The Evaluation of Skin Infiltration in Mycosis Fungoides/Sézary Syndrome Using the High-Frequency Ultrasonography
Abstract
1. Introduction
2. Materials and Methods
2.1. High-Frequency Ultrasonography (HFUS)
2.2. Statistics
3. Results
4. Discussion
4.1. HFUS vs. mSWAT
4.2. HFUS vs. RCM and Dermatoscopy
4.3. HFUS in MF/SS
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polanska, A.; Jenerowicz, D.; Paszynska, E.; Żaba, R.; Adamski, Z.; Dańczak-Pazdrowska, A. High-Frequency Ultrasonography—Possibilities and Perspectives of the Use of 20 MHz in Teledermatology. Front. Med. 2021, 8, 619965. [Google Scholar] [CrossRef]
- Jasaitiene, D.; Valiukeviciene, S.; Linkeviciute, G.; Grigaitiene, J.; Mickeviciene, A. Principles of high-frequency ultrasonography for investigation of skin pathology. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 375–382. [Google Scholar] [CrossRef]
- Polanska, A.; Dańczak-Pazdrowska, A.; Olek-Hrab, K.; Osmola-Mańkowska, A.; Bowszyc-Dmochowska, M.; Żaba, R.; Adamski, Z. High-frequency ultrasonography: New non-invasive method in assessment of skin lymphomas. Skin Res. Technol. 2018, 24, 517–521. [Google Scholar] [CrossRef]
- Hristov, A.C.; Tejasvi, T.; Wilcox, R.A. Cutaneous T-cell lymphomas: 2023 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2023, 98, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Criscione, V.D.; Weinstock, M.A. Incidence of cutaneous T-cell lymphoma in the United States, 1973–2002. Arch. Dermatol. 2007, 143, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.T.; Kartan, S.; Sokol, K.; Nikbakht, N.; Porcu, P. Clinical characteristics and outcomes of Black patients with mycosis fungoides and Sézary syndrome: A subgroup analysis of the phase III MAVORIC trial. Leuk. Lymphoma 2021, 62, 1877–1883. [Google Scholar] [CrossRef] [PubMed]
- Miyagaki, T. Diagnosis of Early Mycosis Fungoides. Diagnostics 2021, 11, 1721. [Google Scholar] [CrossRef]
- Torres-Cabala, C.A. Diagnosis of T-cell lymphoid proliferations of the skin: Putting all the pieces together. Mod. Pathol. 2020, 33, 83–95. [Google Scholar] [CrossRef]
