The Balance Between the Natriuretic Peptides and the Renin-Angiotensin-Aldosterone System in the Preservation of Ideal Cardiovascular Health
Abstract
1. Introduction
2. Experimental and Human Evidence Linking NPs to CVH
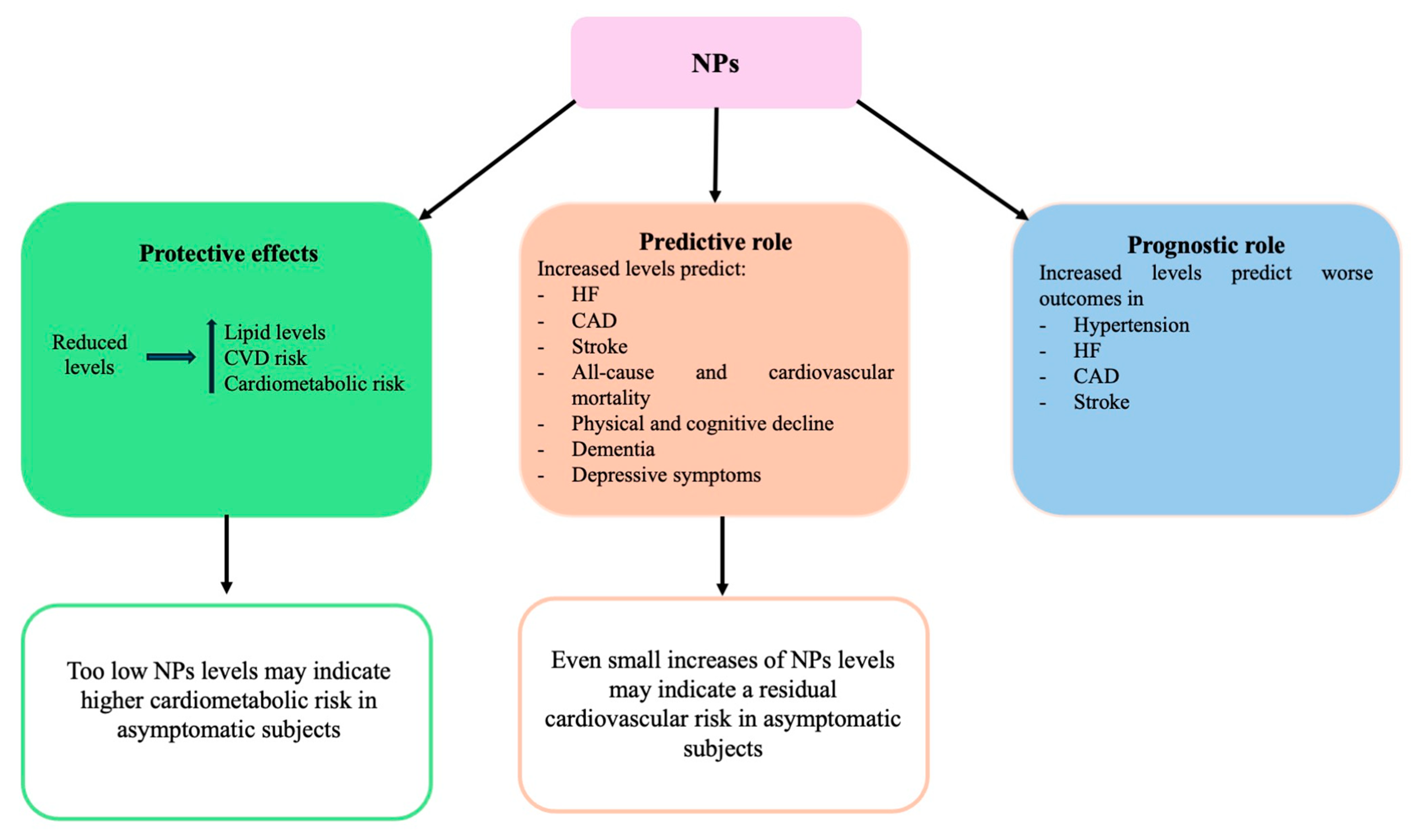
2.1. Evidence Supporting the Cardiovascular and Cardiometabolic Protection Exerted by NPs
2.2. Evidence Supporting the Role of NPs in Cardiovascular Risk Prediction Within General Populations
2.3. Therapeutic Implications of NPs in CVDs
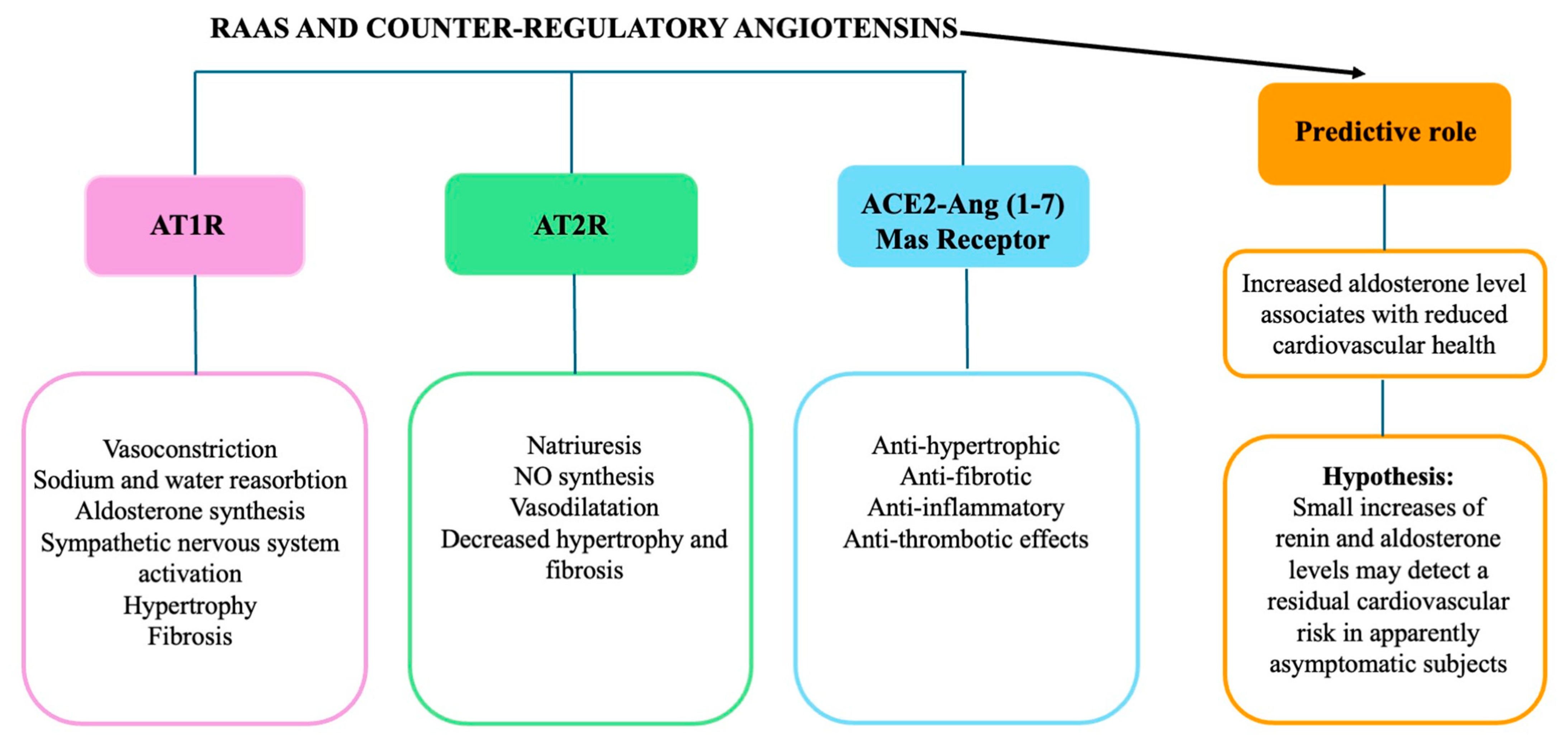
3. The Implications of Both Classical and Counter-Regulatory RAAS in CVH Maintenance
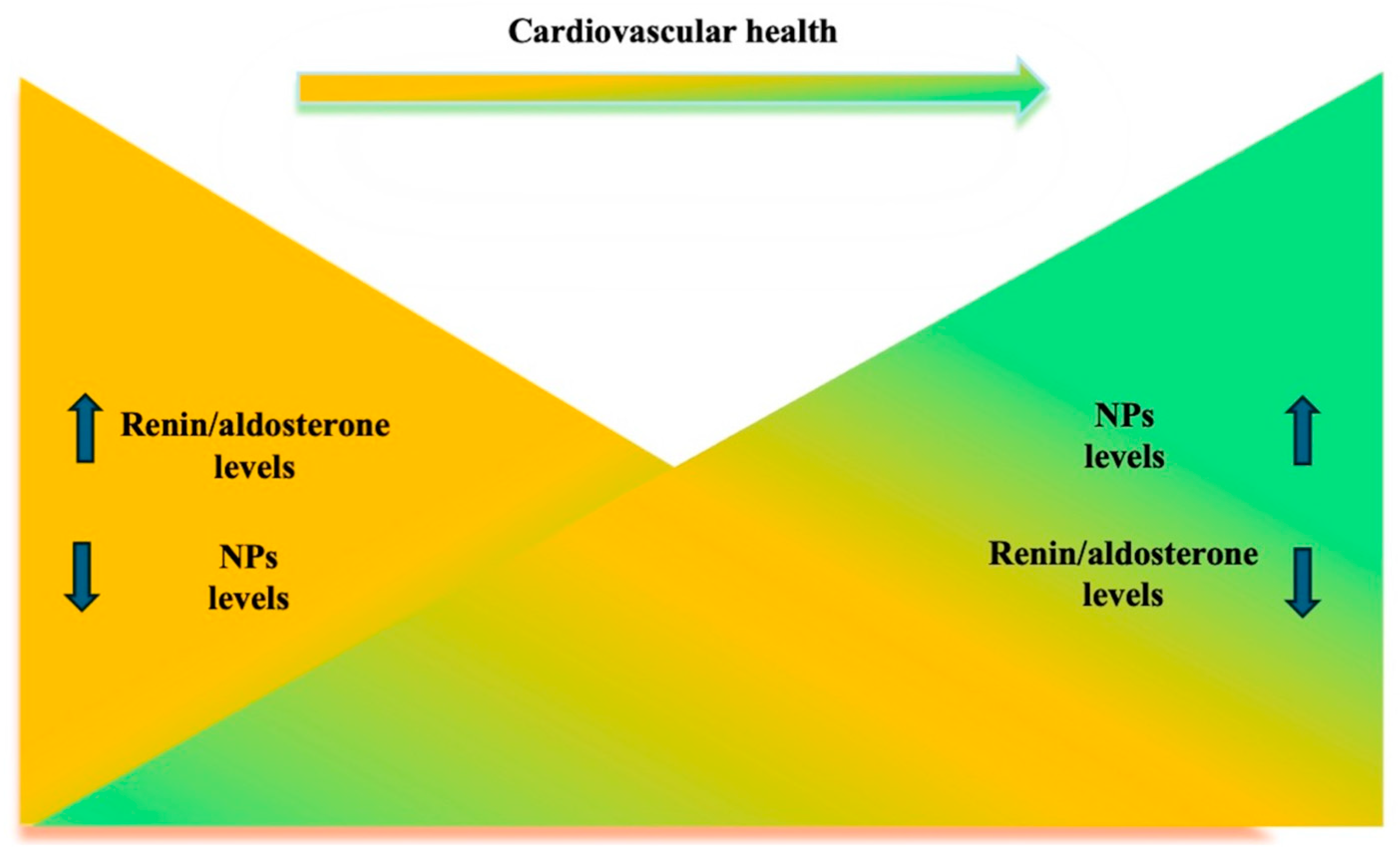
4. Perspectives
5. Study Limitations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Allen, N.B.; Anderson, C.A.M.; Black, T.; Brewer, L.C.; Foraker, R.E.; Grandner, M.A.; Lavretsky, H.; Perak, A.M.; Sharma, G.; et al. Life’s Essential 8: Updating and Enhancing the American Heart Association’s Construct of Cardiovascular Health: A Presidential Advisory from the American Heart Association. Circulation 2022, 146, e18–e43. [Google Scholar] [CrossRef] [PubMed]
- Te Hoonte, F.; Spronk, M.; Sun, Q.; Wu, K.; Fan, S.; Wang, Z.; Bots, M.L.; Van der Schouw, Y.T.; Uijl, A.; Vernooij, R.W.M. Ideal cardiovascular health and cardiovascular-related events: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2024, 31, 966–985. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Vélez, R.; Saavedra, J.M.; Lobelo, F.; Celis-Morales, C.A.; Pozo-Cruz, B.D.; García-Hermoso, A. Ideal cardiovascular health and incident cardiovascular disease among adults: A systematic review and meta-analysis. Mayo Clin. Proc. 2018, 93, 1589–1599. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Mao, Y.; Zhu, F.; Zhang, M.; Pan, M.; Yin, T.; Xu, J.; Chen, R.; Zheng, W. Association of life’s essential 8 with mortality among the individuals with cardiovascular disease. Sci. Rep. 2024, 14, 18520. [Google Scholar] [CrossRef] [PubMed]
- Howard, G.; Cushman, M.; Blair, J.; Wilson, N.R.; Yuan, Y.; Safford, M.M.; Levitan, E.B.; Judd, S.E.; Howard, V.J. Comparative discrimination of Life’s Simple 7 and Life’s Essential 8 to stratify cardiovascular risk: Is the added complexity worth it? Circulation 2024, 149, 905–913. [Google Scholar] [CrossRef]
- Xanthakis, V.; Enserro, D.M.; Murabito, J.M.; Polak, J.F.; Wollert, K.C.; Januzzi, J.L.; Wang, T.J.; Tofler, G.; Vasan, R.S. Ideal cardiovascular health: Associations with biomarkers and subclinical disease and impact on incidence of cardiovascular disease in the Framingham Offspring Study. Circulation 2014, 130, 1676–1683. [Google Scholar] [CrossRef]
- Osibogun, O.; Ogunmoroti, O.; Tibuakuu, M.; Benson, E.M.; Michos, E.D. Sex differences in the association between ideal cardiovascular health and biomarkers of cardiovascular disease among adults in the United States: A cross-sectional analysis from the multiethnic study of atherosclerosis. BMJ Open 2019, 9, e031414. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.E.; Haas, A.V.; Williams, G.H.; Taylor, H.; Seely, E.W.; Adler, G.K. Association between Life’s Simple 7 and biomarkers of cardiovascular disease: Aldosterone, interleukin-6, C-Reactive Protein. J. Am. Heart Assoc. 2023, 12, e028718. [Google Scholar] [CrossRef] [PubMed]
