The Development of a Predictive Model for Postoperative Renal Function in Living Kidney-Transplant Donors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Evaluation of Preoperative Split Renal Function (Pre-SRF) of Remained Kidney
2.3. Comparison of Pre- and Postoperative Renal Functions (Post-RF)
2.4. Development and Validation of Equations
2.5. Statistical Analyses
3. Results
3.1. Characteristics of Participants
3.2. Preoperative Assessment of Split Renal Function Using Computed Tomography Volumetry
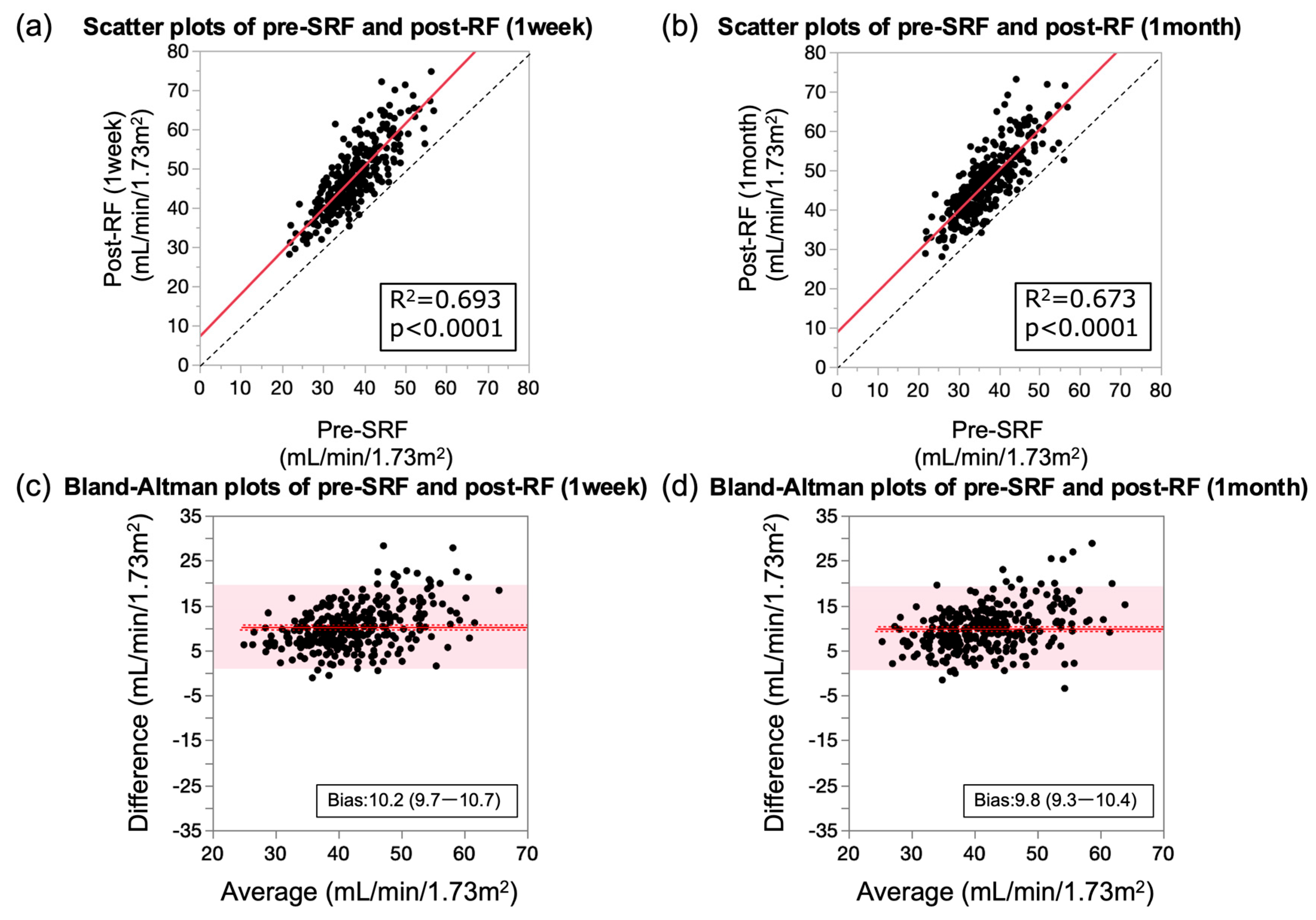
3.3. Preoperative Renal Function Correlates with Post-RF
3.4. Predictors of Change in Pre- and Post-RFs
3.5. Establishment of Prediction Formula for Post-RF
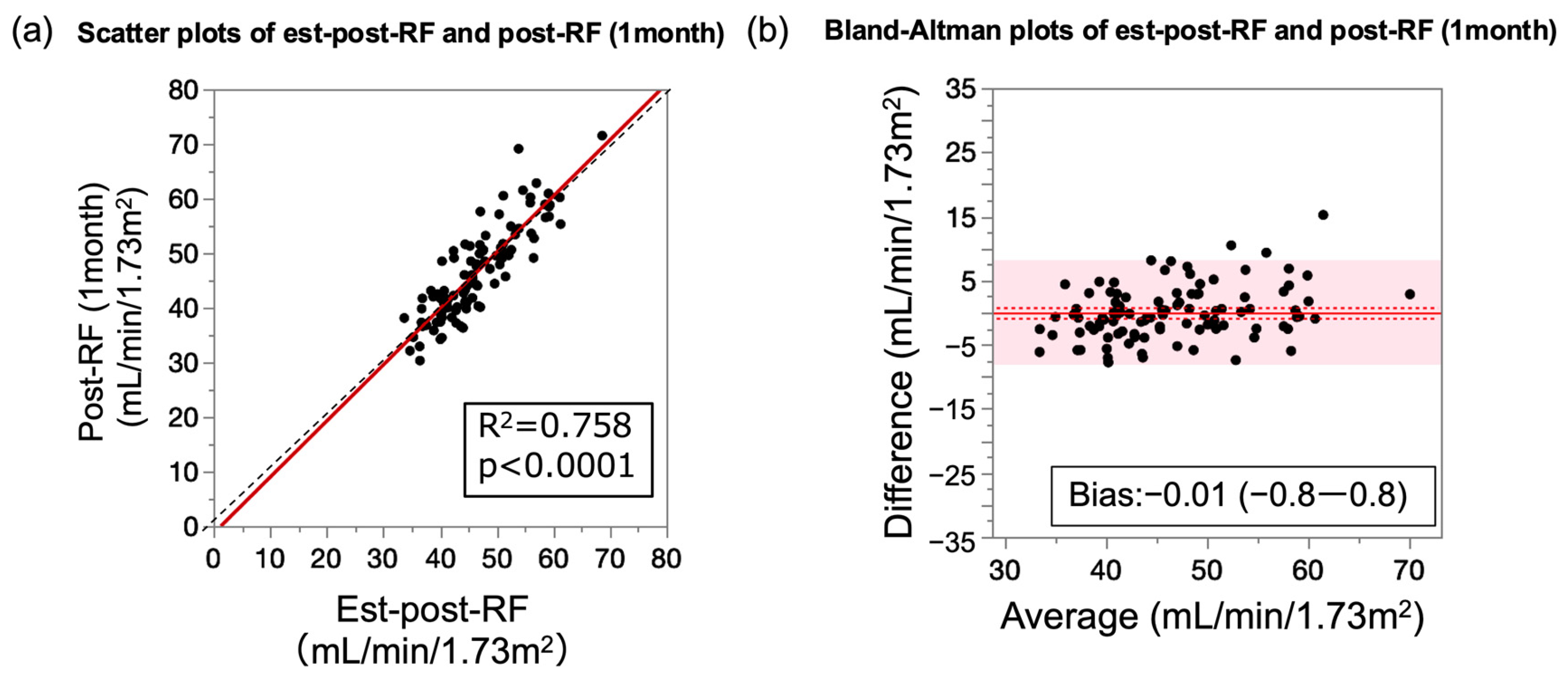
3.6. Performance of Prediction Formula for Post-RF Relative to Measured Post-RF
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Francis, A.; Harhay, M.N.; Ong, A.C.M.; Tummalapalli, S.L.; Ortiz, A.; Fogo, A.B.; Fliser, D.; Roy-Chaudhury, P.; Fontana, M.; Nangaku, M.; et al. Chronic kidney disease and the global public health agenda: An international consensus. Nat. Rev. Nephrol. 2024, 20, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Thurlow, J.S.; Joshi, M.; Yanv, G.; Norris, K.C.; Agodoa, L.Y.; Yuan, C.M.; Nee, R. Global Epidemiology of End-Stage Kidney Disease and Disparities in Kidney Replacement Therapy. Am. J. Nephrol. 2021, 52, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Lentine, K.L.; Kasiske, B.L.; Levey, A.S.; Adams, P.L.; Alberú, J.; Bakr, M.A.; Gallon, L.; Garvey, C.A.; Guleria, S.; Li, P.K.-T.; et al. KDIGO clinical practice guideline on the evaluation and care of living kidney donors. Transplantation 2017, 101, S7–S105. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, A. Why should we implement living donation in renal Transplant? Clin. Nephrol. 2000, 53, Suppl. 55–Suppl. 63. [Google Scholar]
- Ibrahim, H.N.; Foley, R.; Tan, L.; Rogers, T.; Bailey, R.F.; Guo, H.; Gross, C.R.; Matas, A.J. Long-term consequences of kidney donation. N. Engl. J. Med. 2009, 360, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.X.; Muirhead, N.; Knoll, G.; Yang, R.C.; Prasad, G.V.R.; Thiessen-Philbrook, H.; Rosas-Arellano, M.P.; Housawi, A.; Boudville, N.; Donor Nephrectomy Outcomes Research (DONOR) Network. Proteinuria and reduced kidney function in living kidney donors: A systematic review, meta-analysis, and meta-regression. Kidney Int. 2006, 70, 1801–1810. [Google Scholar] [CrossRef]
