Clinical Outcomes of Catheter Ablation for Atrial Fibrillation in Patients with Acute Decompensated Heart Failure
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Exclusion Criteria
2.2. Data Collection, Follow-Up, and Clinical Outcomes
2.3. Procedure Setting
2.4. Statistical Analysis
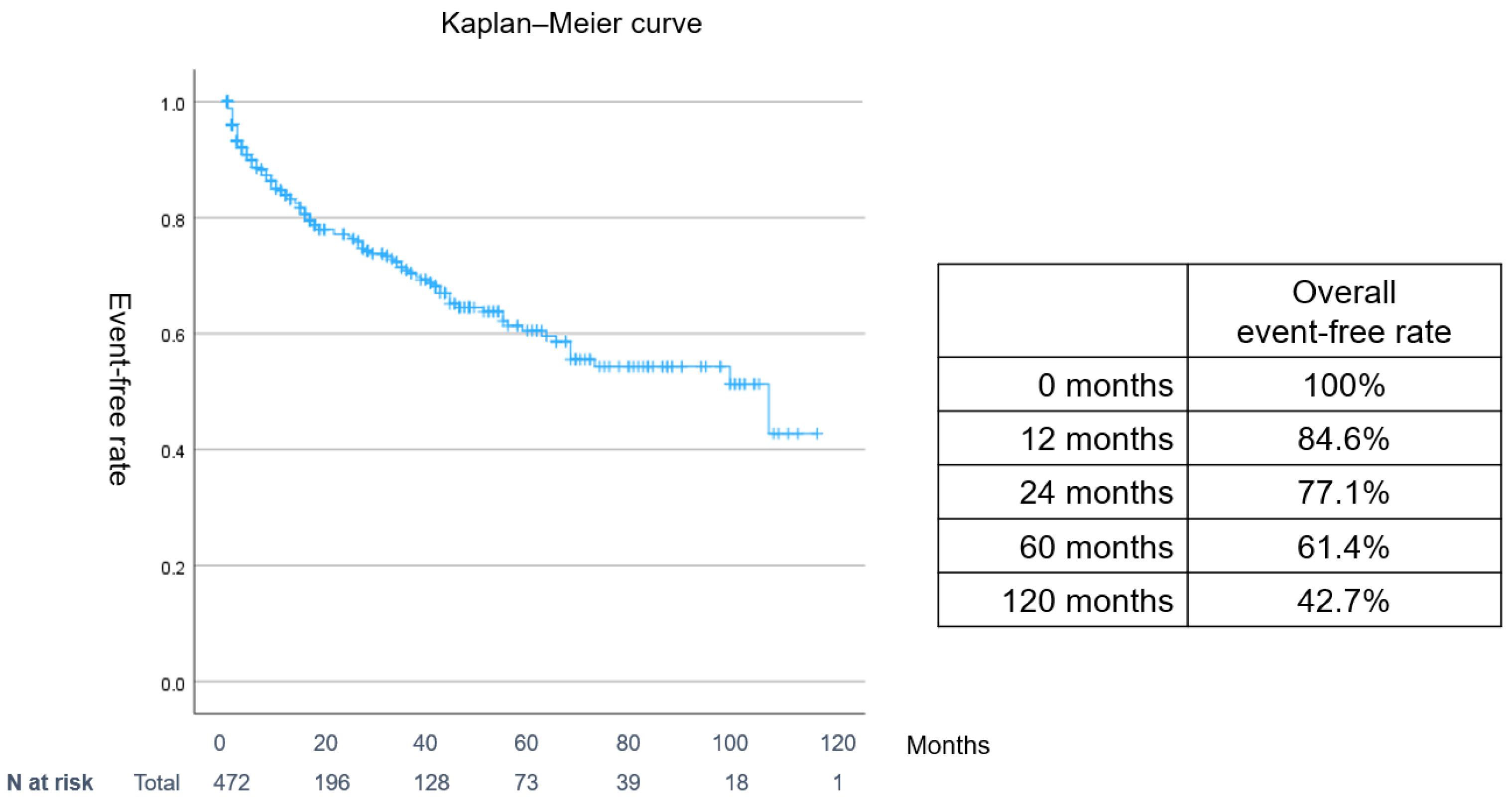
3. Results
Patients
4. Discussion
4.1. Major Findings
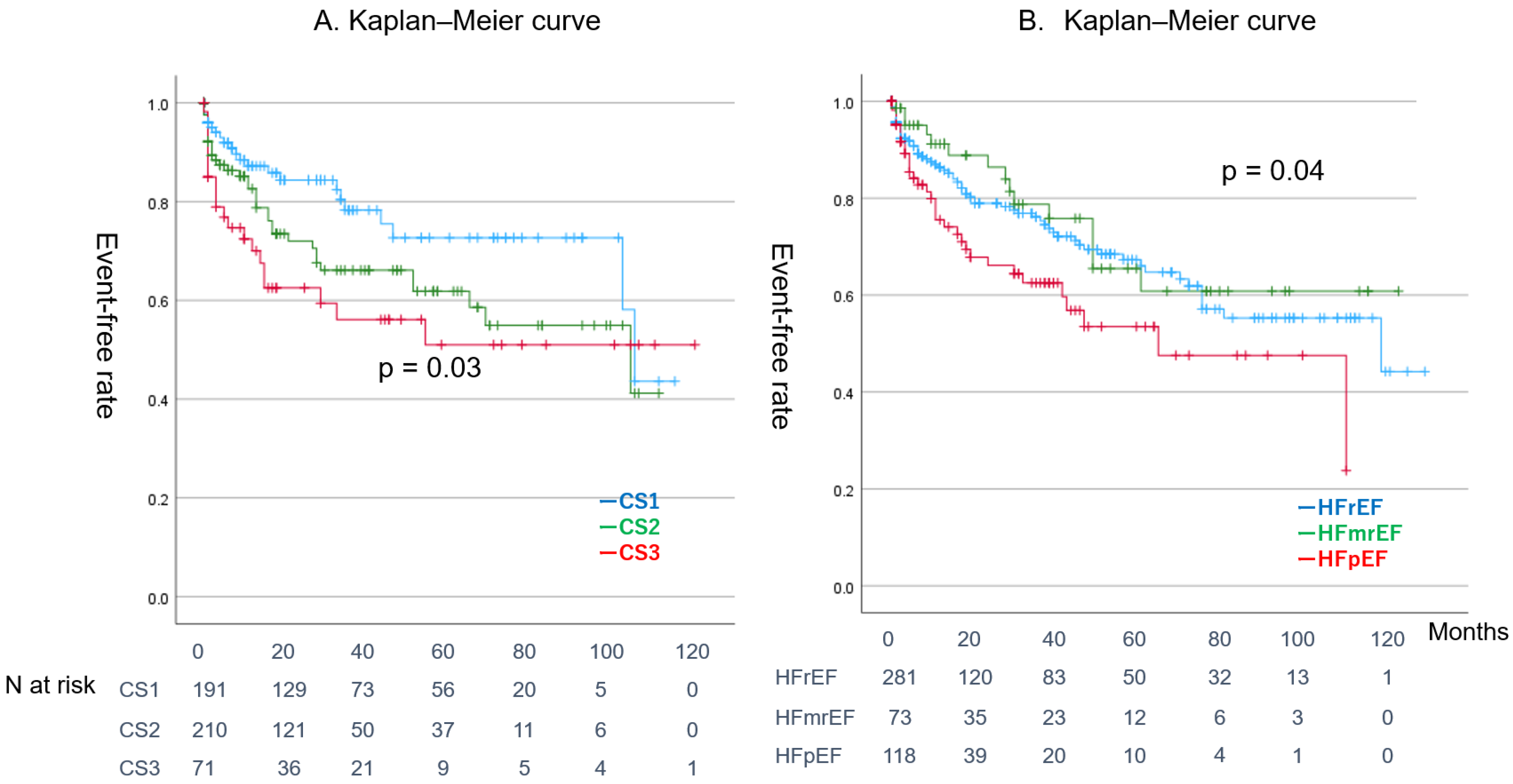
4.2. Relationship Between AF and HF Complexity of Atrial Fibrillation and Heart Failure
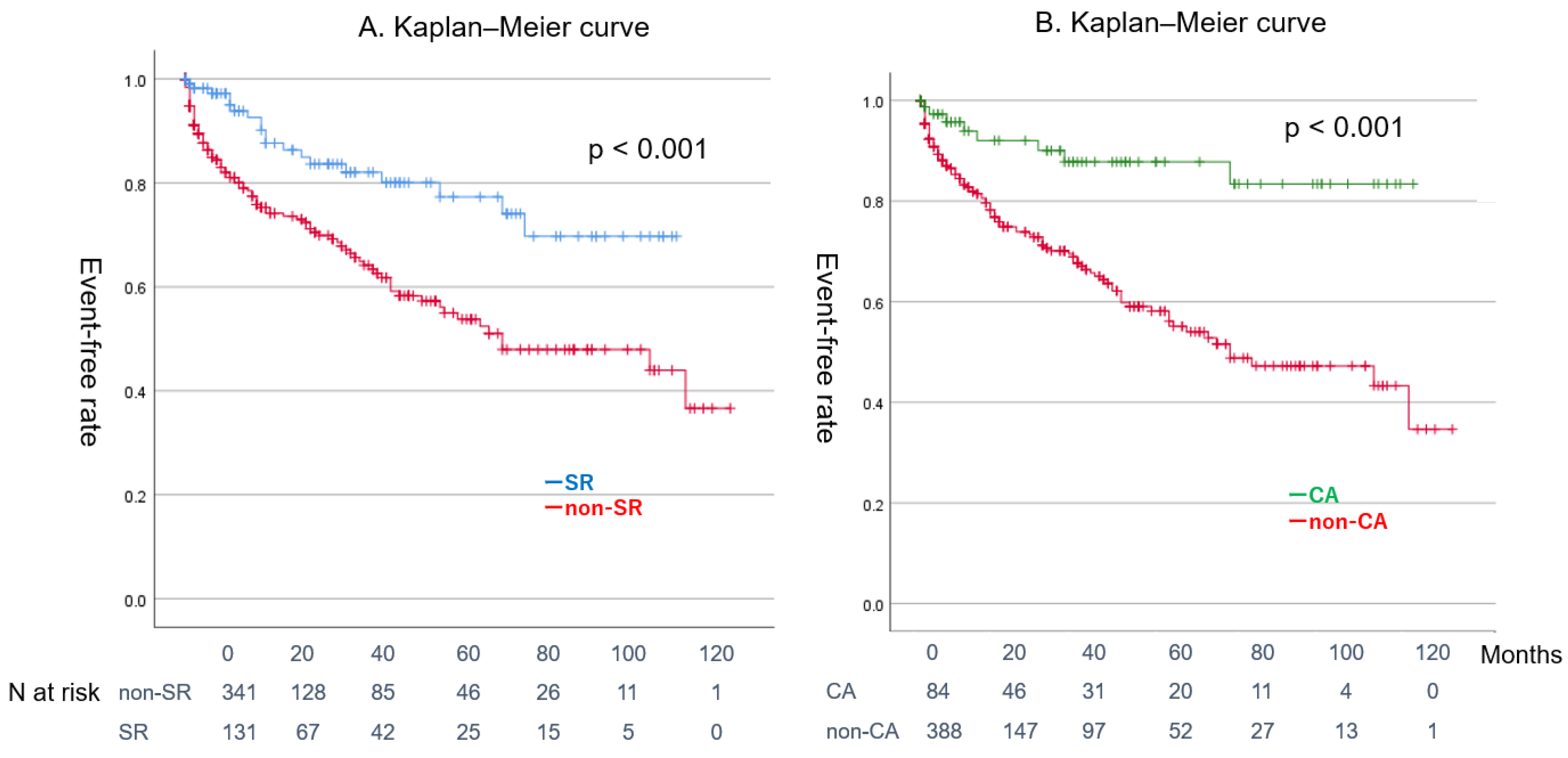
4.3. Clinical Outcomes of Patients with Concurrent ADHF and AF During Follow-Up
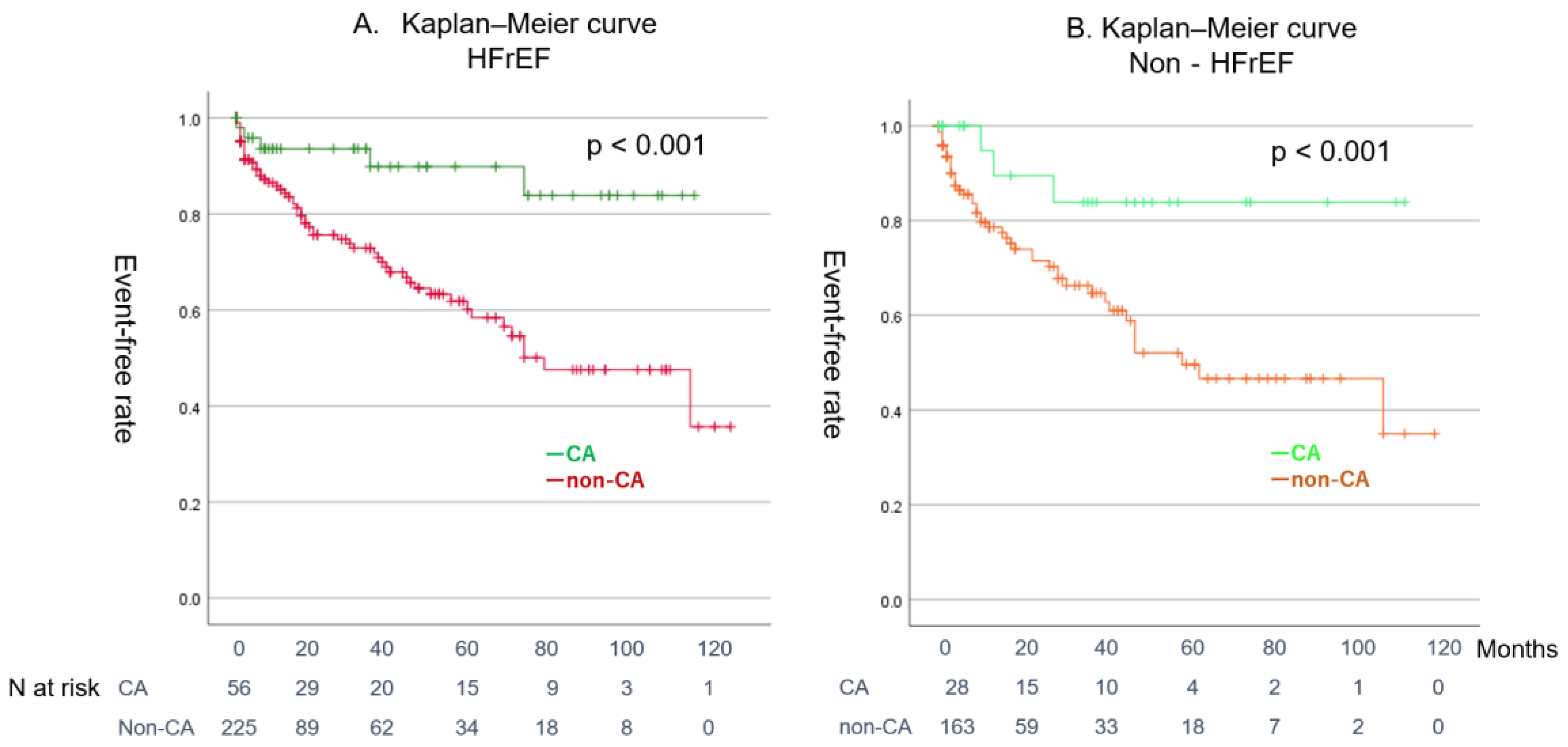
4.4. Effect of CA on Patients with Combined ADHF and AF
4.5. Clinical Implication
4.6. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fauchier, L.; Villejoubert, O.; Clementy, N.; Bernard, A.; Pierre, B.; Angoulvant, D.; Ivanes, F.; Babuty, D.; Lip, G.Y.H. Causes of death and influencing factors in patients with atrial fibrillation. Am. J. Med. 2016, 129, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Zafrir, B.; Lund, L.H.; Laroche, C.; Ruschitzka, F.; Crespo-Leiro, M.G.; Coats, A.J.S.; Anker, S.D.; Filippatos, G.; Seferovic, P.; Maggioni, A.P.; et al. ESC-HFA HF Long-Term Registry Investigators. Prognostic implications of atrial fibrillation in heart failure with reduced, mid-range, and preserved ejection fraction: A report from 14 964 patients in the European Society of Cardiology Heart Failure Long-Term Registry. Eur. Heart J. 2018, 39, 4277–4284. [Google Scholar] [CrossRef] [PubMed]
- CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N. Engl. J. Med. 1987, 316, 1429–1435. [Google Scholar] [CrossRef]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Wittes, J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Coats, A.J.S.; Fowler, M.B.; Katus, H.A.; Krum, H.; Mohacsi, P.; Rouleau, J.L.; Tendera, M.; Castaigne, A.; Roecker, E.B.; et al. Effect of carvedilol on survival in severe chronic heart failure. N. Engl. J. Med. 2001, 344, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Belohlavek, J.; et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutui, H.; Brueckmann, M.; et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Kerlan, J.E.; Sawhney, N.S.; Waggoner, A.D.; Chawla, M.K.; Garhwal, S.; Osborn, J.L.; Faddis, M.N. Prospective comparison of echocardiographic atrioventricular delay optimization methods for cardiac resynchronization therapy. Heart Rhythm. 2006, 3, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Rocca, H.P.B.L.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in heart failure with a preserved ejection fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Levy, D.; Vasan, R.S.; Leip, E.P.; Wolf, P.A.; D’Agosino, R.B.; Murabito, J.M.; Kannel, W.B.; Benjamin, E.J. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: The Framingham Heart Study. Circulation 2003, 107, 2920–2925. [Google Scholar] [CrossRef] [PubMed]