- Olsen, E.A.; Rook, A.H.; Zic, J.; Kim, Y.; Porcu, P.; Querfeld, C.; Wood, G.; Demierre, M.-F.; Pittelkow, M.; Wilson, L.D.; et al. Sézary Syndrome: Immunopathogenesis, Literature Review of Therapeutic Options, and Recommendations for Therapy by the United States Cutaneous Lymphoma Consortium (USCLC). J. Am. Acad. Dermatol. 2011, 64, 352–404. [Google Scholar] [CrossRef]
- Saulite, I.; Hoetzenecker, W.; Weidinger, S.; Cozzio, A.; Guenova, E.; Wehkamp, U. Sézary Syndrome and Atopic Dermatitis: Comparison of Immunological Aspects and Targets. Biomed. Res. Int. 2016, 2016, 9717530. [Google Scholar] [CrossRef]
- Jung, J.M.; Lim, D.J.; Won, C.H.; Chang, S.E.; Lee, M.W.; Lee, W.J. Mycosis Fungoides in Children and Adolescents: A Systematic Review. JAMA Dermatol. 2021, 157, 431–438. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, Z.; Liu, J.; Zhu, Q.; Liu, Z.; Liu, Y.; Jin, H. Value of High-Frequency Ultrasound in Accurate Staging of Mycosis Fungoides/Sézary Syndrome. J. Ultrasound Med. 2020, 39, 1927–1937. [Google Scholar] [CrossRef]
- Taleb, E.; Yélamos, O.; Ardigo, M.; Christensen, R.E.; Geller, S. Non-invasive Skin Imaging in Cutaneous Lymphomas. Am. J. Clin. Dermatol. 2024, 25, 79–89. [Google Scholar] [CrossRef]
- Argalia, G.; Reginelli, A.; Molinelli, E.; Russo, A.; Michelucci, A.; Sechi, A.; Marzano, A.V.; Desyatnikova, S.; Fogante, M.; Patanè, V.; et al. High-Frequency and Ultra-High-Frequency Ultrasound in Dermatologic Diseases and Aesthetic Medicine. Medicina 2025, 61, 220. [Google Scholar] [CrossRef]
- Cisoń, H.; Jankowska-Konsur, A.; Białynicki-Birula, R. Could Residents Adequately Assess the Severity of Skin Lesions in Mycosis Fungoides/Sézary Syndrome? Evaluation of Interrater Agreement and Interrater Reliability of mSWAT. J. Clin. Med. 2024, 14, 75. [Google Scholar] [CrossRef]
- Fernández-de-Misa, R.; Hernández Machín, B.; Aguirre-Jaime, A.; Pérez-Méndez, L.I.; Peñate, Y.; Suárez Hernández, J. Does the new staging system proposed for mycosis fungoides and Sézary syndrome provide reliable agreement for T1 and T2 disease? Dermatology 2015, 230, 40–45. [Google Scholar] [CrossRef]