- Mjøen, G.; Hallan, S.; Hartmann, A.; Foss, A.; Midtvedt, K.; Øyen, O.; Reisæter, A.; Pfeffer, P.; Jenssen, T.; Leivestad, T.; et al. Long-term risks for kidney donors. Kidney Int. 2014, 86, 162–167. [Google Scholar] [CrossRef]
- Muzaale, A.D.; Massie, A.B.; Wang, M.-C.; Montgomery, R.A.; McBride, M.A.; Wainright, J.L.; Segev, D.L. Risk of end-stage renal disease following live kidney donation. JAMA 2014, 311, 579–586. [Google Scholar] [CrossRef]
- Kakuta, Y.; Imamura, R.; Okumi, M.; Horio, M.; Isaka, Y.; Ichimaru, N.; Takahara, S.; Nonomura, N.; Tanabe, K. Assessment of renal function in living kidney donors before and after nephrectomy: A Japanese prospective, observational cohort study. Int. J. Urol. 2019, 26, 499–505. [Google Scholar] [CrossRef]
- Yakoubi, R.; Autorino, R.; Kassab, A.; Long, J.A.; Haber, G.-P.; Kaouk, J.H. Does preserved kidney volume predict 1 year donor renal function after laparoscopic living donor nephrectomy? Int. J. Urol. 2013, 20, 931–934. [Google Scholar] [CrossRef]
- Massie, A.B.; Holscher, C.M.; Henderson, M.L.; Fahmy, L.M.; Thomas, A.G.; Al Ammary, F.; Getsin, S.N.; Snyder, J.J.; Lentine, K.L.; Garg, A.X.; et al. Association of Early postdonation renal function with subsequent risk of end-stage renal disease in living kidney donors. JAMA Surg. 2020, 155, e195472. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, T.; Hariharan, S.; Alon, U.S.; McCarthy, E.T.; Sharma, R.; El-Meanawy, A.; Savin, V.J.; Sharma, M. Hyperfiltration-Mediated Injury in the Remaining Kidney of a Transplant Donor. Transplantation 2018, 102, 1624–1635. [Google Scholar] [CrossRef] [PubMed]
- Benoit, T.; Game, X.; Roumiguie, M.; Sallusto, F.; Doumerc, N.; Beauval, J.B.; Rischmann, P.; Kamar, N.; Soulie, M.; Malavaud, B. Predictive model of 1-year postoperative renal function after living donor nephrectomy. Int. Urol. Nephrol. 2017, 49, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, Y.; Sadahira, T.; Araki, M.; Wada, K.; Tanimoto, R.; Ariyoshi, Y.; Kobayashi, Y.; Watanabe, M.; Watanabe, T.; Nasu, Y. The assessment of renal cortex and parenchymal volume using automated CT volumetry for predicting renal function after donor nephrectomy. Clin. Exp. Nephrol. 2018, 22, 453–458. [Google Scholar] [CrossRef]
