Electrocardiographic Predictors of High-Risk Patent Foramen Ovale Anatomy Defined by Transesophageal Echocardiography
Abstract
1. Introduction
2. Methodology
2.1. Study Design and Population
- Patients who underwent percutaneous PFO closure;
- Age ≥ 18 years;
- Availability of complete medical, clinical, and laboratory records;
- TEE performed as part of the diagnostic evaluation.
- Inadequate echocardiographic or ECG image quality;
- Known pulmonary hypertension;
- Heart failure with either reduced or preserved ejection fraction;
- Severe organic or secondary valvular disease;
- Pre-excitation syndromes (e.g., Wolff–Parkinson–White);
- Systemic illnesses likely to confound outcomes (e.g., advanced hepatic dysfunction, active infection, or malignancy).
2.2. Electrocardiographic Evaluation
2.3. Echocardiographic Assessment
2.4. Laboratory Analysis
2.5. Statistical Analysis
2.6. Study Outcomes
3. Results
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, D.; Shi, Q.; Xu, G.; Hu, Z.; Li, X.; Li, Q.; Guo, Y.; Xu, S.; Lin, Y.; Yu, Z.; et al. Clinical and infarction patterns of PFO-related cryptogenic strokes and a prediction model. Ann. Clin. Transl. Neurol. 2018, 5, 1323–1337. [Google Scholar] [CrossRef]
- Nakayama, R.; Takaya, Y.; Akagi, T.; Watanabe, N.; Ikeda, M.; Nakagawa, K.; Toh, N.; Ito, H. Identification of High-Risk Patent Foramen Ovale Associated with Cryptogenic Stroke: Development of a Scoring System. J. Am. Soc. Echocardiogr. 2019, 32, 811–816. [Google Scholar] [CrossRef]
- Wang, L.; Sun, H.; Shen, H. Anatomical Significance of the Patent Foramen Ovale by Real-Time 3D TEE in Cryptogenic Stroke and Migraine. Echocardiography 2024, 41, e70018. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Gonzalez, J.B.; Testai, F.D. Advances and ongoing controversies in PFO closure and cryptogenic stroke. Handb. Clin. Neurol. 2021, 177, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Ay, H.; Buonanno, F.S.; Abraham, S.A.; Kistler, J.P.; Koroshetz, W.J. An electrocardiographic criterion for diagnosis of patent foramen ovale associated with ischemic stroke. Stroke 1998, 29, 1393–1397. [Google Scholar] [CrossRef] [PubMed]