- Marrouche, N.F.; Brachmann, J.; Andresen, D.; Siebels, J.; Boersma, L.; Jordaens, L.; Merkely, B.; Pokushalov, E.; Sanders, P.; Poff, J.; et al. Catheter ablation for atrial fibrillation with heart failure. N. Engl. J. Med. 2018, 378, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.F.; Fonarow, G.C.; Emerman, C.L.; LeJemtel, T.H.; Costanzo, M.R.; Abraham, W.T.; Berkowitz, R.L.; Galvao, M.S.N.M.; Horton, D.P. Characteristics and outcomes of patients hospitalized for heart failure in the United States: Rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am. Heart J. 2005, 149, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Krum, H.; Gilbert, R.E. Demographics and concomitant disorders in heart failure. Lancet 2003, 362, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Mebazaa, A.; Gheorghiade, M.; Piña, I.L.; Harjola, V.P.; Hollenberg, S.M.; Follath, F.; Rhodes, A.; Plaisance, P.; Roland, E.; Nieminen, M.; et al. Practical recommendations for prehospital and early in-hospital management of patients presenting with acute heart failure syndromes. Crit. Care Med. 2008, 36, S129–S139. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Isobe, M.; Ito, H.; Okumura, K.; Ono, M.; Kitakaze, M.; Kinugawa, K.; Kihara, Y.; Goto, Y.; Komuro, I.; et al. JCS 2017/JHFS 2017 Guideline on Diagnosis and Treatment of Acute and Chronic Heart Failure—Digest Version. Circ. J. 2019, 83, 2084–2184. [Google Scholar] [CrossRef]
- Ahmed, A. A Propensity Matched Study of New York Heart Association Class and Natural History End Points in Heart Failure. Am. J. Cardiol. 2007, 99, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.G.F.; Bunting, K.V.; Flather, M.D.; Douglas, G.; Altman, D.G.; Holmes, J.; Coats, A.J.S.; Manzano, L.; Mcmurray, J.J.V.; Rushitzka, F.; et al. Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: An individual patient-level analysis of double-blind randomized trials. Eur. Heart J. 2018, 39, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Gage, B.F.; Waterman, A.D.; Shannon, W.; Boechler, M.; Rich, M.W.; Radford, M.J. Validation of Clinical Classification Schemes for Predicting Stroke Results From the National Registry of Atrial Fibrillation. JAMA 2001, 285, 2864–2870. [Google Scholar] [CrossRef] [PubMed]
