Traumatic Brain Injury: Advances in Diagnostic Techniques and Treatment Modalities
Abstract
1. Introduction
2. Materials and Methods
3. Results/Findings
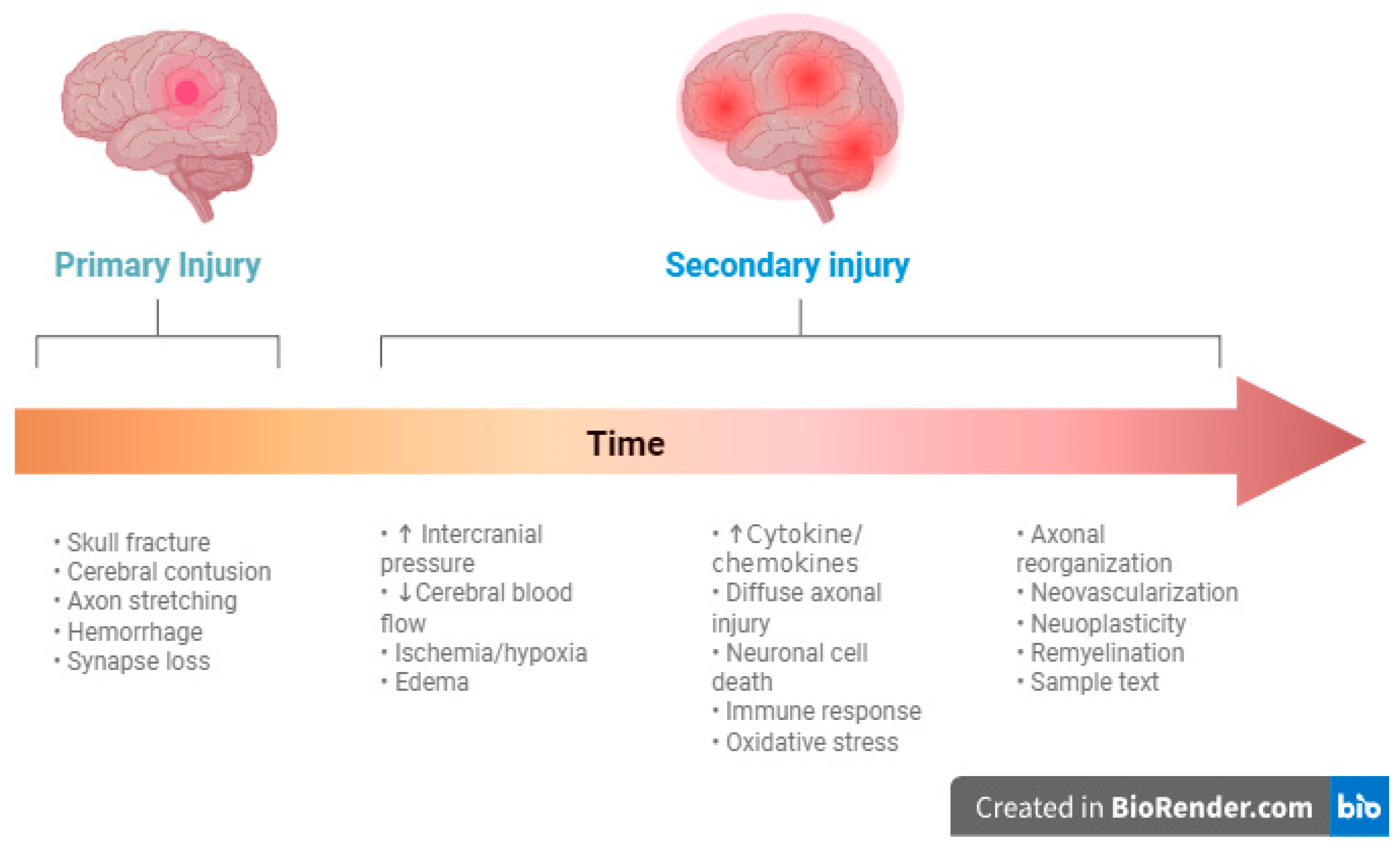
3.1. Pathophysiology
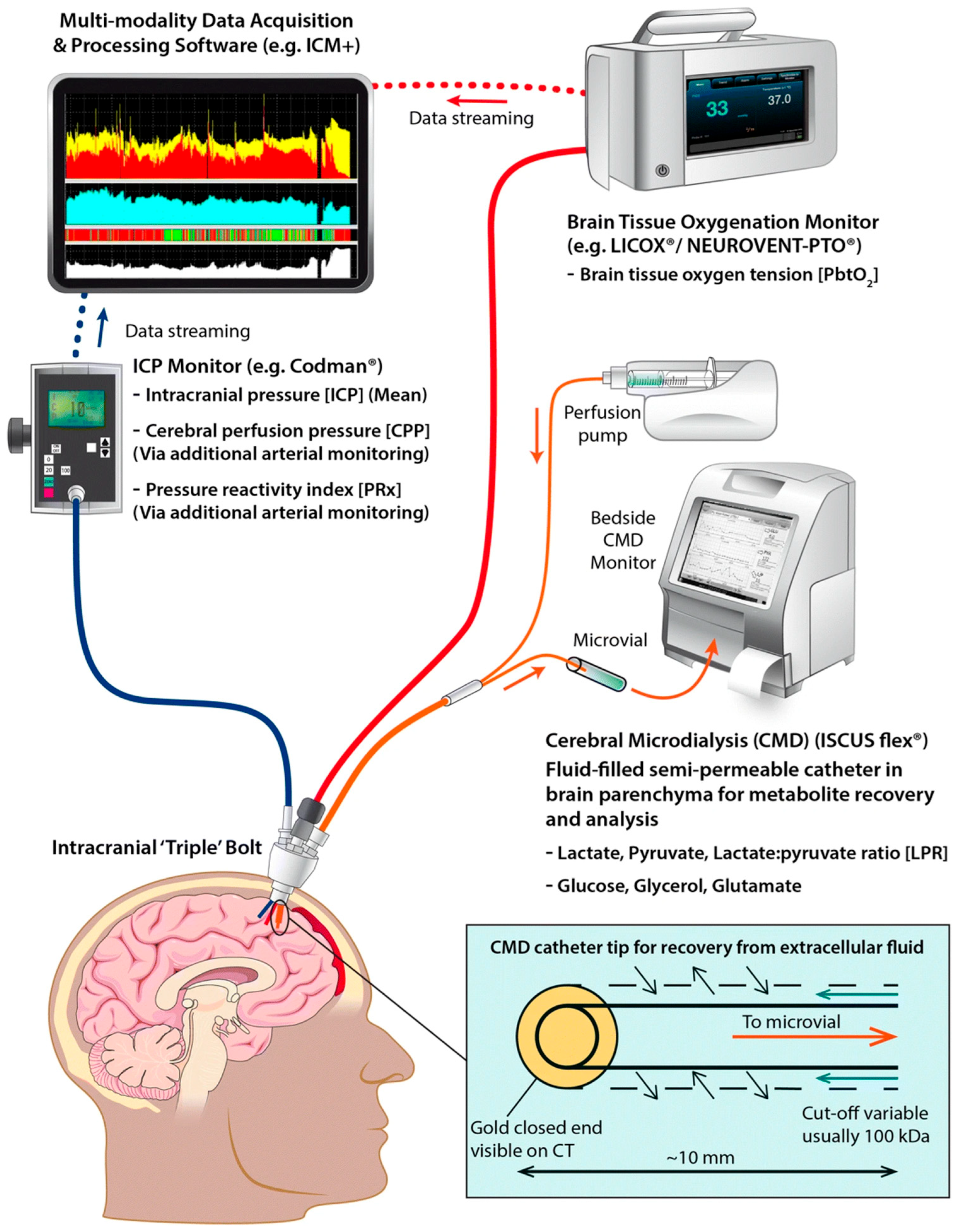
3.2. Advanced Monitoring Techniques
3.2.1. Brain Tissue Oxygenation
3.2.2. Microdialysis
3.2.3. Multimodal Monitoring
3.3. Non-Invasive Monitoring
3.4. Therapeutic Interventions
3.4.1. Blood Pressure Augmentation
3.4.2. Anti-Inflammatory Agents
3.4.3. Hypocapnia
3.4.4. Hypothermia
3.4.5. Hyperosmolar Therapies
3.4.6. Metabolic Suppression
3.4.7. Surgical Management
3.5. Emerging Therapies
3.5.1. Erythropoietin
3.5.2. Progesterone
3.5.3. Stem Cell Therapies
3.6. Advanced Rehabilitation Techniques
3.7. Innovative Techniques for TBI Treatment
3.8. Personalized Medicine in TBI Management
4. Challenges and Controversies
4.1. Variability in Guideline Implementation
4.2. Balancing Benefits and Risks
4.3. Ethical Dilemmas in Invasive Monitoring and Experimental Therapies
4.4. Gaps in Evidence
5. Prevention
6. Future Directions in TBI Management
6.1. Integration of Artificial Intelligence and Big Data
6.2. Global Initiatives
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| TBI | Traumatic brain injury |
| EPO | Erythropoietin |
| WHO | World Health Organization |
| LMIC | Low- and Middle-income countries |
| GFAP | Glial fibrillary acidic protein |
| UCH-L1 | Ubiquitin C-terminal hydrolase-L1 |
| ELISA | Enzyme-linked immunosorbent assay |
| ICPW | ICP waveform |
| TCS | Transcranial stimulation techniques |
| TMS | Transcranial magnetic stimulation |
| MSC | Mesenchymal stem cell |
| FMMS | Fugl-Meyer Motor Scale |