- Kluwe, B.; Pohlman, N.; Kesireddy, V.; Zhao, S.; Tan, Y.; Kline, D.; Brock, G.; Odei, J.B.; Effoe, V.S.; Tcheugui, J.B.E.; et al. The role of aldosterone and ideal cardiovascular health in incident cardiovascular disease: The Jackson heart study. Am. J. Prev. Cardiol. 2023, 14, 100494. [Google Scholar] [CrossRef]
- Kesireddy, V.; Tan, Y.; Kline, D.; Brock, G.; Odei, J.B.; Kluwe, B.; Effoe, V.S.; Echouffo Tcheugui, J.B.; Kalyani, R.R.; Sims, M.; et al. The Association of Life’s Simple 7 with Aldosterone among African Americans in the Jackson Heart Study. Nutrients 2019, 11, 955. [Google Scholar] [CrossRef] [PubMed]
- Mirabito Colafella, K.M.; Bovée, D.M.; Danser, A.H.J. The renin-angiotensin-aldosterone system and its therapeutic targets. Exp. Eye Res. 2019, 186, 107680. [Google Scholar] [CrossRef]
- Volpe, M.; Gallo, G.; Rubattu, S. Endocrine functions of the heart: From bench to bedside. Eur. Heart J. 2023, 44, 643–655. [Google Scholar] [CrossRef]
- Chen, Y.; Qi, Y.; Lu, W. Endogenous Vasoactive Peptides and Vascular Aging-Related Diseases. Oxid. Med. Cell Longev. 2022, 2022, 1534470. [Google Scholar] [CrossRef] [PubMed]
- Loutzenhiser, R.; Hayashi, K.; Epstein, M. Atrial natriuretic peptide reverses afferent arteriolar vasoconstriction and potentiates efferent arteriolar vasoconstriction in the isolated perfused rat kidney. J. Pharmacol. Exp. Ther. 1888, 246, 522–528. [Google Scholar]
- McGrath, M.F.; de Bold, M.L.; de Bold, A.J. The endocrine function of the heart. Trends Endocrinol. Metab. 2005, 16, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Melo, L.G.; Steinhelper, M.E.; Pang, S.C.; Tse, Y.; Ackermann, U. ANP in regulation of arterial pressure and fluid-electrolyte balance: Lessons from genetic mouse models. Physiol. Genom. 2000, 3, 45–58. [Google Scholar] [CrossRef]
- Atchison, D.J.; Ackermann, U. Influence of atrial natriuretic factor on autonomic control of heart rate. Am. J. Phys. 1990, 258, R718–R723. [Google Scholar] [CrossRef] [PubMed]
- Butler, G.C.; Senn, B.L.; Floras, J.S. Influence of atrial natriuretic factor on heart rate variability in normal men. Am. J. Phys. 1994, 267 Pt 2, H500–H505. [Google Scholar] [CrossRef]
- Struthers, A.D.; Anderson, J.V.; Payne, N.; Causon, R.C.; Slater, J.D.; Bloom, S.R. The effect of atrial natriuretic peptide on plasma renin activity, plasma aldosterone, and urinary dopamine in man. Eur. J. Clin. Pharmacol. 1986, 31, 223–226. [Google Scholar] [CrossRef]
- Brenner, B.M.; Ballermann, B.J.; Gunning, M.E.; Zeidel, M.L. Diverse biological actions of atrial natriuretic peptide. Physiol. Rev. 1990, 70, 665–699. [Google Scholar] [CrossRef] [PubMed]