- Scarisbrick, J.J.; Morris, S. How big is your hand and should you use it to score skin in cutaneous T-cell lymphoma? Br. J. Dermatol. 2013, 169, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Melhoranse Gouveia, B.; Wells, J.; Kim, J.; Collgros, H.; Guitera, P.; Longo, C.; Fernandez-Penas, P. Reflectance confocal microscopy role in mycosis fungoides follow-up. Skin Res. Technol. 2020, 27, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.H.; Ramadan, W.M.; Elashmawy, A.A.; Sarsik, S.; Lallas, A. Dermoscopy in the diagnosis of mycosis fungoides: Can it help? Dermatol. Pract. Concept. 2023, 13, e2023284. [Google Scholar] [CrossRef] [PubMed]
- Polanska, A.; Osmola-Mańkowska, A.; Olek-Hrab, K.; Dańczak-Pazdrowska, A.; Żaba, R.; Adamski, Z. High-frequency ultrasonography in objective evaluation of the efficacy of PUVA and UVA1 phototherapy in mycosis fungoides. Arch. Dermatol. Res. 2017, 309, 645–651. [Google Scholar] [CrossRef]
- Polanska, A.; Bowszyc-Dmochowska, M.; Olek-Hrab, K.; Adamski, Z.; Żaba, R.; Dańczak-Pazdrowska, A. High-frequency ultrasonography: A new quantitative method in evaluation of skin lymphomas—First comparative study in relation to histopathology. Skin Res. Technol. 2019, 25, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Wang, Y.; Zhu, Q.; Liu, J.; Liu, Y.; Jin, H. The value of high-frequency ultrasonography in the differential diagnosis of early mycosis fungoides and inflammatory skin diseases: A case-control study. Skin Res. Technol. 2021, 27, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Janowska, A.; Fidanzi, C.; Romanelli, M.; Michelucci, A.; Bevilacqua, M.; Dini, V. Ultra-high-frequency Ultrasound in the Objective Assessment of Chlormethine Gel Efficacy: A Case Report. Acta Dermatovenerol. Croat. 2024, 32, 105–108. [Google Scholar] [PubMed]
- Mandava, A.; Koppula, V.; Wortsman, X.; Catalano, O.; Alfageme, F. The clinical value of imaging in primary cutaneous lymphomas: Role of high resolution ultrasound and PET-CT. Br. J. Radiol. 2019, 92, 20180904. [Google Scholar] [CrossRef]
- Wohlmuth-Wieser, I.; Ramjist, J.M.; Shear, N.; Alhusayen, R. Morphologic Features of Cutaneous T-Cell Lymphomas Using Dermoscopy and High Frequency Ultrasound. J. Clin. Med. 2020, 10, 17. [Google Scholar] [CrossRef]
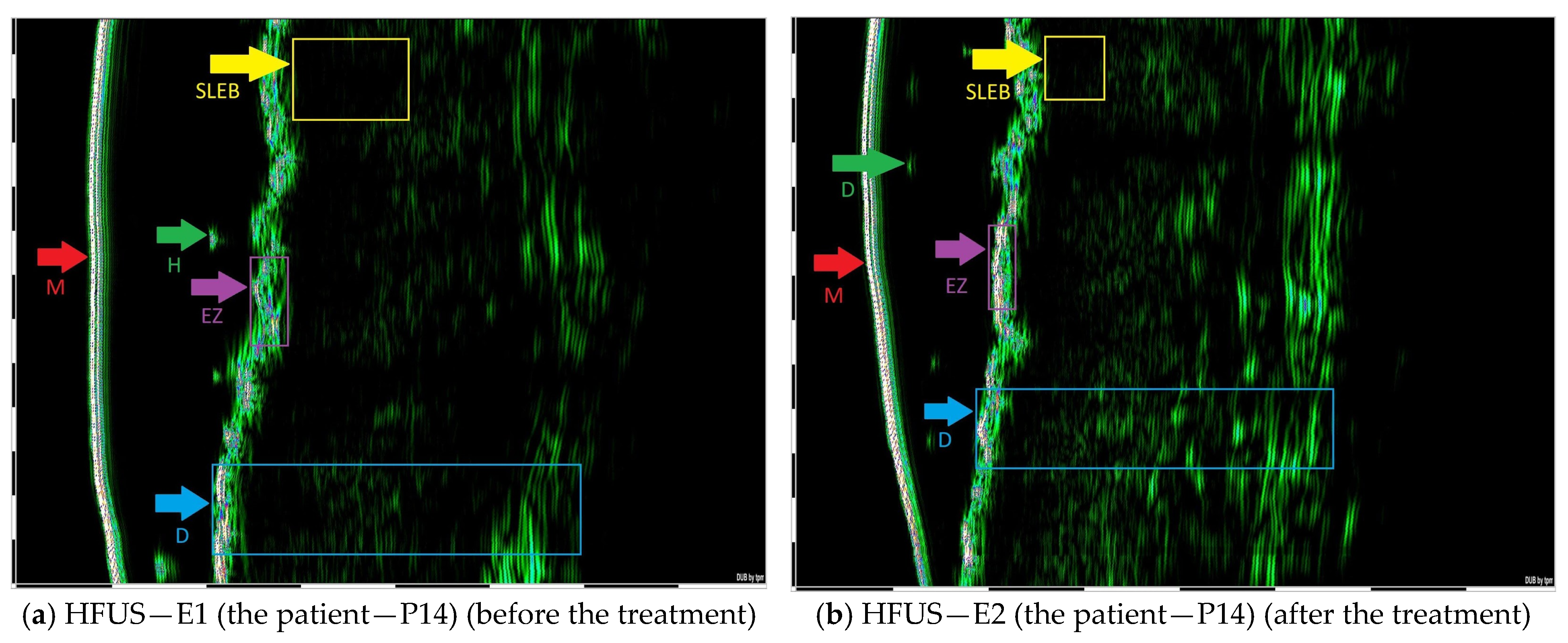
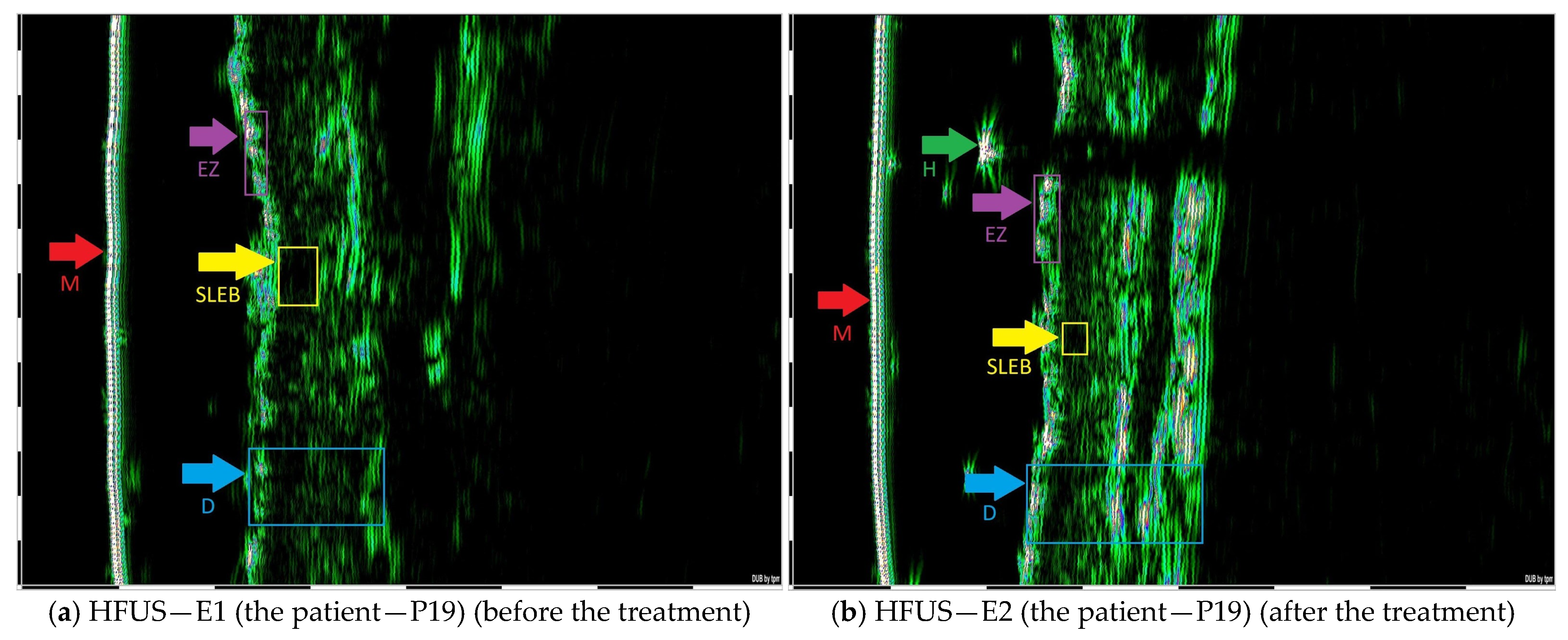
| Selected CTCL Type | HFUS Characteristics |