- Bayar, N.; Arslan, Ş.; Köklü, E.; Cagirci, G.; Cay, S.; Erkal, Z.; Ayoglu, R.U.; Küçükseymen, S. The importance of electrocardiographic findings in the diagnosis of atrial septal defect. Kardiol. Pol. 2015, 73, 331–336. [Google Scholar] [CrossRef]
- Jin, P.; Jiao, P.; Feng, J.; Shi, L.; Ma, L. The predictive value of abnormal electrocardiogram for patent foramen ovale: A retrospective study. Clin. Cardiol. 2023, 46, 1504–1510. [Google Scholar] [CrossRef]
- Belvís, R.; Leta, R.G.; Martínez-Domeño, A.; Planas, F.; Martí-Fàbregas, J.; Carreras, F.; Cocho, D.; Pons-Lladó, G.; Martí-Vilalta, J.L.; Luna, A.B. Electrocardiographic findings in patients with cryptogenic ischemic stroke and patent foramen ovale. J. Electrocardiol. 2007, 40, 168–171. [Google Scholar] [CrossRef]
- Caso, V.; Turc, G.; Abdul-Rahim, A.H.; Castro, P.; Hussain, S.; Lal, A.; Mattle, H.; Korompoki, E.; Søndergaard, L.; Toni, D.; et al. European Stroke Organisation (ESO) Guidelines on the diagnosis and management of patent foramen ovale (PFO) after stroke. Eur. Stroke J. 2024, 9, 800–834. [Google Scholar] [CrossRef]
- Miranda, B.; Fonseca, A.C.; Ferro, J.M. Patent foramen ovale and stroke. J. Neurol. 2018, 265, 1943–1949. [Google Scholar] [CrossRef]
- Torti, S.R.; Billinger, M.; Schwerzmann, M.; Vogel, R.; Zbinden, R.; Windecker, S.; Seiler, C. Risk of decompression illness among 230 divers in relation to the presence and size of patent foramen ovale. Eur. Heart J. 2004, 25, 1014–1020. [Google Scholar] [CrossRef]
- Liu, K.; Wang, B.Z.; Hao, Y.; Song, S.; Pan, M. The Correlation Between Migraine and Patent Foramen Ovale. Front. Neurol. 2020, 11, 543485. [Google Scholar] [CrossRef] [PubMed]
- Allemann, Y.; Hutter, D.; Lipp, E.; Sartori, C.; Duplain, H.; Egli, M.; Cook, S.; Scherrer, U.; Seiler, C. Patent foramen ovale and high-altitude pulmonary edema. JAMA 2006, 296, 2954–2958. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, A.; Vitale, G.; Romano, S.; Sbaraini Zernini, I.; Galeotti, L.; Cescon, M.; Ravaioli, M.; Siniscalchi, A. Transcranial Doppler Ultrasound and Transesophageal Echocardiography for Intraoperative Diagnosis and Monitoring of Patent Foramen Ovale in Non-Cardiac Surgery. Appl. Sci. 2024, 14, 4590. [Google Scholar] [CrossRef]