- Franco-Saenz, R.; Atarashi, K.; Takagi, M.; Mulrow, P.J. Effect of atrial natriuretic factor on renin and aldosterone. J. Cardiovasc. Pharmacol. 1989, 13 (Suppl. S6), S31–S35. [Google Scholar] [CrossRef]
- Burger, A.J. A review of the renal and neurohormonal effects of B-type natriuretic peptide. Congest. Heart Fail. 2005, 11, 30–38. [Google Scholar] [CrossRef]
- Dhingra, H.; Roongsritong, C.; Kurtzman, N.A. Brain natriuretic peptide: Role in cardiovascular and volume homeostasis. Semin. Nephrol. 2002, 22, 423–437. [Google Scholar] [CrossRef]
- Mukoyama, M.; Nakao, K.; Hosoda, K.; Suga, S.; Saito, Y.; Ogawa, Y.; Shirakami, G.; Jougasaki, M.; Obata, K.; Yasue, H.; et al. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J. Clin. Investig. 1991, 87, 1402–1412. [Google Scholar] [CrossRef] [PubMed]
- Vanderheyden, M.; Bartunek, J.; Goethals, M. Brain and other natriuretic peptides: Molecular aspects. Eur. J. Heart Fail. 2004, 6, 261–268. [Google Scholar] [CrossRef]
- Gómez-Virgilio, L.; Silva-Lucero, M.D.; Flores-Morelos, D.S.; Gallardo-Nieto, J.; Lopez-Toledo, G.; Abarca-Fernandez, A.M.; Zacapala-Gómez, A.E.; Luna-Muñoz, J.; Montiel-Sosa, F.; Soto-Rojas, L.O.; et al. Autophagy: A key regulator of homeostasis and disease: An overview of molecular mechanisms and modulators. Cells 2022, 11, 2262. [Google Scholar] [CrossRef]
- Forte, M.; Marchitti, S.; Di Nonno, F.; Stanzione, R.; Schirone, L.; Cotugno, M.; Bianchi, F.; Schiavon, S.; Raffa, S.; Ranieri, D.; et al. NPPA/atrial natriuretic peptide is an extracellular modulator of autophagy in the heart. Autophagy 2023, 19, 1087–1099. [Google Scholar] [CrossRef]
- Forte, M.; Marchitti, S.; di Nonno, F.; Pietrangelo, D.; Stanzione, R.; Cotugno, M.; D’Ambrosio, L.; D’Amico, A.; Cammisotto, V.; Sarto, G.; et al. Atrial natriuretic peptide (ANP) modulates stress-induced autophagy in endothelial cells. BBA Mol. Cell Res. 2025, 1872, 119860. [Google Scholar] [CrossRef] [PubMed]
- Furuya, M.; Yoshida, M.; Hayashi, Y.; Ohnuma, N.; Minamino, N.; Kangawa, K.; Matsuo, H. C-type natriuretic peptide is a growth inhibitor of rat vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 1991, 177, 927–931. [Google Scholar] [CrossRef]
- Nakao, K.; Kuwahara, K.; Nishikimi, T.; Nakagawa, Y.; Kinoshita, H.; Minami, T.; Kuwabara, Y.; Yamada, C.; Yamada, Y.; Tokudome, T.; et al. Endothelium-derived C-type natriuretic peptide contributes to blood pressure regulation by maintaining endothelial integrity. Hypertension 2017, 69, 286–296. [Google Scholar] [CrossRef]
- Moyes, A.J.; Khambata, R.S.; Villar, I.; Bubb, K.J.; Baliga, R.S.; Lumsden, N.G.; Xiao, F.; Gane, P.J.; Rebstock, A.S.; Worthington, R.J.; et al. Endothelial C-type natriuretic peptide maintains vascular homeostasis. J. Clin. Investig. 2014, 124, 4039–4051. [Google Scholar] [CrossRef]