|---|---|
| MF—Patch stage (early stage) | The epidermis with sharply demarcated margins and no detectable posterior acoustic enhancement. SLEB demonstrates a homogeneous echotexture throughout. |
| MF—Plaque stage (early stage) | The epidermis with an irregular, undulating contour and well-defined margins, absence of posterior acoustic enhancement. SLEB is presented with a homogeneous echotexture. |
| MF—Tumor stage (advance stage) | The epidermis with a wavy and irregular contour and poorly defined margins. Hypoechoic infiltration extends into the deep dermis and subcutaneous tissue. |
| Folliculotropic MF | SLEB exhibited well-defined boundaries and a homogeneous echotexture. Patchy hypoechoic foci with ill-defined margins are observed surrounding dermal hair follicles. Additionally, round hyperechoic deposits are noted in association with the hair follicles within the dermis. |
| SS | The epidermis is even and continuous. Patchy hypoechoic foci with indistinct margins are observed in the periadnexal regions surrounding hair follicles. |
| n | % | ||
|---|---|---|---|
| Gender | Male | 27 | 87.1% |
| Female | 4 | 12.9% | |
| Age | To 66 years | 16 | 51.6% |
| From 66 and above | 15 | 48.4% | |
| Treatment during SLEB | Systemic | 22 | 71.0% |
| Topical | 9 | 29.0% |
| Patient | Diagnosis | Gender | Treatment | E1 [mm] | E2 [mm] | E1–E2 [mm] Difference | Examined Body Region |
|---|---|---|---|---|---|---|---|
| P1 | MF | m | MTX 15 mg p.w. plus clobetasol propionate cream 0.05% b.i.d. | 0.42 | 0.29 | 0.13 | E FR L |
| P2 | MF | m | chlormethine gel q.d. | 0.55 | 0.44 | 0.11 | E FR R |
| P3 | MF | m | chlormethine gel q.d. | 0.18 | 0.11 | 0.07 | F SH R |
| P4 | MF | m | chlormethine gel q.d. | 0.67 | 0.48 | 0.19 | F AR R |
| P5 | MF | m | MTX 25 mg p.w. plus chlormethine gel q.d. | 1.02 | 0.81 | 0.21 | E TH L |
| P6 | MF | f | deflazacort 6 mg q.d. plus chlormethine gel q.d. | 0.24 | 0.14 | 0.1 | F AF R |
| P7 | SS | m | brentuksymab plus deflazacort 6 mg q.d. | 0.56 | 0.43 | 0.13 | F AR R |
| P8 | MF | m | MTX 15 mg p.w. | 0.34 | 0.25 | 0.09 | F AF L |
| P9 | MF | m | chlormethine gel q.d. | 0.7 | 0.64 | 0.06 | F AR L |
| P10 | MF | m | MTX 15 mg p.w. | 0.31 | 0.25 | 0.06 | F AF R |