- Teresa SM Tsang, T.S.M.; Barnes, M.E.; Gersh, B.J.; Bailey, K.R.; Seward, J.B. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am. J. Cardiol. 2002, 90, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Bauersachs, J. Heart failure drug treatment: The fantastic four. Eur. Heart J. 2021, 42, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Kohsaka, S.; Sato, N.; Takano, T.; Kitai, T.; Yoshikawa, T.; Matsue, Y. 9-Year trend in the management of acute heart failure in Japan: A report from the National Consortium of Acute Heart Failure Registries. J. Am. Heart Assoc. 2018, 7, e008687. [Google Scholar] [CrossRef] [PubMed]
- Mountantonakis, S.E.; Grau-Sepulveda, M.V.; Bhatt, D.L.; Hernandez, A.F.; Peterson, E.D.; Fonarow, G.C. Presence of atrial fibrillation is independently associated with adverse outcomes in patients hospitalized with heart failure: An analysis of get with the guidelines-heart failure. Circ. Heart Fail. 2012, 5, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Westergaard, L.M.; Alhakak, A.; Rørth, R.; Fosbøl, E.L.; Kristensen, S.L.; Svendsen, J.H.; Graff, C.; Nielsen, J.B.; Gislason, G.H.; Kober, L.; et al. Ventricular rate in atrial fibrillation and the risk of heart failure and death. Europace 2023, 25, euad088. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Lustgarten, D. Beta-blockers in atrial fibrillation-trying to make sense of unsettling results. Europace 2023, 25, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Kucukdurmaz, Z.; Kato, R.; Erdem, A.; Golcuk, E.; Tobiume, T.; Nagase, T.; Ikeda, Y.; Nakajima, Y.; Matsumura, M.; Komiyama, N.; et al. Catheter ablation for atrial fibrillation results in greater improvement in cardiac function in patients with low versus normal left ventricular ejection fraction. J. Interv. Card. Electrophysiol. 2013, 37, 179–187. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Kohsaka, S.; Ikemura, N.; Kimura, T.; Katsumata, Y.; Tanimoto, K.; Suzuki, M.; Ueda, I.; Fukuda, K.; Takatsuki, S. Catheter ablation for patients with atrial fibrillation and heart failure with reduced and preserved ejection fraction: Insights from the KiCS-AF multicentre cohort study. Europace 2023, 25, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.; Nandal, S.; Lüker, J.; Pathak, R.; Mahajan, R.; Twomey, D.; Lau, D.H.; Sanders, P. Catheter ablation of atrial fibrillation in patients with concomitant left ventricular impairment: A systematic review of efficacy and effect on ejection fraction. Heart Lung Circ. 2015, 24, 270–280. [Google Scholar] [CrossRef]