- Ben-Haim, S.; Sopov, V.; Stein, A.; Moskovitz, B.; Front, A.; Mecz, Y.; Las, L.; Kastin, A.; Nativ, O.; Groshar, D. Kidney function after radical nephrectomy: Assessment by quantitative SPECT of 99mTc-DMSA uptake by the kidneys. J. Nucl. Med. 2000, 41, 1025–1029. [Google Scholar]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef]
- Gaillard, F.; Pavlov, P.; Tissier, A.-M.; Harache, B.; Eladari, D.; Timsit, M.-O.; Fournier, C.; Léon, C.; Hignette, C.; Friedlander, G.; et al. Use of computed tomography assessed kidney length to predict split renal GFR in living kidney donors. Eur. Radiol. 2017, 27, 651–659. [Google Scholar] [CrossRef]
- Shimbo, M.; Nojyu, K.; Yonekura, Y.; Nagahama, M.; Komatsu, K.; Endo, F.; Ohyama, T.; Sakuma, Y.; Nakayama, M.; Iwami, D.; et al. Computed tomography renal volumetry better predicts postoperative donor renal function in live kidney donor transplantation than renal scintigraphy: A comparative study. Urol. Int. 2024, 108, 73–79. [Google Scholar] [CrossRef]
- Edgren, J.; Laasonen, L.; Kock, B.; Brotherus, J.W.; Pasternack, A.; Kuhlbäck, B. Kidney function and compensatory growth of the kidney in living kidney donors. Scand. J. Urol. Nephrol. 1976, 10, 134–136. [Google Scholar] [CrossRef]
- Saxena, A.B.; Myers, B.D.; Derby, G.; Blouch, K.L.; Yan, J.; Ho, B.; Tan, J.C. Adaptive hyperfiltration in the aging kidney after contralateral nephrectomy. Am. J. Physiol.-Ren. Physiol. 2006, 291, F629–F634. [Google Scholar] [CrossRef]
- Fang, Y.; Gong, A.Y.; Haller, S.T.; Dworkin, L.D.; Liu, Z.; Gong, R. The ageing kidney: Molecular mechanisms and clinical implications. Ageing Res. Rev. 2020, 63, 101151. [Google Scholar] [CrossRef] [PubMed]
- Denic, A.; Alexander, M.P.; Kaushik, V.; Lerman, L.O.; Lieske, J.C.; Stegall, M.D.; Larson, J.J.; Kremers, W.K.; Vrtiska, T.J.; Chakkera, H.A.; et al. Detection and Clinical Patterns of Nephron Hypertrophy and Nephrosclerosis Among Apparently Healthy Adults. Am. J. Kidney Dis. 2016, 68, 58–67. [Google Scholar] [CrossRef]