| hNSCs | Human neural stem cells |
| ARAT | Action Research Arm Test |
| MWM | Motor Water Maze |
| GOS-E | Glasgow Outcome Scale-Extended |
| DC | Decompressive craniectomy |
| BTF | Brain Trauma Foundation |
| LPR | Lactate-pyruvate ratio |
| CMD | Cerebral microdialysis |
References
- CDC. Facts About TBI. Available online: https://www.cdc.gov/traumatic-brain-injury/data-research/facts-stats/index.html (accessed on 10 December 2024).
- Zhong, H.; Feng, Y.; Shen, J.; Rao, T.; Dai, H.; Zhong, W.; Zhao, G. Global burden of traumatic brain injury in 204 countries and territories from 1990 to 2021. Am. J. Prev. Med. 2025, 68, 754–763. [Google Scholar] [CrossRef]
- Guan, B.; Anderson, D.B.; Chen, L.; Feng, S.; Zhou, H. Global, regional and national burden of traumatic brain injury and spinal cord injury, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. BMJ Open 2023, 13, e075049. [Google Scholar] [CrossRef]
- Huang, X.F.; Ma, S.F.; Jiang, X.H.; Song, R.J.; Li, M.; Zhang, J.; Sun, T.J.; Hu, Q.; Wang, W.R.; Yu, A.Y.; et al. Causes and global, regional, and national burdens of traumatic brain injury from 1990 to 2019. Chin. J. Traumatol. 2024, 27, 311–322. [Google Scholar] [CrossRef]
- CDC. TBI Data. Available online: https://www.cdc.gov/traumatic-brain-injury/data-research/index.html (accessed on 10 December 2024).
- Peterson, C.; Miller, G.F.; Barnett, S.B.L.; Florence, C. Economic Cost of Injury—United States, 2019. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1655–1659. [Google Scholar] [CrossRef]
- CDC. About Potential Effects of a Moderate or Severe TBI. Available online: https://www.cdc.gov/traumatic-brain-injury/about/potential-effects.html (accessed on 10 December 2024).