- Devos, P.; Guedeney, P.; Montalescot, G.; on behalf of the ACTION Study Group. Patent Foramen Ovale Percutaneous Closure: Evolution and Ongoing Challenges. J. Clin. Med. 2024, 13, 54. [Google Scholar] [CrossRef]
- Erdoğan, A.; Genç, Ö.; Demirtola, A.I.; Inan, D.; Şen, F.; Güler, Y.; Güler, A.; Ozkul, A. Assessment of Clinical Outcomes After Percutaneous Patent Foramen Ovale Closure in Adult Patients Diagnosed with Cryptogenic Stroke. East. J. Med. 2023, 28, 783–789. [Google Scholar] [CrossRef]
- Węglarz, P.; Węgiel, M.; Kuszewski, P.; Konarska-Kuszewska, E.; Staszel, M.; Nowok, M.; Bajor, G.; Mizia-Stec, K.; Dziewierz, A.; Rakowski, T. Atrial septum anatomy as a predictor of ischemic neurological episodes in patients with a patent foramen ovale. Kardiol. Pol. 2024, 82, 303–307. [Google Scholar] [CrossRef]
- Lee, P.H.; Song, J.K.; Kim, J.S.; Heo, R.; Lee, S.; Kim, D.-H.; Song, J.-M.; Kang, D.-H.; Kwon, S.U.; Kang, D.-W.; et al. Cryptogenic Stroke and High-Risk Patent Foramen Ovale: The DEFENSE-PFO Trial. J. Am. Coll. Cardiol. 2018, 71, 2335–2342. [Google Scholar] [CrossRef]
- Kalesi, A.E.; Karakosta, M.; Maritsa, D.; Ntiloudi, D.; Gardikioti, V.; Archontikis, A.; Karaminas, N.; Ntalekou, K.; Plaitis, A.; Arnas, N.; et al. Unlocking the mystery. Exploring the relationship between PFO anatomical features and the right to left shunt size. Eur. J. Prev. Cardiol. 2024, 31 (Suppl. S1), zwae175.096. [Google Scholar] [CrossRef]
- Khairy, P.; Marelli, A.J. Clinical use of electrocardiography in adults with congenital heart disease. Circulation 2007, 116, 2734–2746. [Google Scholar] [CrossRef]
- Lim, I.C.Z.Y.; Teo, Y.H.; Fang, J.T.; Teo, Y.N.; Ho, J.S.Y.; Lee, Y.Q.; Chen, X.; Ong, K.H.-X.; Leow, A.S.T.; Ho, A.F.-W.; et al. Association of Shunt Size and Long-Term Clinical Outcomes in Patients with Cryptogenic Ischemic Stroke and Patent Foramen Ovale on Medical Management. J. Clin. Med. 2023, 12, 941. [Google Scholar] [CrossRef]