- Denic, A.; Glassock, R.J.; Rule, A.D. Structural and Functional Changes with the Aging Kidney. Adv. Chronic Kidney Dis. 2016, 23, 19–28. [Google Scholar] [CrossRef]
- Johnson, R.J.; Nakagawa, T.; Jalal, D.; Sánchez-Lozada, L.G.; Kang, D.-H.; Ritz, E. Uric acid and chronic kidney disease: Which is chasing which? Nephrol. Dial. Transpl. 2013, 28, 2221–2228. [Google Scholar] [CrossRef]
- Ejaz, A.A.; Johnson, R.J.; Shimada, M.; Mohandas, R.; Alquadan, K.F.; Beaver, T.M.; Lapsia, V.; Dass, B. The role of uric acid in acute kidney injury. Nephron 2019, 142, 275–283. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, Y.; Li, B.; Xu, S.; Du, H.; Wang, X.; Li, Y. Trends and predictors of changes in renal function after radical nephrectomy for renal tumours. BMC Nephrol. 2024, 25, 174. [Google Scholar] [CrossRef]
- Nishi, S.; Goto, S. Donor evaluation of living kidney transplantation in the aging society. Nephron 2023, 147 (Suppl. S1), 61–66. [Google Scholar] [CrossRef]
- Kakuta, Y.; Okumi, M.; Ichimaru, N.; Abe, T.; Nonomura, N.; Okuyama, A.; Kojima, Y.; Isaka, Y.; Takahara, S.; Imai, E.; et al. Utility of the Japanese GFR estimation equation for evaluating potential donor kidney function. Clin. Exp. Nephrol. 2010, 14, 63–67. [Google Scholar] [CrossRef]
- Kakuta, Y.; Maegawa-Higa, Y.; Matsumura, S.; Fukae, S.; Tanaka, R.; Yonishi, H.; Nakazawa, S.; Yamanaka, K.; Isaka, Y.; Nonomura, N. Performance of the new CKD-EPI creatinine-and cystatin C-based glomerular filtration rate estimation equation in living kidney donor candidate. Transplant. Direct 2024, 10, e1712. [Google Scholar] [CrossRef]
- Delanaye, P.; Weekers, L.; Dubois, B.E.; Cavalier, E.; Detry, O.; Squifflet, J.-P.; Krzesinski, J.-M. Outcome of the living kidney donor. Nephrol. Dial. Transplant. 2012, 27, 41–50. [Google Scholar] [CrossRef]

| Total | Development | Validation | p-Value | |
|---|---|---|---|---|
| n | 310 | 208 | 102 | |
| Age, y | 59.3 ± 11.0 | 58.6 ± 11.5 | 60.7 ± 10.0 | 0.12 |
| Gender (female/male) | 190/120 | 133/75 | 57/45 | 0.17 |
| Body mass index, kg/m2 | 22.8 ± 2.8 | 22.7 ± 2.8 | 23.3 ± 3.4 | 0.26 |
| Body surface area, m2 | 1.62 ± 0.17 | 1.61 ± 0.16 | 1.63 ± 0.18 | 0.36 |
| Diabetes mellitus | 8 (2.6) | 7 (3.4) | 1 (1.0) | 0.18 |
| Hypertension | 83 (26.8) | 53 (25.5) | 30 (29.4) | 0.46 |