- Dams-O’Connor, K.; Juengst, S.B.; Bogner, J.; Chiaravalloti, N.D.; Corrigan, J.D.; Giacino, J.T.; Harrison-Felix, C.L.; Hoffman, J.M.; Ketchum, J.M.; Lequerica, A.H.; et al. Traumatic brain injury as a chronic disease: Insights from the United States Traumatic Brain Injury Model Systems Research Program. Lancet Neurol. 2023, 22, 517–528. [Google Scholar] [CrossRef]
- Maas, A.I.R.; Menon, D.K.; Manley, G.T.; Abrams, M.; Åkerlund, C.; Andelic, N.; Aries, M.; Bashford, T.; Bell, M.J.; Bodien, Y.G.; et al. Traumatic brain injury: Progress and challenges in prevention, clinical care, and research. Lancet Neurol. 2022, 21, 1004–1060. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Board on Health Sciences Policy; Committee on Accelerating Progress in Traumatic Brain Injury Research and Care. Traumatic Brain Injury: A Roadmap for Accelerating Progress; Matney, C., Bowman, K., Berwick, D., Eds.; National Academies Press: Washington, DC, USA, 2022. [Google Scholar]
- Leinonen, V.; Vanninen, R.; Rauramaa, T. Raised intracranial pressure and brain edema. Handb. Clin. Neurol. 2017, 145, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Canac, N.; Jalaleddini, K.; Thorpe, S.G.; Thibeault, C.M.; Hamilton, R.B. Review: Pathophysiology of intracranial hypertension and noninvasive intracranial pressure monitoring. Fluids Barriers CNS 2020, 17, 40. [Google Scholar] [CrossRef] [PubMed]
- White, H.; Venkatesh, B. Cerebral perfusion pressure in neurotrauma: A review. Anesth. Analg. 2008, 107, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Greve, M.W.; Zink, B.J. Pathophysiology of traumatic brain injury. Mt. Sinai J. Med. 2009, 76, 97–104. [Google Scholar] [CrossRef]
- Werner, C.; Engelhard, K. Pathophysiology of traumatic brain injury. Br. J. Anaesth. 2007, 99, 4–9. [Google Scholar] [CrossRef]
- Nakagawa, A.; Manley, G.T.; Gean, A.D.; Ohtani, K.; Armonda, R.; Tsukamoto, A.; Yamamoto, H.; Takayama, K.; Tominaga, T. Mechanisms of primary blast-induced traumatic brain injury: Insights from shock-wave research. J. Neurotrauma 2011, 28, 1101–1119. [Google Scholar] [CrossRef]
- Ladak, A.A.; Enam, S.A.; Ibrahim, M.T. A Review of the Molecular Mechanisms of Traumatic Brain Injury. World Neurosurg. 2019, 131, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Chesnut, R.; Aguilera, S.; Buki, A.; Bulger, E.; Citerio, G.; Cooper, D.J.; Arrastia, R.D.; Diringer, M.; Figaji, A.; Gao, G.; et al. A management algorithm for adult patients with both brain oxygen and intracranial pressure monitoring: The Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 2020, 46, 919–929. [Google Scholar] [CrossRef]
- Lund, A.; Madsen, A.F.; Capion, T.; Jensen, H.R.; Forsse, A.; Hauerberg, J.; Sigurðsson, S.; Mathiesen, T.I.; Møller, K.; Olsen, M.H. Brain hypoxia and metabolic crisis are common in patients with acute brain injury despite a normal intracranial pressure. Sci. Rep. 2024, 14, 23828. [Google Scholar] [CrossRef]
- Häni, L.; Ropelato, M.D.; Wagner, F.; Nowacki, A.; Söll, N.; Haenggi, M.; Raabe, A.; Z’Graggen, W.J. Individualized Brain Tissue Oxygen-Monitoring Probe Placement Helps to Guide Therapy and Optimizes Outcome in Neurocritical Care. Neurocrit. Care 2021, 35, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Meyfroidt, G.; Bouzat, P.; Casaer, M.P.; Chesnut, R.; Hamada, S.R.; Helbok, R.; Hutchinson, P.; Maas, A.I.R.; Manley, G.; Menon, D.K.; et al. Management of moderate to severe traumatic brain injury: An update for the intensivist. Intensive Care Med. 2022, 48, 649–666. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.P. Moderate and Severe Traumatic Brain Injury. Continuum 2021, 27, 1278–1300. [Google Scholar] [CrossRef]
- Hirschi, R.; Hawryluk, G.W.J.; Nielson, J.L.; Huie, J.R.; Zimmermann, L.L.; Saigal, R.; Ding, Q.; Ferguson, A.R.; Manley, G. Analysis of high-frequency PbtO2 measures in traumatic brain injury: Insights into the treatment threshold. J. Neurosurg. 2019, 131, 1216–1226. [Google Scholar] [CrossRef]
- Lin, C.M.; Lin, M.C.; Huang, S.J.; Chang, C.K.; Chao, D.P.; Lui, T.N.; Ma, H.I.; Liu, M.Y.; Chung, W.Y.; Shih, Y.H.; et al. A Prospective Randomized Study of Brain Tissue Oxygen Pressure-Guided Management in Moderate and Severe Traumatic Brain Injury Patients. Biomed. Res. Int. 2015, 2015, 529580. [Google Scholar] [CrossRef]
- Figaji, A.A.; Zwane, E.; Graham Fieggen, A.; Argent, A.C.; Le Roux, P.D.; Peter, J.C. The effect of increased inspired fraction of oxygen on brain tissue oxygen tension in children with severe traumatic brain injury. Neurocrit. Care 2010, 12, 430–437. [Google Scholar] [CrossRef]
- Shen, Y.; Wen, D.; Liang, Z.; Wan, L.; Jiang, Q.; He, H.; He, M. Brain tissue oxygen partial pressure monitoring and prognosis of patients with traumatic brain injury: A meta-analysis. Neurosurg. Rev. 2024, 47, 222. [Google Scholar] [CrossRef] [PubMed]
- Hays, L.M.C.; Udy, A.; Adamides, A.A.; Anstey, J.R.; Bailey, M.; Bellapart, J.; Byrne, K.; Cheng, A.; Jamie Cooper, D.; Drummond, K.J.; et al. Effects of brain tissue oxygen (PbtO(2)) guided management on patient outcomes following severe traumatic brain injury: A systematic review and meta-analysis. J. Clin. Neurosci. 2022, 99, 349–358. [Google Scholar] [CrossRef]
- Stovell, M.G.; Helmy, A.; Thelin, E.P.; Jalloh, I.; Hutchinson, P.J.; Carpenter, K.L.H. An overview of clinical cerebral microdialysis in acute brain injury. Front. Neurol. 2023, 14, 1085540. [Google Scholar] [CrossRef]
- Hutchinson, P.J.; Jalloh, I.; Helmy, A.; Carpenter, K.L.; Rostami, E.; Bellander, B.M.; Boutelle, M.G.; Chen, J.W.; Claassen, J.; Dahyot-Fizelier, C.; et al. Consensus statement from the 2014 International Microdialysis Forum. Intensive Care Med. 2015, 41, 1517–1528. [Google Scholar] [CrossRef]