- Moyes, A.J.; Chu, S.M.; Aubdool, A.A.; Dukinfield, M.S.; Margulies, K.B.; Bedi, K.C.; Hodivala-Dilke, K.; Baliga, R.S.; Hobbs, A.J. C-type natriuretic peptide co-ordinates cardiac structure and function. Eur. Heart J. 2020, 41, 1006–1020. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Redfield, M.M.; Melenovsky, V.; Kane, G.C.; Karon, B.L.; Jacobsen, S.J.; Rodeheffer, R.J. Longitudinal changes in left ventricular stiffness: A community-based study. Circ. Heart Fail. 2013, 6, 944–952. [Google Scholar] [CrossRef]
- Poppe, K.K.; Doughty, R.N.; Gardin, J.M.; Hobbs, F.D.R.; McMurray, J.J.V.; Nagueh, S.F.; Senior, R.; Thomas, L.; Whalley, G.A.; Aune, E.; et al. Echocardiographic Normal Ranges Meta-Analysis of the Left Heart Collaboration Ethnic-specific normative reference values for echocardiographic LA and LV size, LV mass, and systolic function: The EchoNoRMAL Study. JACC Cardiovasc. Imaging 2015, 8, 656–665. [Google Scholar] [CrossRef]
- Cannone, V.; Cabassi, A.; Volpi, R.; Burnett, J.C., Jr. Atrial natriuretic peptide: A molecular target of novel therapeutic approaches to cardio-metabolic disease. Int. J. Mol. Sci. 2019, 20, 3265. [Google Scholar] [CrossRef]
- Patel, N.; Russell, G.K.; Musunuru, K.; Gutierrez, O.M.; Halade, G.; Kain, V.; Lv, W.; Prabhu, S.D.; Margulies, K.B.; Cappola, T.P.; et al. Race, natriuretic peptides, and high-carbohydrate challenge: A clinical trial. Circ. Res. 2019, 125, 957–968. [Google Scholar] [CrossRef]
- Rubattu, S.; Stanzione, R.; Cotugno, M.; Bianchi, F.; Marchitti, S.; Forte, M. Epigenetic control of natriuretic peptides: Implications for health and disease. Cell Mol. Life Sci. 2020, 77, 5121–5130. [Google Scholar] [CrossRef] [PubMed]
- Cannone, V.; Scott, C.G.; Decker, P.A.; Larson, N.B.; Palmas, W.; Taylor, K.D.; Wang, T.J.; Gupta, D.K.; Bielinski, S.J.; Burnett, J.C., Jr. A favorable cardiometabolic profile is associated with the G allele of the genetic variant rs5068 in African Americans: The Multi-Ethnic Study of Atherosclerosis (MESA). PLoS ONE 2017, 12, e0189858. [Google Scholar] [CrossRef]
- Prickett, T.C.R.; Spittlehouse, J.K.; Miller, A.L.; Liau, Y.; Kennedy, M.A.; Cameron, V.A.; Pearson, J.F.; Boden, J.M.; Troughton, R.W.; Espiner, E.A. Contrasting signals of cardiovascular health among natriuretic peptides in subjects without heart disease. Sci. Rep. 2019, 9, 12108. [Google Scholar] [CrossRef]
- Prickett, T.C.R.; Darlow, B.A.; Troughton, R.W.; Cameron, V.A.; Elliott, J.M.; Martin, J.; Horwood, L.J.; Espiner, E.A. New insights into cardiac and vascular natriuretic peptides: Findings from young adults born with very low birth weight. Clin. Chem. 2018, 64, 363–373. [Google Scholar] [CrossRef]
- Prickett, T.C.R.; Pearson, J.F.; Troughton, R.W.; Kennedy, M.A.; Espiner, E.A. The predictive value of A, B, and C-type natriuretic peptides in people at risk of heart disease: Protocol for a longitudinal observational study. JMIR Res. Protoc. 2023, 12, e37011. [Google Scholar] [CrossRef]
- Wang, L.; Yi, J.; Wang, W.; Zhou, Z.; Liu, J.; Zhang, H.; Li, Y.; Ren, X.; Lu, J.; Zheng, X. Impact of first-line antihypertensive drug class and intensity on NT-proBNP improvement and cardiovascular outcomes among hypertensive patients with pre-heart failure: Findings from SPRINT trial. Hypertens. Res. 2024, 47, 3447–3457. [Google Scholar] [CrossRef]