- O’Connor, S.; Recavarren, R.; Nichols, L.C.; Parwani, A.V. Lipomatous hypertrophy of the interatrial septum: An overview. Arch. Pathol. Lab. Med. 2006, 130, 397–399. [Google Scholar] [CrossRef]
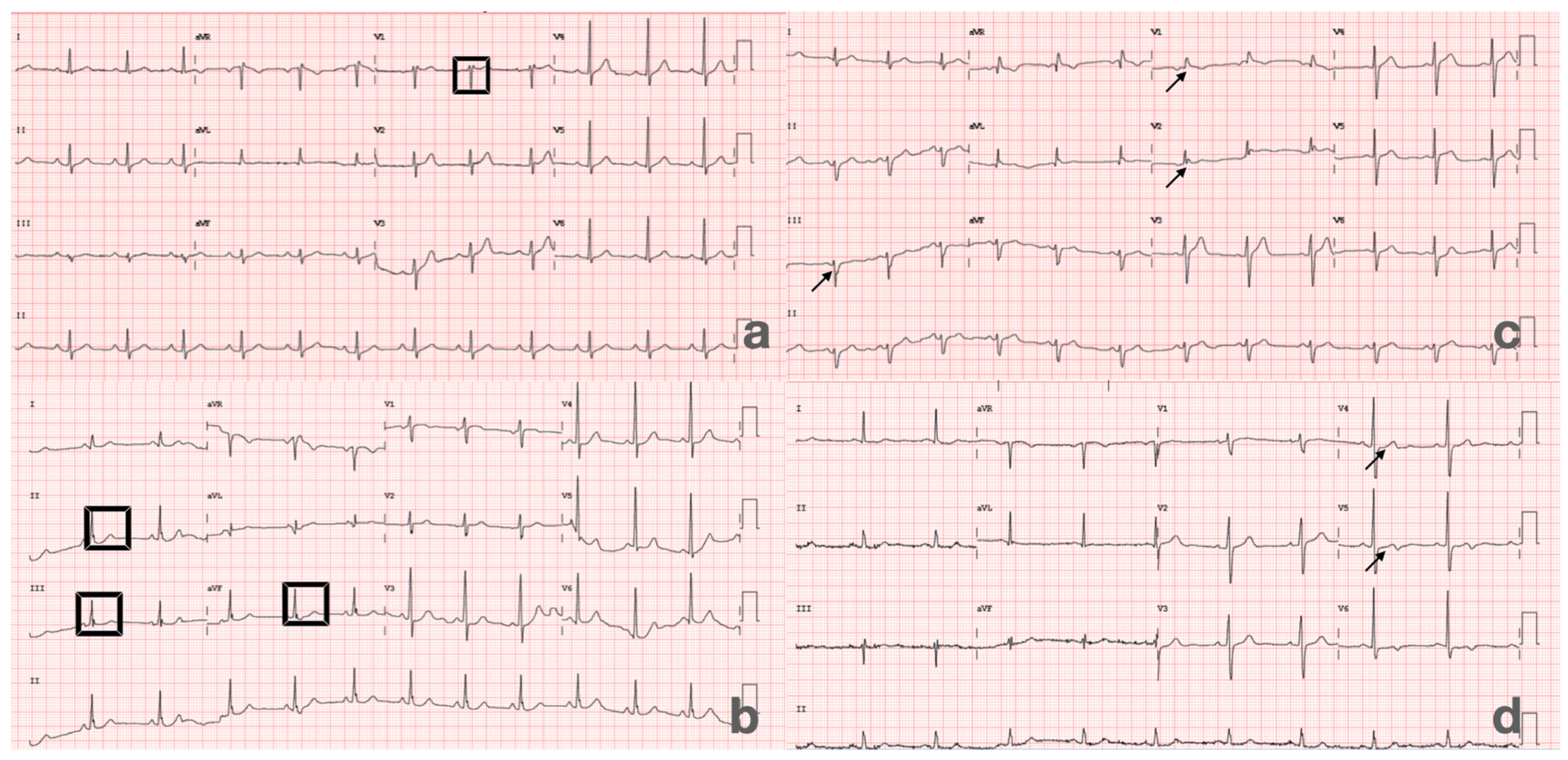
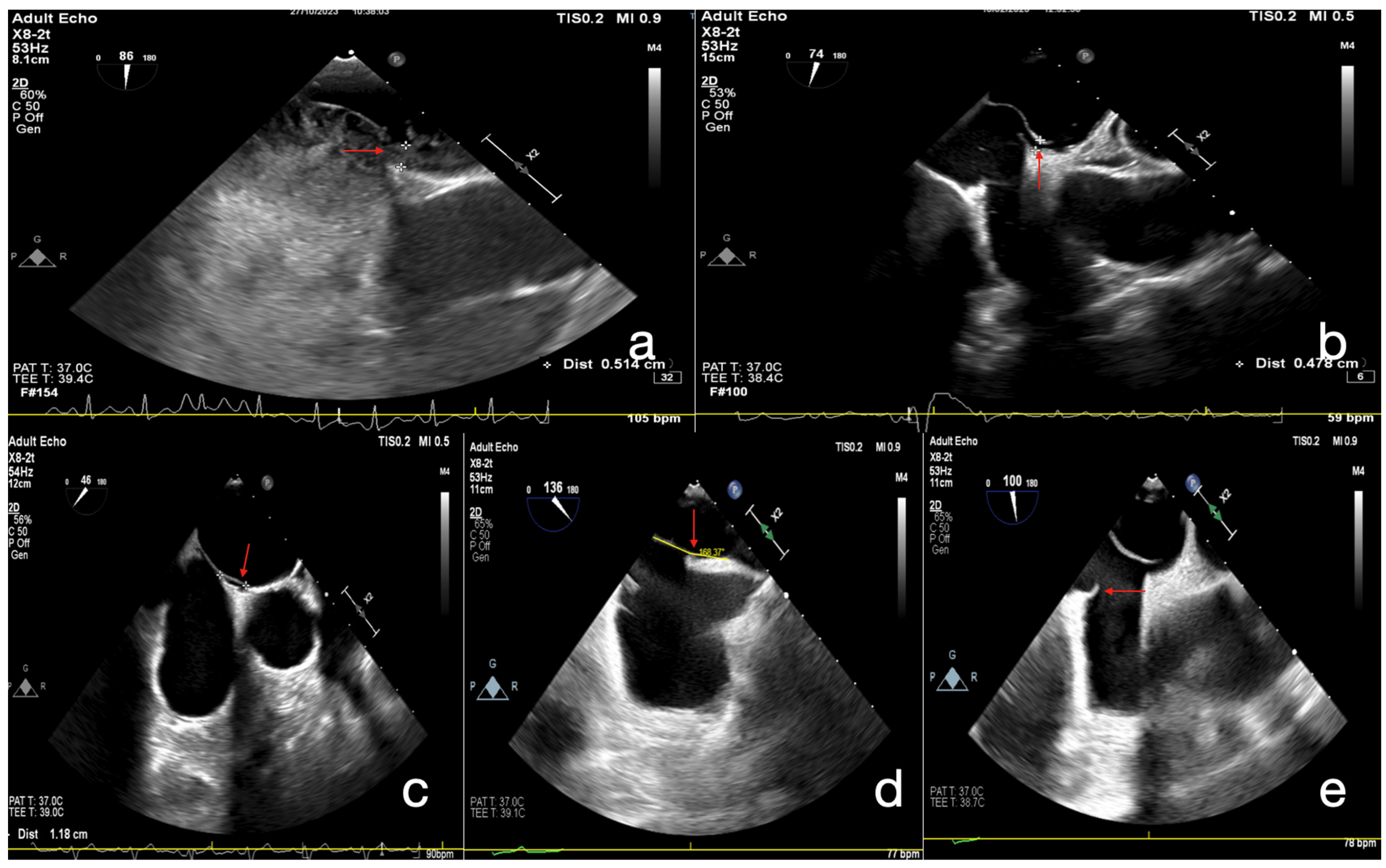
| Variable | Total (n = 207) | Score 0–1 (+) (n = 46) | Score 2–5 (n = 161) | p Value |
|---|---|---|---|---|
| Male Sex (n, %) | 97 (46.9) | 17 (37.0) | 80 (49.7) | 0.127 |
| Age (years) | 45.0 (37.0–52.0) | 46.5 (41.8–53.0) | 44.0 (35.5–50.09) | 0.042 |
| Medication (n, %) | ||||
| ACEi/ARB | 59 (28.5) | 16 (34.7) | 38 (26.7) | 0.183 |
| Beta-blocker | 80 (38.6) | 15 (32.6) | 66 (40.9) | 0.392 |
| Aspirin | 42 (20.2) | 13 (28.2) | 29 (18) | 0.188 |
| Statin | 29 (14) | 10 (21.7) | 19 (11.8) | 0.141 |
| Closure Indication (n, %) | ||||
| Stroke | 198 (95.7) | 43 (93.5) | 155 (96.3) | 0.419 |
| TIA | 9 (4.3) | 3 (6.5) | 6 (3.7) | 0.317 |
| Known Comorbidities (n, %) | ||||
| Diabetes Mellitus | 34 (16.5) | 9 (20.0) | 25 (15.5) | 0.475 |
| Hypertension | 66 (32.0) | 18 (40.0) | 48 (29.8) | 0.195 |
| Coronary Artery Disease | 20 (9.7) | 4 (8.9) | 16 (9.9) | 1.000 |
| Prior Stroke | 13 (6.3) | 4 (8.7) | 9 (5.6) | 0.491 |
| Smoking | 36 (17.7) | 4 (8.9) | 32 (20.3) | 0.078 |
| Hyperlipidemia | 45 (22.1) | 6 (13.3) | 39 (24.5) | 0.110 |
| Migraine | 8 (3.9) | 0 (0) | 8 (5.0) | 0.204 |
| Deep Vein Thrombosis | 6 (2.9) | 0 (0) | 6 (3.7) | 0.342 |
| Atrial Fibrillation | 4 (1.9) | 0 (0) | 4 (2.5) | 0.577 |
| Variable (n, (%) for the First 12 Variable, Median [IQR] for the Rest) | Total (n = 207) | Risk Score 0–1 (n = 46) | Risk Score 2–5 (n = 161) | p Value |
|---|---|---|---|---|
| ASA/Hypermobile septum | 81 (39.5) | 2 (4.4) | 79 (49.4) | <0.001 |
| Chiari/Eustachian Valve | 41 (20.0) | 1 (2.2) | 40 (25.0) | <0.001 |
| Bubble Shunt < 20 | 27 (13.5) | 19 (41.3) | 8 (5) | <0.001 |
| Bubble Shunt ≥ 20 | 180 (86.4) | 27 (58.7) | 153 (95) | <0.001 |
| Lipomatous Hypertrophy | 17 (8.2) | 6 (13.04) | 11 (6.8) | 0.023 |
| Spontaneous Color Doppler Shunt | 93 (48.4) | 13 (31.0) | 80 (53.3) | 0.010 |
| Bubble Shunt at Rest | 112 (57.4) | 24 (54.5) | 88 (58.3) | 0.659 |