- Zeiler, F.A.; Thelin, E.P.; Helmy, A.; Czosnyka, M.; Hutchinson, P.J.A.; Menon, D.K. A systematic review of cerebral microdialysis and outcomes in TBI: Relationships to patient functional outcome, neurophysiologic measures, and tissue outcome. Acta Neurochir. 2017, 159, 2245–2273. [Google Scholar] [CrossRef]
- Foreman, B.; Ngwenya, L.B.; Stoddard, E.; Hinzman, J.M.; Andaluz, N.; Hartings, J.A. Safety and Reliability of Bedside, Single Burr Hole Technique for Intracranial Multimodality Monitoring in Severe Traumatic Brain Injury. Neurocrit. Care 2018, 29, 469–480. [Google Scholar] [CrossRef]
- Okonkwo, D.O.; Shutter, L.A.; Moore, C.; Temkin, N.R.; Puccio, A.M.; Madden, C.J.; Andaluz, N.; Chesnut, R.M.; Bullock, M.R.; Grant, G.A.; et al. Brain Oxygen Optimization in Severe Traumatic Brain Injury Phase-II: A Phase II Randomized Trial. Crit. Care Med. 2017, 45, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Bernard, F.; Barsan, W.; Diaz-Arrastia, R.; Merck, L.H.; Yeatts, S.; Shutter, L.A. Brain Oxygen Optimization in Severe Traumatic Brain Injury (BOOST-3): A multicentre, randomised, blinded-endpoint, comparative effectiveness study of brain tissue oxygen and intracranial pressure monitoring versus intracranial pressure alone. BMJ Open 2022, 12, e060188. [Google Scholar] [CrossRef] [PubMed]
- Payen, J.F.; Launey, Y.; Chabanne, R.; Gay, S.; Francony, G.; Gergele, L.; Vega, E.; Montcriol, A.; Couret, D.; Cottenceau, V.; et al. Intracranial pressure monitoring with and without brain tissue oxygen pressure monitoring for severe traumatic brain injury in France (OXY-TC): An open-label, randomised controlled superiority trial. Lancet Neurol. 2023, 22, 1005–1014. [Google Scholar] [CrossRef]
- Diaz-Arrastia, R.; Bernard, F.; Shutter, L.; Barsan, W.; Silbergleit, R. Monitoring patients with severe traumatic brain injury. Lancet Neurol. 2024, 23, 230–231. [Google Scholar] [CrossRef]
- Khellaf, A.; Khan, D.Z.; Helmy, A. Recent advances in traumatic brain injury. J. Neurol. 2019, 266, 2878–2889. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.; Cody, J.; Maldonado, Y.; Ramakrishna, H. Near-Infrared Spectroscopy (NIRS) for Cerebral and Tissue Oximetry: Analysis of Evolving Applications. J. Cardiothorac. Vasc. Anesth. 2022, 36, 2758–2766. [Google Scholar] [CrossRef]
- Maas, A.I.; Citerio, G. Noninvasive monitoring of cerebral oxygenation in traumatic brain injury: A mix of doubts and hope. Intensive Care Med. 2010, 36, 1283–1285. [Google Scholar] [CrossRef][Green Version]
- Blanco, P.; Abdo-Cuza, A. Transcranial Doppler ultrasound in neurocritical care. J. Ultrasound 2018, 21, 1–16. [Google Scholar] [CrossRef]
- Buccilli, B.; Alan, A.; Baha, A.; Shahzad, A.; Almealawy, Y.F.; Chisvo, N.S.; Ennabe, M.; Weinand, M. Neuroprotection strategies in traumatic brain injury: Studying the effectiveness of different clinical approaches. Surg. Neurol. Int. 2024, 15, 29. [Google Scholar] [CrossRef] [PubMed]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef]
- Vella, M.A.; Crandall, M.; Patel, M.B. Acute management of traumatic brain injury. Surg. Clin. N. Am. 2017, 97, 1015. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.; Malik, R.; Singh, G.; Bhatia, S.; Al-Harrasi, A.; Mohan, S.; Albratty, M.; Albarrati, A.; Tambuwala, M.M. Pathogenesis and management of traumatic brain injury (TBI): Role of neuroinflammation and anti-inflammatory drugs. Inflammopharmacology 2022, 30, 1153–1166. [Google Scholar] [CrossRef]
- Müller, N. COX-2 Inhibitors, Aspirin, and Other Potential Anti-Inflammatory Treatments for Psychiatric Disorders. Front. Psychiatry 2019, 10, 375. [Google Scholar] [CrossRef]
- Bhanja, D.; Hallan, D.R.; Staub, J.; Rizk, E.; Zacko, J.C. Early Celecoxib use in Patients with Traumatic Brain Injury. Neurocrit. Care 2024, 40, 886–897. [Google Scholar] [CrossRef]
- Docherty, A.; Emelifeonwu, J.; Andrews, P.J.D. Hypothermia After Traumatic Brain Injury. JAMA 2018, 320, 2204–2206. [Google Scholar] [CrossRef]
- Cooper, D.J.; Nichol, A.D.; Bailey, M.; Bernard, S.; Cameron, P.A.; Pili-Floury, S.; Forbes, A.; Gantner, D.; Higgins, A.M.; Huet, O.; et al. Effect of Early Sustained Prophylactic Hypothermia on Neurologic Outcomes Among Patients with Severe Traumatic Brain Injury: The POLAR Randomized Clinical Trial. JAMA 2018, 320, 2211–2220. [Google Scholar] [CrossRef]
- Andrews, P.J.; Sinclair, H.L.; Rodriguez, A.; Harris, B.A.; Battison, C.G.; Rhodes, J.K.; Murray, G.D. Hypothermia for Intracranial Hypertension after Traumatic Brain Injury. N. Engl. J. Med. 2015, 373, 2403–2412. [Google Scholar] [CrossRef]
- Wu, X.; Tao, Y.; Marsons, L.; Dee, P.; Yu, D.; Guan, Y.; Zhou, X. The effectiveness of early prophylactic hypothermia in adult patients with traumatic brain injury: A systematic review and meta-analysis. Aust. Crit. Care 2021, 34, 83–91. [Google Scholar] [CrossRef]
- Chen, H.; Wu, F.; Yang, P.; Shao, J.; Chen, Q.; Zheng, R. A meta-analysis of the effects of therapeutic hypothermia in adult patients with traumatic brain injury. Crit. Care 2019, 23, 396. [Google Scholar] [CrossRef]
- Martyniuk, A.; Hart, S.; Lannon, M.; Mastrolonardo, A.; Kabbani, A.; Hafeez, D.A.; Engels, P.T.; Sharma, S. Therapeutic Hypothermia Compared with Normothermia in Adults with Traumatic Brain Injury; Functional Outcome, Mortality, and Adverse Effects: A Systematic Review and Meta-Analysis. Neurocrit. Care 2024, 41, 400–417. [Google Scholar] [CrossRef] [PubMed]