- Xie, Z.; Qi, B.; Wang, Z.; Li, F.; Chen, C.; Li, C.; Yuan, S.; Yao, S.; Zhou, J.; Ge, J. Ablation for atrial fibrillation improves the outcomes in patients with heart failure with preserved ejection fraction. Europace 2023, 26, euad363. [Google Scholar] [CrossRef] [PubMed]
- Tzeis, S.; Gerstenfeld, E.P.; Kalman, J.; Saad, E.B.; Sepehri Shamloo, A.; Andrade, J.G.; Barbhaiya, C.R.; Baykaner, T.; Boveda, S.; Calkins, H.; et al. EHRA/HRS/APHRS consensus document: 2024 European Heart Rhythm Association/Heart Rhythm Society/Asia Pacific Heart Rhythm Society/Latin American Heart Rhythm Society expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2024, 26, euae043. [Google Scholar] [CrossRef] [PubMed]
- Abualnaja, S.; Podder, M.; Hernandez, A.F.; McMurray, J.J.; Starling, R.C.; O’Connor, C.M.; Califf, R.M.; Armstrong, P.W.; Ezekowitz, J.A. Acute heart failure and atrial fibrillation: Insights from the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF) trial. J. Am. Heart Assoc. 2015, 4, e002092. [Google Scholar] [CrossRef]
- Packer, M.; Zannad, F.; Anker, S.D. Heart Failure and a Preserved Ejection Fraction: A Side-by-Side Examination of the PARAGON-HF and EMPEROR-Preserved Trials. Circulation 2021, 144, 1193–1195. [Google Scholar] [CrossRef]
- Deanfield, J.; Verma, S.; Scirica, B.M.; Kahn, S.E.; Emerson, S.S.; Ryan, D.; Lingvay, I.; Colhoun, H.M.; Plutzky, J.; Kosiborod, M.N.; et al. Semaglutide and cardiovascular outcomes in patients with obesity and prevalent heart failure: A prespecified analysis of the SELECT trial. Lancet 2024, 404, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Fragakis, N.; Patoulias, D.; Theofilis, P.; Kassimis, G.; Karamitsos, T.; El-Tanani, M.; Rizzo, M. Effects of Glucagon-Like Peptide 1 Receptor Agonists on Atrial Fibrillation Recurrence After Catheter Ablation: A Systematic Review and Meta-analysis. Adv. Ther. 2024, 41, 3749–3756. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, C.M.; Abraham, W.T.; Albert, N.M.; Clare, R.; Gattis Stough, W.; Mihai Gheorghiade, M.; Greenberg, B.H.; Yancy, C.W.; Young, B.J.; Fanarow, G. Predictors of mortality after discharge in patients hospitalized with heart failure: An analysis from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Am. Heart J. 2008, 156, 662–673. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, H.J.; Han, S.; Yoo, B.S.; Choi, D.J.; Kim, J.J.; Jeon, E.S.; Cho, M.C.; Chae, S.C.; Ryu, K.H. The limited prognostic role of echocardiograms in short-term follow-up after acute decompensated heart failure: An analysis of the Korean Heart Failure (KorHF) Registry. PLoS ONE 2017, 12, e0188938. [Google Scholar] [CrossRef] [PubMed]