| Bubble Shunt Valsalva | 194 (97.5) | 45 (100) | 149 (96.8) | 0.590 |
| Defective T Wave | 2 (1.0) | 0 (0) | 2 (1.2) | 1.000 |
| Crochetage R Wave | 73 (35.3) | 8 (17.4) | 65 (40.4) | 0.004 |
| Incomplete/complete RBBB | 9 (4.3) | 1 (2.2) | 8 (5.0) | 0.687 |
| RSR Pattern | 26 (12.6) | 5 (10.9) | 21 (13.0) | 0.695 |
| Tunnel Length (mm) | 10.0 [9.0–14.0] | 8.1 [7.0–9.1] | 11.0 [9.8–14.0] | <0.001 |
| IVC-PFO Tunnel Angle (°) | 24.0 [15.4–36.0] | 27.5 [18.8–37.0] | 22.0 [13.4–35.0] | 0.059 |
| Tunnel Width (mm) | 4.0 [3.0–5.0] | 3.2 [3.0–4.9] | 4.0 [3.2–5.0] | 0.030 |
| Septum Primum (mm) | 31.0 [28.0–35.0] | 35.5 [27.5–37.8] | 30.0 [28.0–35.0] | 0.163 |
| Septum Secundum (mm) | 20.5 [17.0–24.0] | 22.5 [18.3–25.8] | 20.0 [17.0–24.0] | 0.190 |
| Aortic Rim (mm) | 6.0 [4.3–7.2] | 6.0 [4.5–7.4] | 5.9 [4.2–7.1] | 0.321 |
| Sinus Valsalva Diameter (mm) | 32.0 [29.0–34.5] | 31.0 [28.0–35.0] | 32.0 [29.0–34.0] | 0.710 |
| Ascending Aorta Diameter (mm) | 30.0 [27.0–33.0] | 28.0 [25.5–34.5] | 30.0 [28.0–33.0] | 0.369 |
| LA Diameter (mm) | 34.0 [31.9–38.3] | 33.0 [31.5–38.5] | 34.0 [31.9–38.5] | 0.585 |
| RA Diameter (mm) | 33.0 [29.8–37.0] | 33.0 [32.0–35.5] | 33.0 [29.0–37.0] | 0.345 |
| LA/RA Ratio | 1.05 [0.97–1.13] | 1.0 [0.94–1.08] | 1.06 [1.00–1.14] | 0.112 |
| SPAP (mmHg) | 25.0 [22.0–28.0] | 28.0 [26.0–53.0] | 25.0 [21.5–28.0] | 0.105 |
| Variable | Total (n = 207) (Median [IQR]) | Risk Score 0–1 (n = 46) Median [IQR] | Risk Score 2–5 (n = 161) Median [IQR] | p Value |
|---|---|---|---|---|
| White Blood Cell (×103/µL) | 7.8 [6.6–9.5] | 8.0 [6.2–10.0] | 7.8 [6.6–9.1] | 0.742 |
| Hemoglobin (g/dL) | 13.0 [12.0–14.2] | 13.0 [12.0–15.0] | 13.3 [12.0–14.2] | 0.960 |
| Platelet (×103/µL) | 261.0 [229.0–305.0] | 272 [242–308] | 256 [225–305] | 0.073 |
| Fasting glucose (mg/dL) | 96.0 [86.0–114.0] | 97.5 [85–117.5] | 96.0 [86–114] | 0.836 |
| BUN (mg/dL) | 26.0 [21.0–32.0] | 28.0 [21–37] | 26.0 [21–32] | 0.305 |
| Creatinine (mg/dL) | 0.80 [0.69–0.90] | 0.80 [0.70–0.96] | 0.78 [0.67–0.90] | 0.132 |
| GFR (mL/dk/1.73 m2) | 101.0 [88.0–112.0] | 97.0 [83–110] | 102 [90–112] | 0.224 |
| Total protein (g/dL) | 69.0 [66.0–74.0] | 7.0 [6.7–7.6] | 7.0 [6.5–7.3] | 0.663 |