- Natriuretic Peptides Studies Collaboration; Willeit, P.; Kaptoge, S.; Welsh, P.; Butterworth, A.S.; Chowdhury, R.; Spackman, S.A.; Pennells, L.; Gao, P.; Burgess, S.; et al. Natriuretic peptides and integrated risk assessment for cardiovascular disease: An individual-participant-data meta-analysis. Lancet Diabetes Endocrinol. 2016, 4, 840–849. [Google Scholar] [CrossRef]
- Neumann, J.T.; Twerenbold, R.; Weimann, J.; Ballantyne, C.M.; Benjamin, E.J.; Costanzo, S.; de Lemos, J.A.; deFilippi, C.R.; Di Castelnuovo, A.; Donfrancesco, C.; et al. Prognostic value of cardiovascular biomarkers in the population. JAMA 2024, 331, 1898–1909. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, S.; Lampa, E.; Jensevik Eriksson, K.; Butterworth, A.S.; Elmståhl, S.; Engström, G.; Hveem, K.; Johansson, M.; Langhammer, A.; Lind, L.; et al. Markers of imminent myocardial infarction. Nat. Cardiovasc. Res. 2024, 3, 130–139. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, C.; Li, J.; Sun, L. Association of N-terminal pro-B natriuretic peptide with all-cause mortality and cardiovascular mortality in obese and non-obese populations and the development of a machine learning prediction model: National Health and Nutrition Examination Survey (NHANES) 1999–2004. Diabetes Obes. Metab. 2024, 26, 5609–5620. [Google Scholar]
- Segar, M.W.; Khan, M.S.; Patel, K.V.; Vaduganathan, M.; Kannan, V.; Willett, D.; Peterson, E.; Tang, W.H.W.; Butler, J.; Everett, B.M.; et al. Incorporation of natriuretic peptides with clinical risk scores to predict heart failure among individuals with dysglycaemia. Eur. J. Heart Fail. 2022, 24, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Jehn, S.; Mahabadi, A.A.; Pfohl, C.; Vogel, L.; Al-Rashid, F.; Luedike, P.; Totzeck, M.; Rassaf, T.; Dykun, I. BNP and NT-proBNP thresholds for the assessment of prognosis in patients without heart failure. JACC Adv. 2023, 2, 100688. [Google Scholar] [CrossRef]
- Volpe, M.; Gallo, G.; Rubattu, S. BNP/NT-proBNP levels are sensitive markers of impaired prognosis in patients without heart failure. JACC Adv. 2023, 2, 100691. [Google Scholar] [CrossRef]
- Oh, H.S.; Rutledge, J.; Nachun, D.; Pálovics, R.; Abiose, O.; Moran-Losada, P.; Channappa, D.; Urey, D.Y.; Kim, K.; Sung, Y.J.; et al. Organ aging signatures in the plasma proteome track health and disease. Nature 2023, 624, 164–172. [Google Scholar] [CrossRef]
- Kuh, D.; Cooper, R.; Sattar, N.; Welsh, P.; Hardy, R.; Ben-Shlomo, Y. Systemic inflammation and cardio-renal organ damage biomarkers in middle age are associated with physical capability up to 9 years later. Circulation 2019, 139, 1988–1999. [Google Scholar] [CrossRef]