- Lahoz, R.; Fagan, A.; McSharry, M.; Proudfoot, C.; Corda, S.; Studer, R. Recurrent heart failure hospitalizations are associated with increased cardiovascular mortality in patients with heart failure in Clinical Practice Research Datalink. ESC Heart Fail. 2020, 7, 1688–1699. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Qadir, H.M.; Tu, J.V.; Yun, L.; Austin, P.C.; Newton, G.E.; Lee, D.S. Diuretic dose and long-term outcomes in elderly patients with heart failure after hospitalization. Am. Heart J. 2010, 160, 264–271.e1. [Google Scholar] [CrossRef]
- Metra, M.; Davison, B.; Bettari, L.; Sun, H.; Edwards, C.; Lazzarini, V.; Piovanelli, B.; Carubelli, V.; Bugatti, S.; Lombardi, C.; et al. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ. Heart Fail. 2012, 5, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Corley, S.D.; Epstein, A.E.; DiMarco, J.P.; Domanski, M.J.; Geller, N.; Greene, H.L.; Josephson, R.A.; Kallen, J.C.; Klein, R.C.; Krahn, A.D.; et al. AFFIRM Investigators. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation 2004, 109, 1509–1513. [Google Scholar] [CrossRef] [PubMed]
- Brachmann, J.; Sohns, C.; Andresen, D.; Siebels, J.; Sehner, S.; Boersma, L.; Merkely, B.; Pokushalov, E.; Sanders, P.; Schunkert, H.; et al. Atrial fibrillation burden and clinical outcomes in heart failure: The CASTLE-AF Trial. JACC Clin. Electrophysiol. 2021, 7, 594–603. [Google Scholar] [CrossRef]
- Lo, L.W.; Chen, S.A. Cardiac remodeling after atrial fibrillation ablation. J. Atr. Fibrillation 2013, 6, 877. [Google Scholar] [CrossRef] [PubMed]
- Kramer, D.G.; Trikalinos, T.A.; Kent, D.M.; Antonopoulos, G.V.; Konstam, M.A.; Udelson, J.E. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: A meta-analytic approach. J. Am. Coll. Cardiol. 2010, 56, 392–406. [Google Scholar] [CrossRef] [PubMed]
- Van Deutekom, C.; Van Gelder, I.C.; Rienstra, M. Atrial fibrillation and heart failure temporality: Does it matter? Europace 2023, 2, 247–248. [Google Scholar] [CrossRef] [PubMed]
- Komuro, J.; Nagatomo, Y.; Mahara, K.; Isobe, M.; Goda, A.; Sujino, Y.; Mizuno, A.; Shiraishi, Y.; Kohno, T.; Kohsaka, S.; et al. West Tokyo Heart Failure (WET-HF) Registry Investigators. Clinical scenario classification for characterization and outcome prediction of acute decompensated heart failure under contemporary phenotyping. Circ. Rep. 2019, 1, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Tobushi, T.; Sakemi, T.; Honda, N.; Mukai, Y. Emergent catheter ablation for atrial fibrillation in a patient with acute decompensated heart failure on a mechanical haemodynamic support: A case report. Eur. Heart J. Case Rep. 2021, 5, ytab350. [Google Scholar] [CrossRef] [PubMed]