| Albumin (g/dL) | 4.3 [4.0–4.5] | 4.2 [4.1–4.5] | 4.3 [4.0–4.6] | 0.463 |
| Triglyceride (mg/dL) | 117.5 [85.0–167.0] | 108 [80–159] | 119 [86–168] | 0.437 |
| Total Cholesterol (mg/dL) | 166.0 [140.0–196.0] | 167 [139–201] | 166 [140–194] | 0.835 |
| HDL (mg/dL) | 43.0 [36.0–50.0] | 40 [35–46] | 44 [38–50] | 0.088 |
| LDL (mg/dL) | 99.0 [74.0–124.8] | 104 [70–130] | 98 [74–123] | 0.872 |
| HbA1c (%) | 5.5 [5.2–5.9] | 5.8 [5.4–6.5] | 5.5 [5.2–5.8] | 0.475 |
| Homosistein (µmol/L) | 12.5 [9.8–14.9] | 13.1 [10–17] | 12.3 [9.6–14.6] | 0.443 |
| Variable | p Value | Odds Ratio | Min–Max (95% CI) |
|---|---|---|---|
| Male Sex | 0.129 | 0.594 | 0.303–1.164 |
| Age | 0.1 | 0.974 | 0.944–1.005 |
| Diabetes Mellitus | 0.476 | 0.735 | 0.316–1.713 |
| Hypertension | 0.197 | 0.637 | 0.321–1.264 |
| Coronary Artery Disease | 0.834 | 1.131 | 0.358–3.569 |
| Smoking | 0.088 | 2.603 | 0.869–7.801 |
| Hyperlipidemia | 0.116 | 2.112 | 0.832–5.367 |
| Stroke history | 0.418 | 1.802 | 0.433–7.505 |
| Migraine | 0.999 | ||
| Deep Vein Thrombosis | 0.999 | ||
| Atrial Fibrillation | 0.999 | ||
| White Blood Cell (×103/µL) | 0.509 | 0.954 | 0.831–1.096 |
| Hemoglobin (g/dL) | 0.680 | 1.015 | 0.945–1.090 |
| Platelet (×103/µL) | 0.088 | 0.996 | 0.991–1.001 |
| Fasting glucose (mg/dL) | 0.622 | 1.003 | 0.992–1.013 |
| BUN (mg/dL) | 0.049 | 0.973 | 0.948–1.000 |
| Creatinine (mg/dL) | 0.180 | 0.484 | 0.167–1.399 |
| GFR (mL/dk/1.73 m2) | 0.161 | 1.012 | 0.995–1.029 |
| Total protein (g/dL) | 0.992 | 1.0 | 0.933–1.071 |
| Albumin (g/dL) | 0.677 | 0.981 | 0.894–1.075 |
| Triglyceride (mg/dL) | 0.589 | 1.001 | 0.996–1.006 |
| Total Cholesterol (mg/dL) | 0.438 | 1.003 | 0.995–1.012 |
| HDL (mg/dL) | 0.128 | 1.026 | 0.993–1.060 |
| LDL (mg/dL) | 0.966 | 1.0 | 0.990–1.010 |
| HbA1c (%) | 0.171 | 0.838 | 0.651–1.079 |
| Homosistein (µmol/L) | 0.146 | 0.961 | 0.911–1.014 |
| Septum primum (mm) | 0.302 | 0.956 | 0.877–1.041 |
| Septum secundum (mm) | 0.259 | 0.93 | 0.819–1.055 |
| Aortic rim (mm) | 0.122 | 0.895 | 0.777–1.030 |
| Sinus valsalva diameter (mm) | 0.747 | 1.022 | 0.894–1.170 |