- van Peet, P.G.; de Craen, A.J.; Gussekloo, J.; de Ruijter, W. Plasma NT-proBNP as predictor of change in functional status, cardiovascular morbidity and mortality in the oldest old: The Leiden 85-plus study. Age 2014, 36, 9660. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hozawa, A.; Sugawara, Y.; Tomata, Y.; Kakizaki, M.; Ohmori-Matsuda, K.; Nakaya, N.; Kuriyama, S.; Fukao, A.; Tsuji, I. Relationships between N-terminal pro B-type natriuretic peptide and incident disability and mortality in older community-dwelling adults: The Tsurugaya study. J. Am. Geriatr. Soc. 2010, 58, 2439–2441. [Google Scholar] [CrossRef] [PubMed]
- Gallo, G.; Bianchi, F.; Cotugno, M.; Volpe, M.; Rubattu, S. Natriuretic peptides, cognitive impairment and dementia: An intriguing pathogenic link with implications in hypertension. J. Clin. Med. 2020, 9, 2265. [Google Scholar] [CrossRef] [PubMed]
- Ostovaneh, M.R.; Moazzami, K.; Yoneyama, K.; Venkatesh, B.A.; Heckbert, S.R.; Wu, C.O.; Shea, S.; Post, W.S.; Fitzpatrick, A.L.; Burke, G.L.; et al. Change in NT-proBNP (N-Terminal pro-B-type natriuretic peptide) level and risk of dementia in multi-ethnic study of atherosclerosis (MESA). Hypertension 2020, 75, 316–323. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [PubMed]
- Raffa, S.; Forte, M.; Gallo, G.; Ranieri, D.; Marchitti, S.; Magrì, D.; Testa, M.; Stanzione, R.; Bianchi, F.; Cotugno, M.; et al. Atrial natriuretic peptide stimulates autophagy/mitophagy and improves mitochondrial function in chronic heart failure. Cell Mol. Life Sci. 2023, 80, 134. [Google Scholar] [CrossRef]
- Rubattu, S.; Cotugno, M.; Forte, M.; Stanzione, R.; Bianchi, F.; Madonna, M.; Marchitti, S.; Volpe, M. Effects of dual angiotensin type 1 receptor/neprilysin inhibition vs. angiotensin type 1 receptor inhibition on target organ injury in the stroke-prone spontaneously hypertensive rat. J. Hypertens. 2018, 36, 1902–1914. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhou, Z.Y.; Sun, J.C.; Xing, W.; Yan, J.; Xu, W.J.; Lu, Y.S.; Liu, T.; Jin, Y. Protective effect of novel angiotensin receptor neprilysin inhibitor S086 on target organ injury in spontaneously hypertensive rats. Biomed. Pharmacother. 2024, 170, 115968. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Wan, S.H.; Iyer, S.R.; Cannone, V.; Sangaralingham, S.J.; Nuetel, J.; Burnett, J.C., Jr. First-in-human study of MANP: A novel ANP (Atrial Natriuretic Peptide) analog in human hypertension. Hypertension 2021, 78, 1859–1867. [Google Scholar] [CrossRef]
- Ma, X.; McKie, P.M.; Iyer, S.R.; Scott, C.; Bailey, K.; Johnson, B.K.; Benike, S.L.; Chen, H.; Miller, W.L.; Cabassi, A.; et al. MANP in hypertension with metabolic syndrome: Proof-of-concept study of natriuretic peptide-based therapy for cardiometabolic disease. JACC Basic. Transl. Sci. 2023, 9, 18–29. [Google Scholar] [CrossRef]
- Ferrario, C.M. The renin-angiotensin system: Importance in physiology and pathology. J. Cardiovasc. Pharmacol. 1990, 15 (Suppl. S3), S1–S5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, X.C.; Campbell, D.J.; Ohishi, M.; Yuan, S.; Zhuo, J.L. AT1 receptor-activated signaling mediates angiotensin IV-induced renal cortical vasoconstriction in rats. Am. J. Physiol. Ren. Physiol. 2006, 290, F1024–F1033. [Google Scholar] [CrossRef] [PubMed]
