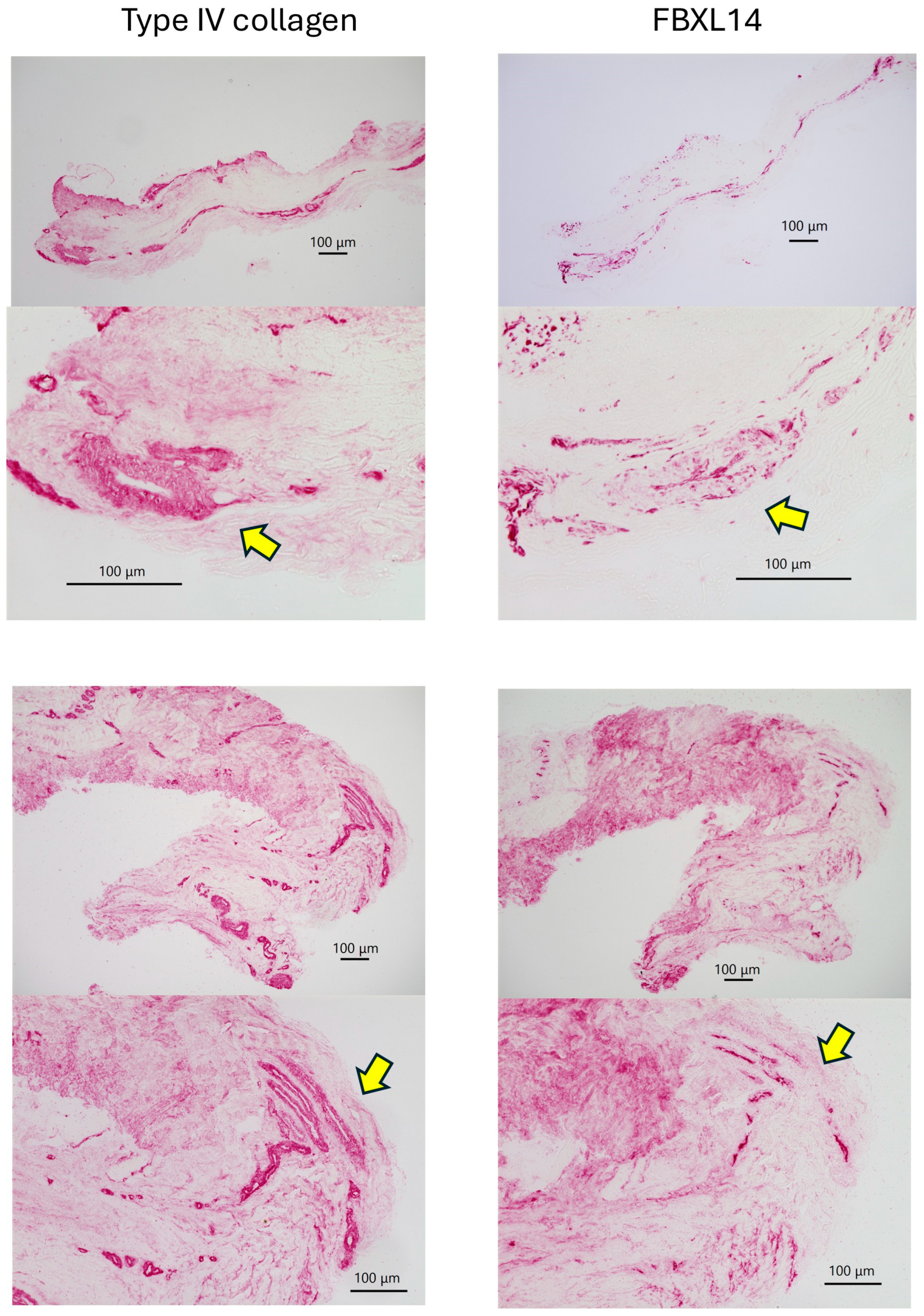

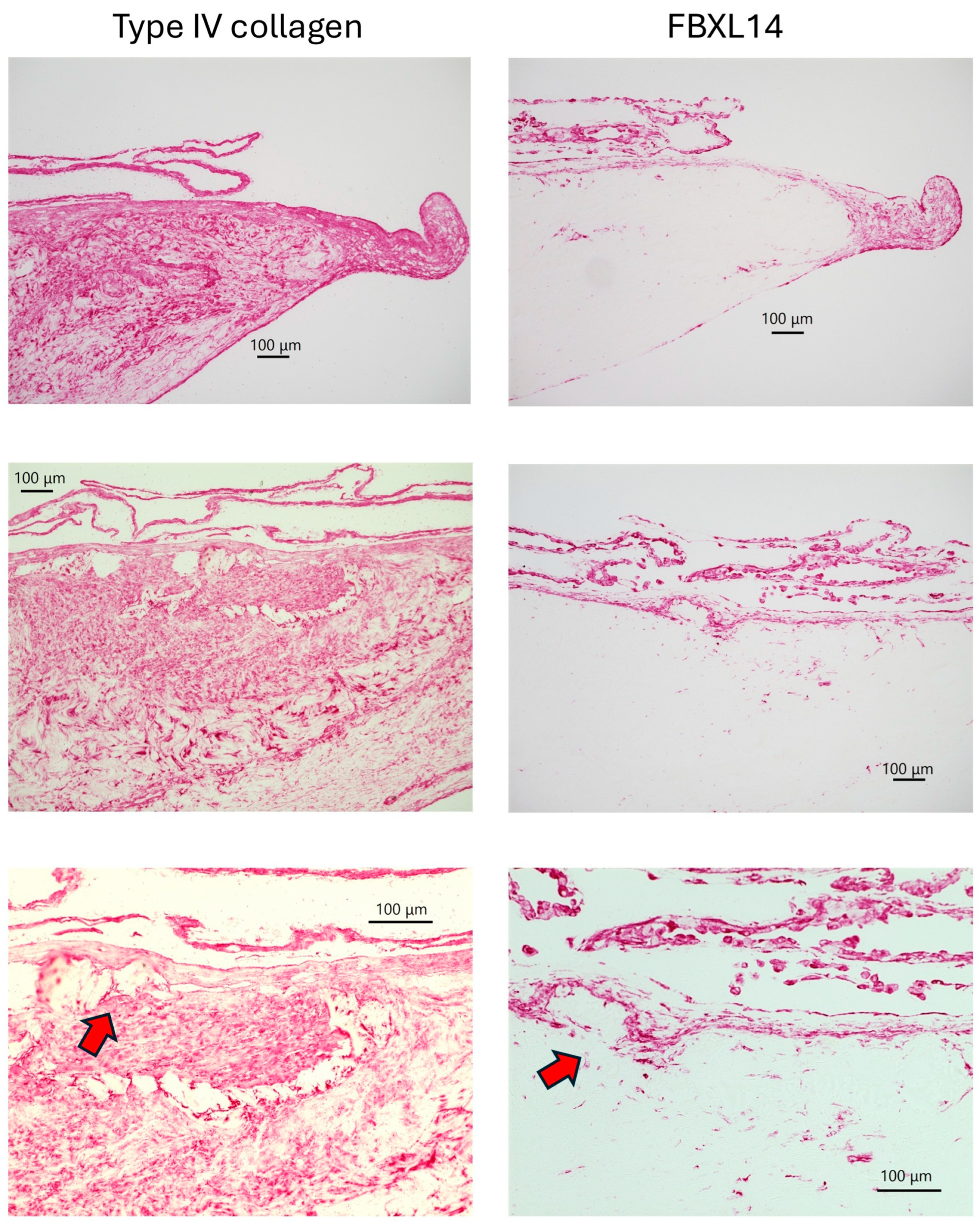
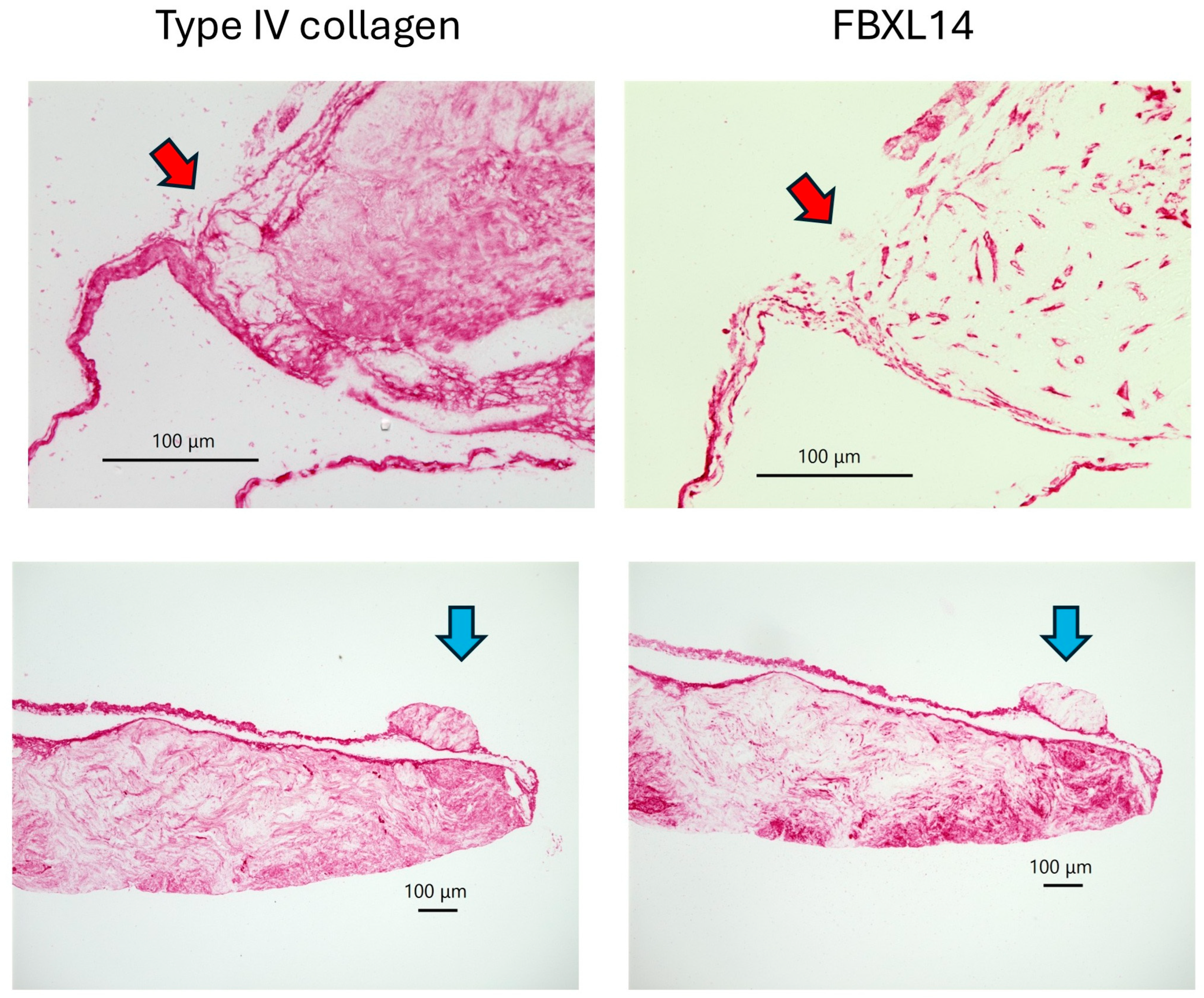
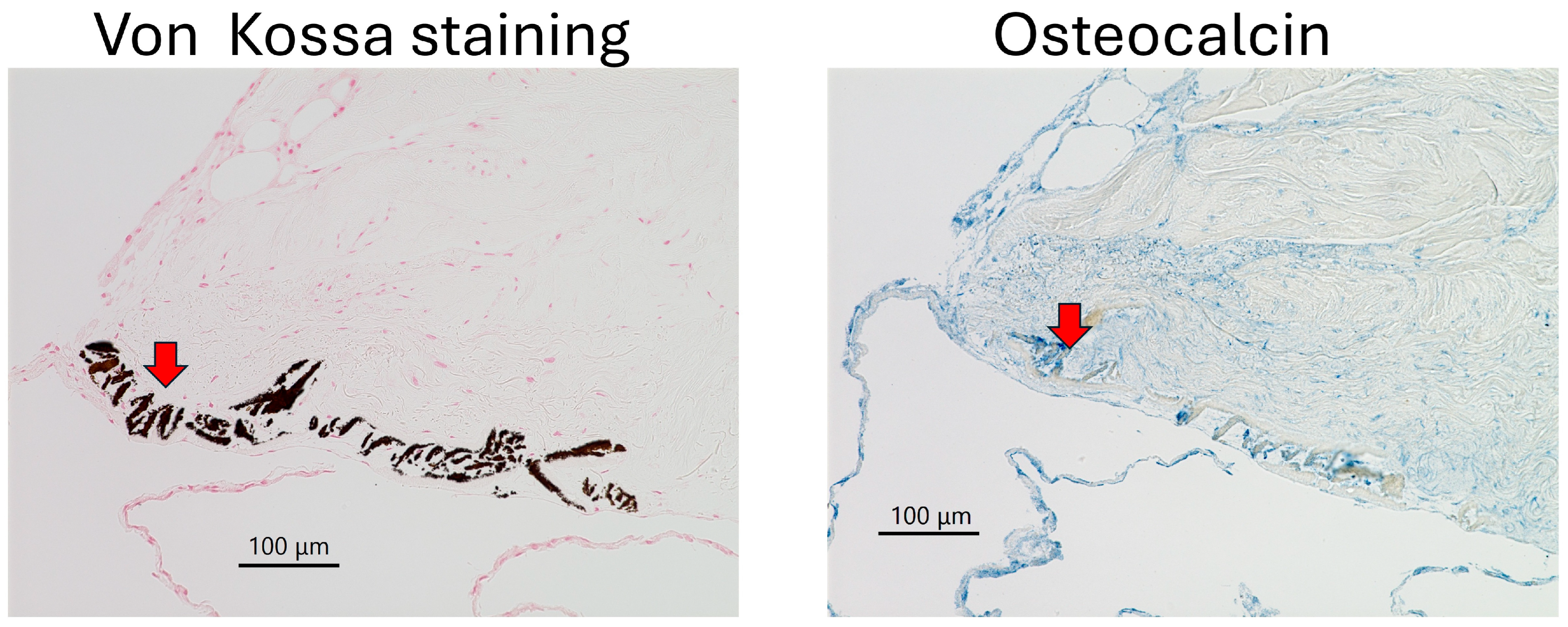
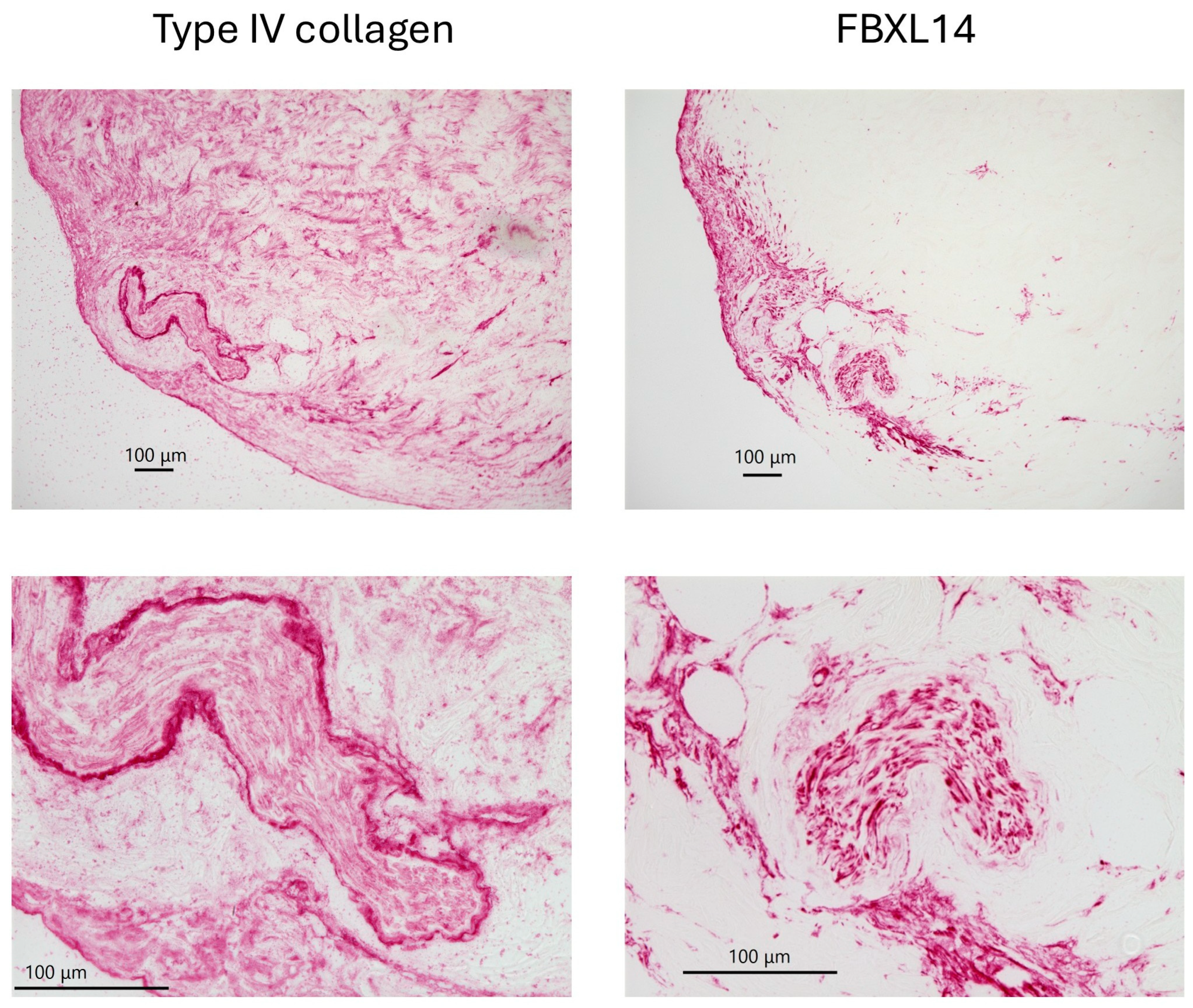
| Cow 1. | PDCs with Type IV collagen and FBxL14, crystal |