| Ascending aorta diameter (mm) | 0.718 | 1.026 | 0.892–1.180 |
| Lipomatous hypertrophy | 0.018 | 0.238 | 0.072–0.785 |
| Spontaneous Color Doppler shunt | 0.012 | 2.549 | 1.230–5.283 |
| Bubble Shunt at Rest | 0.660 | 1.164 | 0.592–2.288 |
| Bubble Shunt Valsalva | 0.999 | ||
| Transcranial Doppler (Ref:No) | 0.208 | ||
| 10 HITS | 0.076 | 8.143 | 0.802–82.678 |
| >10 HITS | 0.998 | ||
| LA Diameter (apicobasal) | 0.391 | 1.050 | 0.939–1.175 |
| RA Diameter (apicobasal) | 0.526 | 0.969 | 0.878–1.069 |
| LA/RA ratio | 0.089 | 54.115 | 0.548–5345.092 |
| SPAP (mm-Hg) | 0.136 | 0.811 | 0.615–1.069 |
| Defective T Wave | 0.999 | ||
| Crochetage R Wave | 0.006 | 3.216 | 1.410–7.338 |
| Incomplete/complete RBBB | 0.426 | 2.353 | 0.287–19.316 |
| RSR Pattern | 0.695 | 4.492 | 0.437–3.464 |
| Variable | p Value | Odds Ratio | Min (95% CI) | Max (95% CI) |
|---|---|---|---|---|
| Crochetage Rw | 0.007 | 32.390 | 2.638 | 397.663 |
| Lipomatous hypertrophy | 0.022 | 0.095 | 0.013 | 0.714 |
| Spontaneous color Doppler shunt | 0.039 | 5.373 | 1.093 | 26.419 |
| Homocysteine | 0.187 | 0.948 | 0.876 | 1.026 |
| Age | 0.020 | 0.899 | 0.821 | 0.983 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalkan, S.; Tekin, M. Electrocardiographic Predictors of High-Risk Patent Foramen Ovale Anatomy Defined by Transesophageal Echocardiography. J. Clin. Med. 2025, 14, 7138. https://doi.org/10.3390/jcm14207138
Kalkan S, Tekin M. Electrocardiographic Predictors of High-Risk Patent Foramen Ovale Anatomy Defined by Transesophageal Echocardiography. Journal of Clinical Medicine. 2025; 14(20):7138. https://doi.org/10.3390/jcm14207138
Chicago/Turabian StyleKalkan, Semih, and Muhammet Tekin. 2025. "Electrocardiographic Predictors of High-Risk Patent Foramen Ovale Anatomy Defined by Transesophageal Echocardiography" Journal of Clinical Medicine 14, no. 20: 7138. https://doi.org/10.3390/jcm14207138
APA StyleKalkan, S., & Tekin, M. (2025). Electrocardiographic Predictors of High-Risk Patent Foramen Ovale Anatomy Defined by Transesophageal Echocardiography. Journal of Clinical Medicine, 14(20), 7138. https://doi.org/10.3390/jcm14207138