- Volpe, M.; Rubattu, S.; Gigante, B.; Ganten, D.; Porcellini, A.; Russo, R.; Romano, M.; Enea, I.; Lee, M.A.; Trimarco, B. Regulation of aldosterone biosynthesis by adrenal renin is mediated through AT1 receptors in renin transgenic rats. Circ. Res. 1995, 77, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.M. Update on angiotensin AT2 receptors. Curr. Opin. Nephrol. Hypertens. 2017, 26, 91–96. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Albiston, A.L.; McDowall, S.G.; Matsacos, D.; Sim, P.; Clune, E.; Mustafa, T.; Lee, J.; Mendelsohn, F.A.; Simpson, R.J.; Connolly, L.M.; et al. Evidence that the angiotensin IV (AT (4)) receptor is the enzyme insulin-regulated aminopeptidase. J. Biol. Chem. 2001, 276, 48623–48626. [Google Scholar] [CrossRef]
- Matavelli, L.C.; Siragy, H.M. AT2 receptor activities and pathophysiological implications. J. Cardiovasc. Pharmacol. 2015, 65, 226–232. [Google Scholar] [CrossRef]
- Kemp, B.A.; Howell, N.L.; Gildea, J.J.; Keller, S.R.; Padia, S.H.; Carey, R.M. AT2 receptor activation induces natriuresis and lowers blood pressure. Circ. Res. 2014, 115, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Gigante, B.; Rubattu, S.; Russo, R.; Porcellini, A.; Enea, I.; De Paolis, P.; Savoia, C.; Natale, A.; Piras, O.; Volpe, M. Opposite feedback control of renin and aldosterone biosynthesis in the adrenal cortex by angiotensin II AT1-subtype receptors. Hypertension 1997, 30, 563–568. [Google Scholar] [CrossRef]
- De Paolis, P.; Porcellini, A.; Gigante, B.; Giliberti, R.; Lombardi, A.; Savoia, C.; Rubattu, S.; Volpe, M. Modulation of the AT2 subtype receptor gene activation and expression by the AT1 receptor in endothelial cells. J. Hypertens. 1999, 17 Pt 2, 1873–1877. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.H. Age-related changes in the renin-aldosterone system. Physiological effects and clinical implications. Drugs Aging 1993, 3, 238–245. [Google Scholar] [CrossRef]
- Zhuo, J.L.; Ferrao, F.M.; Zheng, Y.; Li, X.C. New frontiers in the intrarenal Renin-Angiotensin system: A critical review of classical and new paradigms. Front. Endocrinol. 2013, 4, 166. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Gul, R.; Yuan, K.; Gao, S.; Oh, Y.B.; Kim, U.H.; Kim, S.H. Angiotensin-(1-7) stimulates high atrial pacing-induced ANP secretion via Mas/PI3-kinase/Akt axis and Na+/H+ exchanger. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H1365–H1374. [Google Scholar] [CrossRef] [PubMed]