- Gharizadeh, N.; Ghojazadeh, M.; Naseri, A.; Dolati, S.; Tarighat, F.; Soleimanpour, H. Hypertonic saline for traumatic brain injury: A systematic review and meta-analysis. Eur. J. Med. Res. 2022, 27, 254. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeong, H.; Choo, Y.H.; Kim, M.; Ha, E.J.; Oh, J.; Shim, Y.; Kim, S.B.; Jung, H.G.; Park, S.H.; et al. Optimizing Mannitol Use in Managing Increased Intracranial Pressure: A Comprehensive Review of Recent Research and Clinical Experiences. Korean J. Neurotrauma 2023, 19, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Boone, M.D.; Oren-Grinberg, A.; Robinson, T.M.; Chen, C.C.; Kasper, E.M. Mannitol or hypertonic saline in the setting of traumatic brain injury: What have we learned? Surg. Neurol. Int. 2015, 6, 177. [Google Scholar] [CrossRef] [PubMed]
- Patil, H.; Gupta, R. A Comparative Study of Bolus Dose of Hypertonic Saline, Mannitol, and Mannitol Plus Glycerol Combination in Patients with Severe Traumatic Brain Injury. World Neurosurg. 2019, 125, e221–e228. [Google Scholar] [CrossRef]
- Bernhardt, K.; McClune, W.; Rowland, M.J.; Shah, A. Hypertonic Saline Versus Other Intracranial-Pressure-Lowering Agents for Patients with Acute Traumatic Brain Injury: A Systematic Review and Meta-analysis. Neurocrit. Care 2024, 40, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Ferrer, R.; Taccone, F.S.; Wiedermann, C.J.; Reinstrup, P. Re-evaluating albumin use in traumatic brain injury. J. Intensive Care 2025, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Wiedermann, C.J. Use of Hyperoncotic Human Albumin Solution in Severe Traumatic Brain Injury Revisited—A Narrative Review and Meta-Analysis. J. Clin. Med. 2022, 11, 2662. [Google Scholar] [CrossRef] [PubMed]
- Choo, Y.H.; Seo, Y.; Oh, H.J. Deep Sedation in Traumatic Brain Injury Patients. Korean J. Neurotrauma 2023, 19, 185–194. [Google Scholar] [CrossRef]
- Roberts, I.; Sydenham, E. Barbiturates for acute traumatic brain injury. Cochrane Database Syst. Rev. 2012, 12, Cd000033. [Google Scholar] [CrossRef]
- Eisenberg, H.M.; Frankowski, R.F.; Contant, C.F.; Marshall, L.F.; Walker, M.D. High-dose barbiturate control of elevated intracranial pressure in patients with severe head injury. J. Neurosurg. 1988, 69, 15–23. [Google Scholar] [CrossRef]
- Tomita, H.; Kimura, K.; Ono, Y.; Yamada, O.; Yagihara, T.; Echigo, S. Life-threatening pulmonary edema following unilateral stent implantation for bilateral branch pulmonary stenosis: Recovery after contralateral stent implantation. Jpn. Circ. J. 2001, 65, 688–690. [Google Scholar] [CrossRef][Green Version]
- Bor-Seng-Shu, E.; Figueiredo, E.G.; Amorim, R.L.; Teixeira, M.J.; Valbuza, J.S.; de Oliveira, M.M.; Panerai, R.B. Decompressive craniectomy: A meta-analysis of influences on intracranial pressure and cerebral perfusion pressure in the treatment of traumatic brain injury. J. Neurosurg. 2012, 117, 589–596. [Google Scholar] [CrossRef]
- Hutchinson, P.J.; Kolias, A.G.; Timofeev, I.S.; Corteen, E.A.; Czosnyka, M.; Timothy, J.; Anderson, I.; Bulters, D.O.; Belli, A.; Eynon, C.A.; et al. Trial of Decompressive Craniectomy for Traumatic Intracranial Hypertension. N. Engl. J. Med. 2016, 375, 1119–1130. [Google Scholar] [CrossRef]
- Ponce, L.L.; Navarro, J.C.; Ahmed, O.; Robertson, C.S. Erythropoietin neuroprotection with traumatic brain injury. Pathophysiology 2013, 20, 31–38. [Google Scholar] [CrossRef]
- Blixt, J.; Gunnarson, E.; Wanecek, M. Erythropoietin Attenuates the Brain Edema Response after Experimental Traumatic Brain Injury. J. Neurotrauma 2018, 35, 671–680. [Google Scholar] [CrossRef]
- Nichol, A.; French, C.; Little, L.; Haddad, S.; Presneill, J.; Arabi, Y.; Bailey, M.; Cooper, D.J.; Duranteau, J.; Huet, O.; et al. Erythropoietin in traumatic brain injury (EPO-TBI): A double-blind randomised controlled trial. Lancet 2015, 386, 2499–2506. [Google Scholar] [CrossRef]
- Skrifvars, M.B.; Luethi, N.; Bailey, M.; French, C.; Nichol, A.; Trapani, T.; McArthur, C.; Arabi, Y.M.; Bendel, S.; Cooper, D.J.; et al. The effect of recombinant erythropoietin on long-term outcome after moderate-to-severe traumatic brain injury. Intensive Care Med. 2023, 49, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Ma, L.; Chen, H.; You, C. Pharmacological components with neuroprotective effects in the management of traumatic brain injury: Evidence from network meta-analysis. Neurol. Sci. 2023, 44, 1665–1678. [Google Scholar] [CrossRef] [PubMed]
- Tetel, M.J.; Lange, C.A. 44—Molecular Genomics of Progestin Actions. In Hormones, Brain and Behavior (Second Edition); Pfaff, D.W., Arnold, A.P., Etgen, A.M., Fahrbach, S.E., Rubin, R.T., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 1439–1466. [Google Scholar]
- Skolnick, B.E.; Maas, A.I.; Narayan, R.K.; van der Hoop, R.G.; MacAllister, T.; Ward, J.D.; Nelson, N.R.; Stocchetti, N. A clinical trial of progesterone for severe traumatic brain injury. N. Engl. J. Med. 2014, 371, 2467–2476. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, Y.; Peng, R.; Shi, M.; Gao, W.; Lei, P.; Zhang, J. Progesterone induces neuroprotection associated with immune/inflammatory modulation in experimental traumatic brain injury. Neuroreport 2024, 35, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.G. Embracing failure: What the Phase III progesterone studies can teach about TBI clinical trials. Brain Inj. 2015, 29, 1259–1272. [Google Scholar] [CrossRef]
- Pan, Z.Y.; Zhao, Y.H.; Huang, W.H.; Xiao, Z.Z.; Li, Z.Q. Effect of progesterone administration on the prognosis of patients with severe traumatic brain injury: A meta-analysis of randomized clinical trials. Drug Des. Dev. Ther. 2019, 13, 265–273. [Google Scholar] [CrossRef]
- Bazgir, R.; Siahposht-Khachaki, A.; Akbari, E.; Farzin, D. A study of the therapeutic effects of progesterone in patients with traumatic brain injury: A systematic review and meta-analysis. Arch. Trauma Res. 2021, 10, 53–58. [Google Scholar] [CrossRef]