- Todoroki, W.; Takemoto, M.; Sakai, T.; Tsuchihashi, T. Successful recovery from acute decompensated heart failure associated with left ventricular diastolic dysfunction and atrial fibrillation by urgent radiofrequency catheter ablation using mechanical haemodynamic support: A case report. Eur. Heart J. Case Rep. 2023, 7, ytad086. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Tohyama, T.; Ide, T.; Mukai, Y.; Enzan, N.; Nagata, T.; Ikeda, M.; Takase, S.; Nagayama, Y.; Fujino, T.; et al. Efficacy of early catheter ablation for atrial fibrillation after admission for heart failure. JACC Clin. Electrophysiol. 2023, 9, 1948–1959. [Google Scholar] [CrossRef] [PubMed]

| Total Number | Total (n = 472) | Event Group (n = 115) | Control Group (n = 357) | p-Value | |
|---|---|---|---|---|---|
| Male | 274 (57%) | 74 (64%) | 200 (56%) | 0.116 | NS |
| Age | 69.5 ± 13.0 | 72.1 ± 11.0 | 68.8 ± 13.4 | 0.008 | |
| CHADS2 score | 2.2 ± 1.2 | 2.4 ± 1.3 | 2.2 ± 1.2 | 0.073 | NS |
| NYHA classification | 3.6 ± 0.6 | 3.8± 0.4 | 3.6 ± 0.6 | <0.001 | |
| BMI | 21.9 ± 3.5 | 21.2 ± 3.5 | 22.3 ± 4.6 | 0.221 | NS |
| Systolic BP(mmHg) | 134.1 ± 36.0 | 132.0 ± 37.3 | 135.6 ± 36.6 | 0.565 | NS |
| HR (bpm) | 111.9 ± 36.8 | 105.1± 37.2 | 110.3 ± 35.2 | 0.364 | NS |
| Categories | |||||
| CS1 | 191 (40%) | 44 (38%) | 147 (30%) | 0.580 | NS |
| CS2 | 210 (44%) | 43 (37%) | 167 (47%) | 0.078 | NS |
| CS3 | 71 (15%) | 28 (24%) | 43 (12%) | 0.001 | |
| HFrEF | 281 (58%) | 66 (64%) | 215 (65%) | 0.590 | NS |
| HFmrEF | 73 (15%) | 15 (15%) | 58 (18%) | 0.409 | NS |
| HFpEF | 118 (24%) | 34 (30%) | 84 (24%) | 0.194 | NS |
| Examinations | |||||
| LVEF (%) | 41.5 ± 19.3 | 42.9 ± 21.8 | 41.4± 18.5 | 0.531 | NS |
| LVESV (mL) | 73.6 ± 48.4 | 83.8 ± 55.3 | 70.4 ± 42.5 | 0.031 | |
| LAD (mm) | 45.2 ± 9.1 | 48.2 ± 10.2 | 44.8 ± 8.4 | 0.003 | |
| Cr (mg/dL) | 1.5 ± 3.3 | 1.4 ± 1.2 | 1.6 ± 3.8 | 0.532 | NS |
| Hb (g/dL) | 12.9 ± 2.5 | 12.4 ± 2.3 | 14.2 ± 21.9 | 0.168 | NS |
| BNP (pg/mL) | 744.2 ± 732.8 | 758.7 ± 703.1 | 748.4 ± 756.3 | 0.905 | NS |
| Comorbidities | |||||
| PAF | 110 (23%) | 33 (33%) | 77 (22%) | 0.116 | NS |
| DM | 83 (18%) | 27 (23%) | 56 (16%) | 0.056 | NS |
| HT | 199 (42%) | 48 (42%) | 151 (42%) | 0.916 | NS |
| CVD | 67 (14%) | 21 (18%) | 46 (13%) | 0.151 | NS |
| CAD | 58 (12%) | 24 (21%) | 34 (10%) | <0.001 | |
| CKD | 133 (28%) | 41 (36%) | 92 (26%) | 0.040 | |
| Total | Event Group | Control Group | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| Total Number | (n = 472) | (n = 115) | (n = 357) | |||||