| Cow 2. | PDCs with Type IV collagen and FBxL14 |
| Cow 3. | PDCs with Type IV collagen and FBxL14, crystal |
| Cow 4. | PDCs with Type IV collagen and FBxL14, crystal |
| Cow 5. | PDCs with Type IV collagen and FBxL14, crystal |
| Cow 6. | PDCs not observed. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akiyama, M. Scaffold-Free Bone Regeneration Through Collaboration Between Type IV Collagen and FBXL14. J. Clin. Med. 2025, 14, 7160. https://doi.org/10.3390/jcm14207160
Akiyama M. Scaffold-Free Bone Regeneration Through Collaboration Between Type IV Collagen and FBXL14. Journal of Clinical Medicine. 2025; 14(20):7160. https://doi.org/10.3390/jcm14207160
Chicago/Turabian StyleAkiyama, Mari. 2025. "Scaffold-Free Bone Regeneration Through Collaboration Between Type IV Collagen and FBXL14" Journal of Clinical Medicine 14, no. 20: 7160. https://doi.org/10.3390/jcm14207160
APA StyleAkiyama, M. (2025). Scaffold-Free Bone Regeneration Through Collaboration Between Type IV Collagen and FBXL14. Journal of Clinical Medicine, 14(20), 7160. https://doi.org/10.3390/jcm14207160