- Nasre-Nasser, R.G.; Severo, M.M.R.; Pires, G.N.; Hort, M.A.; Arbo, B.D. Effects of Progesterone on Preclinical Animal Models of Traumatic Brain Injury: Systematic Review and Meta-analysis. Mol. Neurobiol. 2022, 59, 6341–6362. [Google Scholar] [CrossRef]
- Biehl, J.K.; Russell, B. Introduction to stem cell therapy. J. Cardiovasc. Nurs. 2009, 24, 98–103; quiz 104–105. [Google Scholar] [CrossRef] [PubMed]
- Pischiutta, F.; Caruso, E.; Lugo, A.; Cavaleiro, H.; Stocchetti, N.; Citerio, G.; Salgado, A.; Gallus, S.; Zanier, E.R. Systematic review and meta-analysis of preclinical studies testing mesenchymal stromal cells for traumatic brain injury. NPJ Regen. Med. 2021, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Wu, J.; Wang, J.; Li, Y.; Hu, X.; Luo, S.; Xiang, D. Extracellular vesicles derived from different sources of mesenchymal stem cells: Therapeutic effects and translational potential. Cell Biosci. 2020, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.L.; Escayg, A. Extracellular vesicles in the treatment of neurological disorders. Neurobiol. Dis. 2021, 157, 105445. [Google Scholar] [CrossRef]
- Reis, C.; Gospodarev, V.; Reis, H.; Wilkinson, M.; Gaio, J.; Araujo, C.; Chen, S.; Zhang, J.H. Traumatic Brain Injury and Stem Cell: Pathophysiology and Update on Recent Treatment Modalities. Stem Cells Int. 2017, 2017, 6392592. [Google Scholar] [CrossRef]
- Muir, K.W.; Bulters, D.; Willmot, M.; Sprigg, N.; Dixit, A.; Ward, N.; Tyrrell, P.; Majid, A.; Dunn, L.; Bath, P.; et al. Intracerebral implantation of human neural stem cells and motor recovery after stroke: Multicentre prospective single-arm study (PISCES-2). J. Neurol. Neurosurg. Psychiatry 2020, 91, 396–401. [Google Scholar] [CrossRef]
- Kawabori, M.; Weintraub, A.H.; Imai, H.; Zinkevych, I.; McAllister, P.; Steinberg, G.K.; Frishberg, B.M.; Yasuhara, T.; Chen, J.W.; Cramer, S.C.; et al. Cell Therapy for Chronic TBI. Neurology 2021, 96, e1202–e1214. [Google Scholar] [CrossRef]
- Marklund, N.; Bellander, B.M.; Godbolt, A.K.; Levin, H.; McCrory, P.; Thelin, E.P. Treatments and rehabilitation in the acute and chronic state of traumatic brain injury. J. Intern. Med. 2019, 285, 608–623. [Google Scholar] [CrossRef]
- Maggio, M.G.; De Bartolo, D.; Calabrò, R.S.; Ciancarelli, I.; Cerasa, A.; Tonin, P.; Di Iulio, F.; Paolucci, S.; Antonucci, G.; Morone, G.; et al. Computer-assisted cognitive rehabilitation in neurological patients: State-of-art and future perspectives. Front. Neurol. 2023, 14, 1255319. [Google Scholar] [CrossRef]
- Gray, S.N. An Overview of the Use of Neurofeedback Biofeedback for the Treatment of Symptoms of Traumatic Brain Injury in Military and Civilian Populations. Med. Acupunct. 2017, 29, 215–219. [Google Scholar] [CrossRef]
- Calderone, A.; Carta, D.; Cardile, D.; Quartarone, A.; Rifici, C.; Calabrò, R.S.; Corallo, F. Use of Virtual Reality in Patients with Acquired Brain Injury: A Systematic Review. J. Clin. Med. 2023, 12, 7680. [Google Scholar] [CrossRef]
- Karunakaran, K.K.; Pamula, S.D.; Bach, C.P.; Legelen, E.; Saleh, S.; Nolan, K.J. Lower extremity robotic exoskeleton devices for overground ambulation recovery in acquired brain injury—A review. Front. Neurorobot. 2023, 17, 1014616. [Google Scholar] [CrossRef] [PubMed]
- Gassert, R.; Dietz, V. Rehabilitation robots for the treatment of sensorimotor deficits: A neurophysiological perspective. J. Neuroeng. Rehabil. 2018, 15, 46. [Google Scholar] [CrossRef] [PubMed]
- Clayton, E.; Kinley-Cooper, S.K.; Weber, R.A.; Adkins, D.L. Brain stimulation: Neuromodulation as a potential treatment for motor recovery following traumatic brain injury. Brain Res. 2016, 1640, 130–138. [Google Scholar] [CrossRef]
- Stein, K.Y.; Froese, L.; Gomez, A.; Sainbhi, A.S.; Vakitbilir, N.; Ibrahim, Y.; Zeiler, F.A. Intracranial Pressure Monitoring and Treatment Thresholds in Acute Neural Injury: A Narrative Review of the Historical Achievements, Current State, and Future Perspectives. Neurotrauma Rep. 2023, 4, 478–494. [Google Scholar] [CrossRef]
- Chesnut, R.M.; Videtta, W. Situational Intracranial Pressure Management: An Argument Against a Fixed Treatment Threshold. Crit. Care Med. 2020, 48, 1214–1216. [Google Scholar] [CrossRef]
- Zeiler, F.A.; Ercole, A.; Cabeleira, M.; Beqiri, E.; Zoerle, T.; Carbonara, M.; Stocchetti, N.; Menon, D.K.; Lazaridis, C.; Smielewski, P.; et al. Patient-specific ICP Epidemiologic Thresholds in Adult Traumatic Brain Injury: A CENTER-TBI Validation Study. J. Neurosurg. Anesthesiol. 2021, 33, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Czosnyka, M.; Czosnyka, Z.; Smielewski, P. Pressure reactivity index: Journey through the past 20 years. Acta Neurochir. 2017, 159, 2063–2065. [Google Scholar] [CrossRef]
- Tsigaras, Z.A.; Weeden, M.; McNamara, R.; Jeffcote, T.; Udy, A.A. The pressure reactivity index as a measure of cerebral autoregulation and its application in traumatic brain injury management. Crit. Care Resusc. 2023, 25, 229–236. [Google Scholar] [CrossRef]
- Sánchez-Porras, R.; Santos, E.; Czosnyka, M.; Zheng, Z.; Unterberg, A.W.; Sakowitz, O.W. ‘Long’ pressure reactivity index (L-PRx) as a measure of autoregulation correlates with outcome in traumatic brain injury patients. Acta Neurochir. 2012, 154, 1575–1581. [Google Scholar] [CrossRef]
- Uryga, A.; Ziółkowski, A.; Kazimierska, A.; Pudełko, A.; Mataczyński, C.; Lang, E.W.; Czosnyka, M.; Kasprowicz, M. Analysis of intracranial pressure pulse waveform in traumatic brain injury patients: A CENTER-TBI study. J. Neurosurg. 2023, 139, 201–211. [Google Scholar] [CrossRef]
- Ghaith, H.S.; Nawar, A.A.; Gabra, M.D.; Abdelrahman, M.E.; Nafady, M.H.; Bahbah, E.I.; Ebada, M.A.; Ashraf, G.M.; Negida, A.; Barreto, G.E. A Literature Review of Traumatic Brain Injury Biomarkers. Mol. Neurobiol. 2022, 59, 4141–4158. [Google Scholar] [CrossRef]