| P11 | MF | f | nbUVB (311 nm) | 0.29 | 0.26 | 0.03 | F AF R |
| P12 | MF | m | nbUVB (311 nm) | 0.35 | 0.21 | 0.14 | F AF L |
| P13 | MF | m | clobetasol propionate cream 0.05% b.i.d. | 0.41 | 0.27 | 0.14 | F AF R |
| P14 | MF | m | MTX 15 mg p.w. | 1.28 | 0.78 | 0.5 | E TH R |
| P15 | MF | f | clobetasol propionate cream 0.05% b.i.d. | 0.4 | 0.27 | 0.13 | E FR L |
| P16 | MF | m | nbUVB (311 nm) | 0.41 | 0.36 | 0.05 | E FR R |
| P17 | MF | m | MTX 15 mg p.w. | 0.56 | 0.44 | 0.12 | E FR L |
| P18 | MF | m | clobetasol propionate cream 0.05% b.i.d. | 0.73 | 0.65 | 0.08 | E FR R |
| P19 | MF | m | MTX 15 mg p.w. | 0.47 | 0.3 | 0.17 | E FR L |
| P20 | MF | m | PUVA | 1.12 | 0.76 | 0.36 | E TH R |
| P21 | MF | m | MTX 15 mg p.w. | 0.59 | 0.48 | 0.11 | E FR L |
| P22 | MF | m | MTX 20 mg p.w. | 0.52 | 0.45 | 0.07 | E FR L |
| P23 | MF | m | MTX 15 mg p.w. | 0.47 | 0.35 | 0.12 | E FR L |
| P24 | MF | m | MTX 20 mg p.w. | 1.01 | 0.93 | 0.08 | E TH L |
| P25 | SS | m | PUVA | 0.33 | 0.28 | 0.05 | F AF R |
| P26 | MF | m | PUVA, MTX 15 mg p.w. | 3.17 | 1.45 | 1.72 | E TH R |
| P27 | MF | m | MTX 15 mg p.w. | 2.6 | 2.32 | 0.28 | E TH R |
| P28 | MF | m | MTX 15 mg p.w. | 0.3 | 0.17 | 0.13 | F AF L |
| P29 | MF | m | PUVA | 0.51 | 0.31 | 0.2 | E TH R |
| P30 | MF | m | MTX 20 mg p.w. | 0.55 | 0.41 | 0.14 | E AR R |
| P31 | fMF | m | chlormethine gel q.d. and RT | 5.71 | 4.85 | 0.86 | E TR L |
| P32 | MF | m | chlormethine gel q.d. | 1.3 | 1.19 | 0.11 | E TH R |
| P33 | MF | f | chlormethine gel q.d. | 0.83 | 0.64 | 0.19 | E TH L |
| N | M | SD | F | p | η2 | Post Hoc | ||
|---|---|---|---|---|---|---|---|---|
| A | before treatment | 31 | 0.90 | 1.10 | 8.88 | 0.006 | 0.23 | B < A ** |
| B | after treatment | 31 | 0.69 | 0.89 | ||||
| I | systemic | 44 | 0.88 | 1.16 | 0.58 | 0.452 | 0.02 | n.s. |
| II | topical | 18 | 0.58 | 0.31 | ||||
| A.I | before treatment systemic | 22 | 1.01 | 1.29 | 1.18 | 0.286 | 0.03 | n.s. |
| A.II | before treatment topical | 9 | 0.64 | 0.32 | ||||
| B.I | after treatment systemic | 22 | 0.75 | 1.04 | ||||
| B.II | after treatment topical | 9 | 0.52 | 0.32 |
| N | M | SD | F | p | η2 | Post Hoc | ||
|---|---|---|---|---|---|---|---|---|