| Hyperuricemia | 28 (9.0) | 15 (7.2) | 13 (12.8) | 0.12 |
| Removed kidney side (left/right) | 268/42 | 185/23 | 83/19 | 0.08 |
| Serum creatinine level, mg/dL | 0.71 ± 0.14 | 0.70 ± 0.14 | 0.73 ± 0.15 | 0.13 |
| eGFR, mL/min/1.73 m2 | 75.2 ± 12.6 | 75.7 ± 12.4 | 74.2 ± 13.1 | 0.31 |
| Pre-SRF, mL/min/1.73 m2 | 37.1 ± 6.6 | 37.2 ± 6.5 | 37.0 ± 6.9 | 0.49 |
| Post-RF (1 week), mL/min/1.73 m2 | 47.3 ± 8.6 | 47.5 ± 8.4 | 46.8 ± 9.0 | 0.47 |
| Post-RF (1 month), mL/min/1.73 m2 | 46.9 ± 8.3 | 47.2 ± 8.3 | 46.4 ± 8.4 | 0.45 |
| Equation | R2 | p-Value | Slope (95% CI) | Bias (95% CI) |
|---|---|---|---|---|
| Post-RF (1 week) | 0.69 | <0.0001 | 1.09 (1.01–1.17) | 10.2 (9.7–10.7) |
| Post-RF (1 month) | 0.67 | <0.0001 | 1.03 (0.95–1.11) | 9.8 (9.3–10.4) |
| Equation | R2 | p-Value | Slope (95% CI) | Bias (95% CI) | RMSE (95% CI) |
|---|---|---|---|---|---|
| Est-post-RF | 0.76 | <0.0001 | 1.03 (0.92–1.14) | −0.01 (−0.83–0.82) | 4.15 (3.61–4.91) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, R.; Taniguchi, A.; Higa-Maegawa, Y.; Matsumura, S.; Fukae, S.; Nakazawa, S.; Kakuta, Y.; Nonomura, N. The Development of a Predictive Model for Postoperative Renal Function in Living Kidney-Transplant Donors. J. Clin. Med. 2024, 13, 7090. https://doi.org/10.3390/jcm13237090
Tanaka R, Taniguchi A, Higa-Maegawa Y, Matsumura S, Fukae S, Nakazawa S, Kakuta Y, Nonomura N. The Development of a Predictive Model for Postoperative Renal Function in Living Kidney-Transplant Donors. Journal of Clinical Medicine. 2024; 13(23):7090. https://doi.org/10.3390/jcm13237090
Chicago/Turabian StyleTanaka, Ryo, Ayumu Taniguchi, Yoko Higa-Maegawa, Soichi Matsumura, Shota Fukae, Shigeaki Nakazawa, Yoichi Kakuta, and Norio Nonomura. 2024. "The Development of a Predictive Model for Postoperative Renal Function in Living Kidney-Transplant Donors" Journal of Clinical Medicine 13, no. 23: 7090. https://doi.org/10.3390/jcm13237090
APA StyleTanaka, R., Taniguchi, A., Higa-Maegawa, Y., Matsumura, S., Fukae, S., Nakazawa, S., Kakuta, Y., & Nonomura, N. (2024). The Development of a Predictive Model for Postoperative Renal Function in Living Kidney-Transplant Donors. Journal of Clinical Medicine, 13(23), 7090. https://doi.org/10.3390/jcm13237090