- Nielson, J.L.; Cooper, S.R.; Yue, J.K.; Sorani, M.D.; Inoue, T.; Yuh, E.L.; Mukherjee, P.; Petrossian, T.C.; Paquette, J.; Lum, P.Y.; et al. Uncovering precision phenotype-biomarker associations in traumatic brain injury using topological data analysis. PLoS ONE 2017, 12, e0169490. [Google Scholar] [CrossRef]
- Abdelhak, A.; Foschi, M.; Abu-Rumeileh, S.; Yue, J.K.; D’Anna, L.; Huss, A.; Oeckl, P.; Ludolph, A.C.; Kuhle, J.; Petzold, A.; et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat. Rev. Neurol. 2022, 18, 158–172. [Google Scholar] [CrossRef]
- Frankel, M.; Fan, L.; Yeatts, S.D.; Jeromin, A.; Vos, P.E.; Wagner, A.K.; Wolf, B.J.; Pauls, Q.; Lunney, M.; Merck, L.H.; et al. Association of Very Early Serum Levels of S100B, Glial Fibrillary Acidic Protein, Ubiquitin C-Terminal Hydrolase-L1, and Spectrin Breakdown Product with Outcome in ProTECT III. J. Neurotrauma 2019, 36, 2863–2871. [Google Scholar] [CrossRef]
- Bazarian, J.J.; Biberthaler, P.; Welch, R.D.; Lewis, L.M.; Barzo, P.; Bogner-Flatz, V.; Gunnar Brolinson, P.; Büki, A.; Chen, J.Y.; Christenson, R.H.; et al. Serum GFAP and UCH-L1 for prediction of absence of intracranial injuries on head CT (ALERT-TBI): A multicentre observational study. Lancet Neurol. 2018, 17, 782–789. [Google Scholar] [CrossRef]
- Korley, F.K.; Jain, S.; Sun, X.; Puccio, A.M.; Yue, J.K.; Gardner, R.C.; Wang, K.K.W.; Okonkwo, D.O.; Yuh, E.L.; Mukherjee, P.; et al. Prognostic value of day-of-injury plasma GFAP and UCH-L1 concentrations for predicting functional recovery after traumatic brain injury in patients from the US TRACK-TBI cohort: An observational cohort study. Lancet Neurol. 2022, 21, 803–813. [Google Scholar] [CrossRef]
- Wang, K.K.; Yang, Z.; Zhu, T.; Shi, Y.; Rubenstein, R.; Tyndall, J.A.; Manley, G.T. An update on diagnostic and prognostic biomarkers for traumatic brain injury. Expert. Rev. Mol. Diagn. 2018, 18, 165–180. [Google Scholar] [CrossRef]
- Volovici, V.; Ercole, A.; Citerio, G.; Stocchetti, N.; Haitsma, I.K.; Huijben, J.A.; Dirven, C.M.F.; van der Jagt, M.; Steyerberg, E.W.; Nelson, D.; et al. Variation in Guideline Implementation and Adherence Regarding Severe Traumatic Brain Injury Treatment: A CENTER-TBI Survey Study in Europe. World Neurosurg. 2019, 125, e515–e520. [Google Scholar] [CrossRef]
- Cnossen, M.C.; Scholten, A.C.; Lingsma, H.F.; Synnot, A.; Tavender, E.; Gantner, D.; Lecky, F.; Steyerberg, E.W.; Polinder, S. Adherence to Guidelines in Adult Patients with Traumatic Brain Injury: A Living Systematic Review. J. Neurotrauma 2021, 38, 1072–1085. [Google Scholar] [CrossRef]
- English, S.W.; Turgeon, A.F.; Owen, E.; Doucette, S.; Pagliarello, G.; McIntyre, L. Protocol management of severe traumatic brain injury in intensive care units: A systematic review. Neurocrit. Care 2013, 18, 131–142. [Google Scholar] [CrossRef]
- Dheansa, S.; Rajwani, K.M.; Pang, G.; Bench, S.; Kailaya-Vasan, A.; Maratos, E.; Lavrador, J.P.; Bhangoo, R.; Tolias, C.M. Relationship between guideline adherence and outcomes in severe traumatic brain injury. Ann. R. Coll. Surg. Engl. 2023, 105, 400–406. [Google Scholar] [CrossRef]
- Svedung Wettervik, T.M.; Lewén, A.; Enblad, P. Fine Tuning of Traumatic Brain Injury Management in Neurointensive Care—Indicative Observations and Future Perspectives. Front. Neurol. 2021, 12, 638132. [Google Scholar] [CrossRef]
- Kompanje, E.J.; Maas, A.I.; Hilhorst, M.T.; Slieker, F.J.; Teasdale, G.M. Ethical considerations on consent procedures for emergency research in severe and moderate traumatic brain injury. Acta Neurochir. 2005, 147, 633–639; discussion 639–640. [Google Scholar] [CrossRef]
- Bekar, A.; Doğan, S.; Abaş, F.; Caner, B.; Korfali, G.; Kocaeli, H.; Yilmazlar, S.; Korfali, E. Risk factors and complications of intracranial pressure monitoring with a fiberoptic device. J. Clin. Neurosci. 2009, 16, 236–240. [Google Scholar] [CrossRef]
- Sahuquillo, J.; Biestro, A. Is intracranial pressure monitoring still required in the management of severe traumatic brain injury? Ethical and methodological considerations on conducting clinical research in poor and low-income countries. Surg. Neurol. Int. 2014, 5, 86. [Google Scholar] [CrossRef]
- Chesnut, R.M.; Temkin, N.; Carney, N.; Dikmen, S.; Rondina, C.; Videtta, W.; Petroni, G.; Lujan, S.; Pridgeon, J.; Barber, J.; et al. A trial of intracranial-pressure monitoring in traumatic brain injury. N. Engl. J. Med. 2012, 367, 2471–2481. [Google Scholar] [CrossRef]
- Stiver, S.I. Complications of decompressive craniectomy for traumatic brain injury. Neurosurg. Focus 2009, 26, E7. [Google Scholar] [CrossRef]
- Coronado, V.G.; McGuire, L.C.; Sarmiento, K.; Bell, J.; Lionbarger, M.R.; Jones, C.D.; Geller, A.I.; Khoury, N.; Xu, L. Trends in traumatic brain injury in the US and the public health response: 1995–2009. J. Saf. Res. 2012, 43, 299–307. [Google Scholar] [CrossRef]
- Gilchrist, J.; Thomas, K.; Wald, M.; Langlois, J. Nonfatal traumatic brain injuries from sports and recreation activities--United States, 2001–2005. MMWR Morb. Mortal. Wkly. Rep. 2007, 56, 733. [Google Scholar]
- Parker, E.M.; Gilchrist, J.; Schuster, D.; Lee, R.; Sarmiento, K. Reach and knowledge change among coaches and other participants of the online course:“Concussion in sports: What you need to know”. J. Head. Trauma. Rehabil. 2015, 30, 198–206. [Google Scholar] [CrossRef]
- Baldwin, G.; Breiding, M.; Sleet, D. Using the public health model to address unintentional injuries and TBI: A perspective from the Centers for Disease Control and Prevention (CDC). NeuroRehabilitation 2016, 39, 345–349. [Google Scholar] [CrossRef]