| A | before treatment | 31 | 0.90 | 1.10 | 16.59 | 0.000 | 0.33 | B < A ** |
| B | after treatment | 31 | 0.69 | 0.89 | ||||
| I | to 66 years | 32 | 0.44 | 0.26 | 4.66 | 0.039 | 0.14 | I < II * |
| II | from 66 and above | 30 | 1.17 | 1.32 | ||||
| A.I | before treatment to 66 years | 16 | 0.50 | 0.26 | 4.79 | 0.037 | 0.10 | A.I < A.II * |
| A.II | before treatment from 66 and above | 15 | 1.34 | 1.46 | B.I < B.II † | |||
| B.I | after treatment to 66 years | 16 | 0.39 | 0.25 | B.II < A.II ** | |||
| B.II | after treatment from 66 and above | 15 | 1.00 | 1.20 |
| N | M | SD | F | p | η2 | Post Hoc | ||
|---|---|---|---|---|---|---|---|---|
| A | before treatment | 31 | 0.90 | 1.10 | 3.97 | 0.056 | 0.12 | B < A |
| B | after treatment | 31 | 0.69 | 0.89 | ||||
| I | Male | 54 | 0.86 | 1.06 | 0.79 | 0.383 | 0.03 | n.s. |
| II | Female | 8 | 0.38 | 0.23 | ||||
| A.I | before treatment male | 27 | 0.97 | 1.16 | 0.48 | 0.492 | 0.01 | n.s. |
| A.II | before treatment female | 4 | 0.44 | 0.27 | ||||
| B.I | after treatment male | 27 | 0.74 | 0.95 | ||||
| B.II | after treatment female | 4 | 0.33 | 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cisoń, H.; Jankowska-Konsur, A.; Białynicki-Birula, R. The Evaluation of Skin Infiltration in Mycosis Fungoides/Sézary Syndrome Using the High-Frequency Ultrasonography. J. Clin. Med. 2025, 14, 7143. https://doi.org/10.3390/jcm14207143
Cisoń H, Jankowska-Konsur A, Białynicki-Birula R. The Evaluation of Skin Infiltration in Mycosis Fungoides/Sézary Syndrome Using the High-Frequency Ultrasonography. Journal of Clinical Medicine. 2025; 14(20):7143. https://doi.org/10.3390/jcm14207143
Chicago/Turabian StyleCisoń, Hanna, Alina Jankowska-Konsur, and Rafał Białynicki-Birula. 2025. "The Evaluation of Skin Infiltration in Mycosis Fungoides/Sézary Syndrome Using the High-Frequency Ultrasonography" Journal of Clinical Medicine 14, no. 20: 7143. https://doi.org/10.3390/jcm14207143
APA StyleCisoń, H., Jankowska-Konsur, A., & Białynicki-Birula, R. (2025). The Evaluation of Skin Infiltration in Mycosis Fungoides/Sézary Syndrome Using the High-Frequency Ultrasonography. Journal of Clinical Medicine, 14(20), 7143. https://doi.org/10.3390/jcm14207143