| Single HFH | 311 (57%) | 65 (57%) | 246 (69%) | 0.015 | ||||
| Multiple HFH | 161 (34%) | 50 (43%) | 111 (31%) | 0.015 | ||||
| Total N of HFH | 1.3 ± 1.1 | 1.8 ± 1.9 | 1.2 ± 0.6 | 0.002 | ||||
| Latest LVEF(%) | 48.7 ± 16.7 | 42.0 ± 17.8 | 51.9 ± 15.1 | <0.001 | ||||
| Latest LVESV(mL) | 63.8 ± 50.2 | 91.4 ± 50.2 | 50.7 ± 28.0 | <0.001 | ||||
| Latest LAD(mm) | 46.8 ± 9.1 | 49.5 ± 9.1 | 45.5 ± 8.4 | 0.006 | ||||
| SR maintenance | 131 (28%) | 20 (17%) | 111 (31%) | <0.001 | ||||
| Procedures | ||||||||
| CA | 84 (17%) | 10 (9%) | 74 (21%) | 0.003 | ||||
| before contact force | 70 (14%) | 8 (7%) | 62 (18%) | 0.373 | NS | |||
| after contact force | 14 (3%) | 2 (2%) | 12 (3%) | 0.373 | NS | |||
| Pacemaker | 10 (2%) | 3 (3%) | 7 (2%) | 0.675 | NS | |||
| ICD | 21 (4%) | 8 (7%) | 13 (4%) | 0.134 | NS | |||
| CRT | 15 (3%) | 7 (6%) | 8 (2%) | 0.041 | ||||
| other intervention | 16 (3%) | 7 (6%) | 9 (3%) | 0.066 | NS | |||
| Medications | ||||||||
| Anti-platelet | 131 (28%) | 33 (29%) | 98 (27%) | 0.795 | NS | |||
| OAC | 224 (47%) | [DOAC16%] | 52 (45%) | [DOAC13%] | 172 (48%) | [DOAC17%] | 0.580 | NS |
| BB | 224 (47%) | 51 (45%) | 173 (48%) | 0.443 | NS | |||
| RAAS antagonist | 249 (52%) | 64 (56%) | 185 (52%) | 0.474 | NS | |||
| AAD | 156 (32%) | 36 (31%) | 120 (34%) | 0.980 | NS | |||
| Amiodarone | 98 (21%) | 26 (23%) | 72 (20%) | 0.575 | NS | |||
| Diuretics | 297 (62%) | 83 (72%) | 214 (60%) | 0.018 |
| Univariate Analysys | Multivariate Analysys | |||||
|---|---|---|---|---|---|---|
| Categories | HR | CI | p-Value | HR | CI | p-Value |
| Baseline | ||||||
| Age > 74 y | 1.20 | (0.78–1.83) | 0.403 | |||
| NYHA classification 3/4 | 1.53 | (0.97–2.43) | 0.070 | |||
| CS 3 | 2.20 | (1.28–3.75) | 0.004 | |||
| LVEF < 40% | 0.75 | (0.50–1.14) | 0.180 | |||
| LVESV > 84 mL | 1.85 | (1.25–2.80) | 0.003 | |||
| LAD > 44 mm | 1.74 | (1.13–2.69) | 0.012 | |||
| CAD | 2.53 | (1.42–4.53) | 0.002 | |||
| CKD | 1.66 | (1.04–2.67) | 0.036 | |||
| Follow up | ||||||
| multiple hospitalizatioin | 4.53 | (2.60–7.91) | <0.001 | 2.26 | (1.41–3.61) | <0.001 |
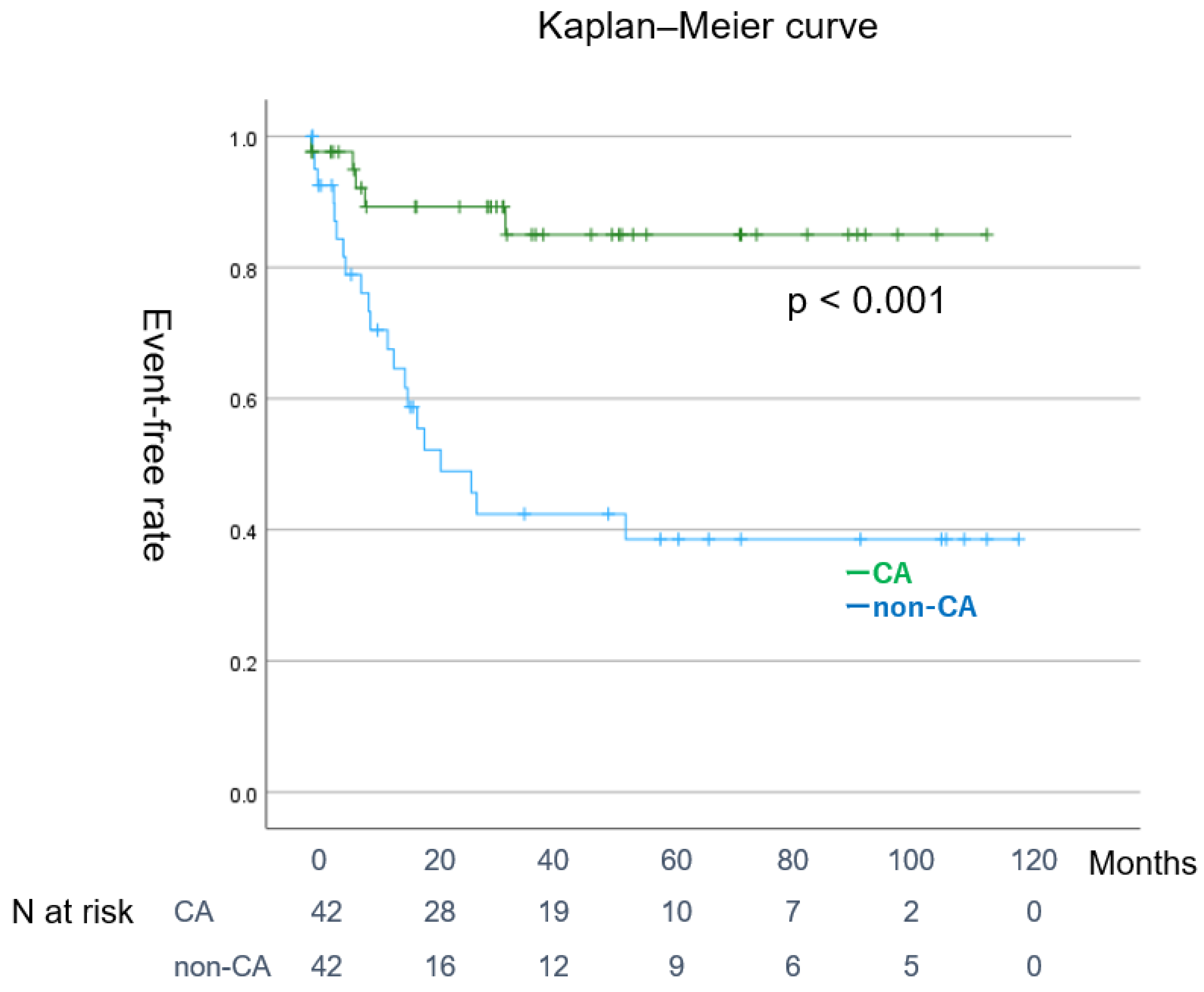
| CA | 0.36 | (0.18–0.71) | 0.004 | 0.41 | (0.21–0.82) | 0.012 |
| SR maintenance | 0.46 | (0.27–0.78) | 0.004 | |||
| Deuretics | 3.02 | (1.69–5.41) | <0.001 | 2.20 | (1.27–3.80) | 0.005 |
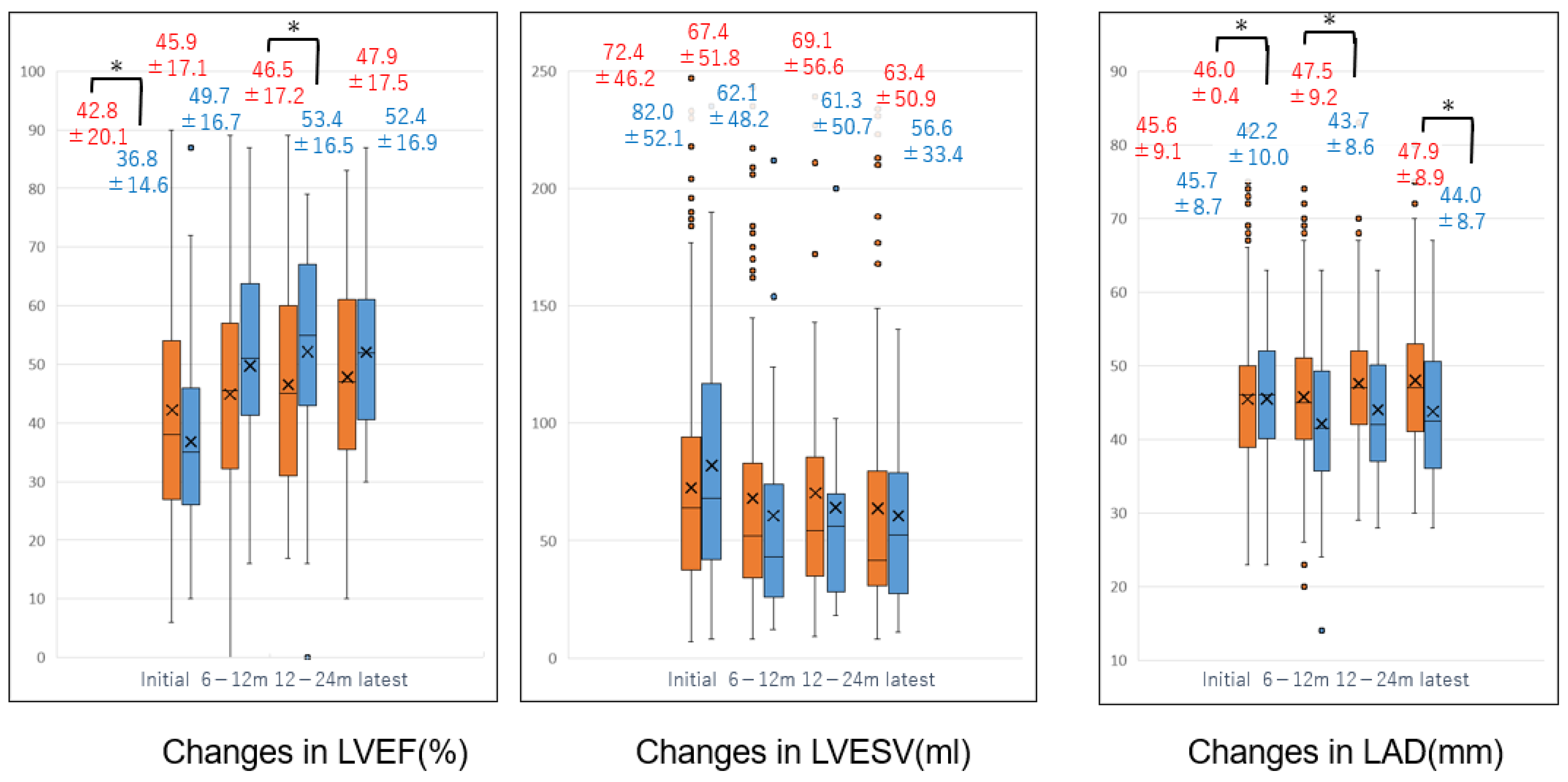
| Pre Matching | CA Group (n = 84) | Non-CA Group (n = 388) | p-Value | |
|---|---|---|---|---|
| Age | 61.5 ± 13.0 | 66.2 ± 11.2 | <0.001 | |
| Male | 56 (67%) | 218 (56%) | 0.440 | NS |
| CHADS2 score | 1.6 ± 1.0 | 2.3 ± 1.2 | <0.001 | |