- Lee, R. The CDC’s STEADI initiative: Promoting older adult health and independence through fall prevention. Am. Fam. Physician 2017, 96, 220. [Google Scholar]
- Casey, C.M.; Parker, E.M.; Winkler, G.; Liu, X.; Lambert, G.H.; Eckstrom, E. Lessons learned from implementing CDC’s STEADI falls prevention algorithm in primary care. Gerontologist 2017, 57, 787–796. [Google Scholar] [CrossRef]
- Olson, Z.; Staples, J.A.; Mock, C.; Nguyen, N.P.; Bachani, A.M.; Nugent, R.; Verguet, S. Helmet regulation in Vietnam: Impact on health, equity and medical impoverishment. Inj. Prev. 2016, 22, 233–238. [Google Scholar] [CrossRef]
- Popescu, C.; Anghelescu, A.; Daia, C.; Onose, G. Actual data on epidemiological evolution and prevention endeavours regarding traumatic brain injury. J. Med. Life 2015, 8, 272. [Google Scholar]
- Fatuki, T.A.; Zvonarev, V.; Rodas, A.W.; Bellman, V.; Rodas, A. Prevention of traumatic brain injury in the United States: Significance, new findings, and practical applications. Cureus 2020, 12, e11225. [Google Scholar] [CrossRef]
- Fong, N.; Feng, J.; Hubbard, A.; Dang, L.E.; Pirracchio, R. IntraCranial pressure prediction AlgoRithm using machinE learning (I-CARE): Training and Validation Study. Crit. Care Explor. 2024, 6, e1024. [Google Scholar] [CrossRef]
- Myers, R.B.; Lazaridis, C.; Jermaine, C.M.; Robertson, C.S.; Rusin, C.G. Predicting Intracranial Pressure and Brain Tissue Oxygen Crises in Patients With Severe Traumatic Brain Injury. Crit. Care Med. 2016, 44, 1754–1761. [Google Scholar] [CrossRef]
- Hu, X.; Xu, P.; Asgari, S.; Vespa, P.; Bergsneider, M. Forecasting ICP elevation based on prescient changes of intracranial pressure waveform morphology. IEEE Trans. Biomed. Eng. 2010, 57, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Schroder, A.; Lawrence, T.; Voets, N.; Garcia-Gonzalez, D.; Jones, M.; Peña, J.M.; Jerusalem, A. A Machine Learning Enhanced Mechanistic Simulation Framework for Functional Deficit Prediction in TBI. Front. Bioeng. Biotechnol. 2021, 9, 587082. [Google Scholar] [CrossRef]
- Raj, R.; Luostarinen, T.; Pursiainen, E.; Posti, J.P.; Takala, R.S.K.; Bendel, S.; Konttila, T.; Korja, M. Machine learning-based dynamic mortality prediction after traumatic brain injury. Sci. Rep. 2019, 9, 17672. [Google Scholar] [CrossRef]
- Raj, R.; Wennervirta, J.M.; Tjerkaski, J.; Luoto, T.M.; Posti, J.P.; Nelson, D.W.; Takala, R.; Bendel, S.; Thelin, E.P.; Luostarinen, T.; et al. Dynamic prediction of mortality after traumatic brain injury using a machine learning algorithm. npj Digit. Med. 2022, 5, 96. [Google Scholar] [CrossRef]
- Courville, E.; Kazim, S.F.; Vellek, J.; Tarawneh, O.; Stack, J.; Roster, K.; Roy, J.; Schmidt, M.; Bowers, C. Machine learning algorithms for predicting outcomes of traumatic brain injury: A systematic review and meta-analysis. Surg. Neurol. Int. 2023, 14, 262. [Google Scholar] [CrossRef]
- Mainali, S.; Park, S. Artificial Intelligence and Big Data Science in Neurocritical Care. Crit. Care Clin. 2023, 39, 235–242. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019, 18, 56–87. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.K.; Winkler, E.A.; Sharma, S.; Vassar, M.J.; Ratcliff, J.J.; Korley, F.K.; Seabury, S.A.; Ferguson, A.R.; Lingsma, H.F.; Deng, H.; et al. Temporal profile of care following mild traumatic brain injury: Predictors of hospital admission, follow-up referral and six-month outcome. Brain Inj. 2017, 31, 1820–1829. [Google Scholar] [CrossRef]
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Büki, A.; Chesnut, R.M.; et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef]
- Jiang, J.Y.; Gao, G.Y.; Feng, J.F.; Mao, Q.; Chen, L.G.; Yang, X.F.; Liu, J.F.; Wang, Y.H.; Qiu, B.H.; Huang, X.J. Traumatic brain injury in China. Lancet Neurol. 2019, 18, 286–295. [Google Scholar] [CrossRef]
- Amorim, R.L.; Oliveira, L.M.; Malbouisson, L.M.; Nagumo, M.M.; Simoes, M.; Miranda, L.; Bor-Seng-Shu, E.; Beer-Furlan, A.; De Andrade, A.F.; Rubiano, A.M.; et al. Prediction of Early TBI Mortality Using a Machine Learning Approach in a LMIC Population. Front. Neurol. 2019, 10, 1366. [Google Scholar] [CrossRef] [PubMed]
- Clavijo, A.; Khan, A.A.; Mendoza, J.; Montenegro, J.H.; Johnson, E.D.; Adeleye, A.O.; Rubiano, A.M. The Role of Decompressive Craniectomy in Limited Resource Environments. Front. Neurol. 2019, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Kanmounye, U.S. The Rise of Inflow Cisternostomy in Resource-Limited Settings: Rationale, Limitations, and Future Challenges. Emerg. Med. Int. 2021, 2021, 6630050. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarmer, L.; Khan, M.S.; Islat, G.; Alameddin, H.; Massey, M.; Kazui, S.; Chaudhry, R. Traumatic Brain Injury: Advances in Diagnostic Techniques and Treatment Modalities. J. Clin. Med. 2025, 14, 7145. https://doi.org/10.3390/jcm14207145
Zarmer L, Khan MS, Islat G, Alameddin H, Massey M, Kazui S, Chaudhry R. Traumatic Brain Injury: Advances in Diagnostic Techniques and Treatment Modalities. Journal of Clinical Medicine. 2025; 14(20):7145. https://doi.org/10.3390/jcm14207145
Chicago/Turabian StyleZarmer, Lori, Maaz S. Khan, Glenn Islat, Hanan Alameddin, Maria Massey, Saki Kazui, and Rabail Chaudhry. 2025. "Traumatic Brain Injury: Advances in Diagnostic Techniques and Treatment Modalities" Journal of Clinical Medicine 14, no. 20: 7145. https://doi.org/10.3390/jcm14207145
APA StyleZarmer, L., Khan, M. S., Islat, G., Alameddin, H., Massey, M., Kazui, S., & Chaudhry, R. (2025). Traumatic Brain Injury: Advances in Diagnostic Techniques and Treatment Modalities. Journal of Clinical Medicine, 14(20), 7145. https://doi.org/10.3390/jcm14207145