| NYHA | 3.2 ± 0.6 | 3.7 ± 0.5 | <0.001 | |
| preLVEF (%) | 36.8 ± 14.6 | 42.8 ± 20.1 | 0.003 | |
| latestLVEF (%) | 50.9 ± 15.4 | 47.7 ± 17.2 | 0.225 | NS |
| preLAD (mm) | 45.7 ± 8.7 | 45.6 ± 9.1 | 0.951 | NS |
| latestLAD (mm) | 43.8 ± 9.3 | 47.9 ± 8.9 | 0.009 | |
| preBNP (pg/mL) | 567.5 ± 511.2 | 787.7 ± 777.5 | 0.005 | |
| latestBNP (pg/mL) | 71.6 ± 87.0 | 1041.0 ± 4870.1 | <0.001 | |
| Recurence after 1stCA | 48 (57%) | NA | NA | |
| Second ablation | 27 (32%) | NA | NA | |
| SR maintenance | 54 (64%) | 77 (20%) | <0.001 | |
| Post Matching | (n = 42) | (n = 42) | ||
| Age | 60.3 ± 11.3 | 66.2 ± 11.2 | 0.110 | NS |
| Male | 30 (62%) | 34 (70%) | 0.440 | NS |
| CHADS2 score | 1.6 ± 0.8 | 1.5 ± 1.0 | 0.790 | NS |
| NYHA | 2.4 ± 1.0 | 2.8 ± 0.8 | 0.340 | NS |
| preLVEF (%) | 35.6 ± 15.9 | 40.0 ± 17.0 | 0.470 | NS |
| latestLVEF (%) | 59.1 ± 13.8 | 43.9 ± 18.5 | <0.001 | |
| preLAD (mm) | 44.3 ± 9.2 | 44.2 ± 11.2 | 0.230 | NS |
| latestLAD (mm) | 42.1 ± 13.8 | 49.1 ± 13.5 | 0.047 | |
| preBNP (pg/mL) | 463.1 ± 407.4 | 713.2 ± 722.6 | 0.070 | NS |
| latestBNP (pg/mL) | 71.6 ± 87.0 | 671.9 ± 806.2 | <0.001 | |
| Recurence after 1st CA | 17 (40%) | NA | NA | |
| Second ablation | 13 (31%) | NA | NA | |
| SR maintenance | 31 (73%) | 14 (33%) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeda, Y.; Kato, R.; Mori, H.; Tsutsui, K.; Matsumoto, K.; Narita, M.; Sasaki, W.; Kudo, D.; Tanaka, N.; Matsumoto, K. Clinical Outcomes of Catheter Ablation for Atrial Fibrillation in Patients with Acute Decompensated Heart Failure. J. Clin. Med. 2025, 14, 629. https://doi.org/10.3390/jcm14020629
Ikeda Y, Kato R, Mori H, Tsutsui K, Matsumoto K, Narita M, Sasaki W, Kudo D, Tanaka N, Matsumoto K. Clinical Outcomes of Catheter Ablation for Atrial Fibrillation in Patients with Acute Decompensated Heart Failure. Journal of Clinical Medicine. 2025; 14(2):629. https://doi.org/10.3390/jcm14020629
Chicago/Turabian StyleIkeda, Yoshifumi, Ritsushi Kato, Hitoshi Mori, Kenta Tsutsui, Kazuhisa Matsumoto, Masataka Narita, Wataru Sasaki, Daisuke Kudo, Naomichi Tanaka, and Kazuo Matsumoto. 2025. "Clinical Outcomes of Catheter Ablation for Atrial Fibrillation in Patients with Acute Decompensated Heart Failure" Journal of Clinical Medicine 14, no. 2: 629. https://doi.org/10.3390/jcm14020629
APA StyleIkeda, Y., Kato, R., Mori, H., Tsutsui, K., Matsumoto, K., Narita, M., Sasaki, W., Kudo, D., Tanaka, N., & Matsumoto, K. (2025). Clinical Outcomes of Catheter Ablation for Atrial Fibrillation in Patients with Acute Decompensated Heart Failure. Journal of Clinical Medicine, 14(2), 629. https://doi.org/10.3390/jcm14020629