- Foote, K.; Reinhold, J.; Yu, E.P.K.; Figg, N.L.; Finigan, A.; Murphy, M.P.; Bennett, M.R. Restoring mitochondrial DNA copy number preserves mitochondrial function and delays vascular aging in mice. Aging Cell 2018, 17, e12773. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yin, A.; Zhang, Q.; Zhong, T.; O’Rourke, S.T.; Sun, C. Angiotensin-(1-7) attenuates angiotensin II-induced cardiac hypertrophy via a Sirt3-dependent mechanism. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H980–H991. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, S.V.; Ferreira, A.J.; Kitten, G.T.; da Silveira, K.D.; da Silva, D.A.; Santos, S.H.; Gava, E.; Castro, C.H.; Magalhães, J.A.; da Mota, R.K.; et al. Genetic deletion of the angiotensin-(1–7) receptor Mas leads to glomerular hyperfiltration and microalbuminuria. Kidney Int. 2009, 75, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, J.; He, Q.; Shou, Z.; Zhang, P.; Pen, W.; Zhu, Y.; Chen, J. Angiotensin (1–7) prevents heart dysfunction and left ventricular remodeling caused by renal dysfunction in 5/6 nephrectomy mice. Hypertens. Res. 2009, 32, 369–374. [Google Scholar] [CrossRef]
- Oliveira Andrade, J.M.; de Farias Lelis, D.; Mafra, V.; Cota, J. The Angiotensin Converting Enzyme 2 (ACE2), Gut microbiota, and cardiovascular health. Protein Pept. Lett. 2017, 24, 827–832. [Google Scholar] [CrossRef]
- Gamino-Gutierrez, J.A.; Teran-Hernandez, I.M.; Castellar-Lopez, J.; Villamizar-Villamizar, W.; Osorio-Llanes, E.; Palacios-Cruz, M.; Rosales, W.; Chang, A.Y.; Diaz-Ariza, L.A.; Ospino, M.C.; et al. Novel insights into the cardioprotective effects of the peptides of the counter-regulatory renin–angiotensin system. Biomedicines 2024, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.A.; Park, B.M.; Gao, S.; Kim, S.H. Stimulation of ANP by angiotensin-(1-9) via the angiotensin type 2 receptor. Life Sci. 2013, 93, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Park, B.M.; Phuong, H.T.A.; Yu, L.; Kim, S.H. Alamandine protects the heart against reperfusion injury via the MrgD receptor. Circ. J. 2018, 82, 2584–2593. [Google Scholar] [CrossRef]
- Campbell, D.J. Critical review of prorenin and (pro)renin receptor research. Hypertension 2008, 51, 1259–1264. [Google Scholar] [CrossRef]
- Neves, M.F.; Cunha, A.R.; Cunha, M.R.; Gismondi, R.A.; Oigman, W. The role of renin-angiotensin-aldosterone system and its new components in arterial stiffness and vascular aging. High Blood Press Cardiovasc. Prev. 2018, 25, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Feldman, J.M.; Frishman, W.H.; Aronow, W.S. Emerging therapies for treatment-resistant hypertension: A review of lorundrostat and related selective aldosterone synthase inhibitors. Cardiol. Rev. 2024; in press. [Google Scholar] [CrossRef] [PubMed]
- Roth, L.; Dogan, S.; Tuna, B.G.; Aranyi, T.; Benitez, S.; Borrell-Pages, M.; Bozaykut, P.; De Meyer, G.R.Y.; Duca, L.; Durmus, N.; et al. Pharmacological modulation of vascular ageing: A review from VascAgeNet. Ageing Res. Rev. 2023, 92, 102122. [Google Scholar] [CrossRef] [PubMed]
- de Cavanagh, E.M.; Inserra, F.; Ferder, L. Angiotensin II blockade: How its molecular targets may signal to mitochondria and slow aging. Coincidences with calorie restriction and mTOR inhibition. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H15–H44. [Google Scholar] [CrossRef] [PubMed]
- de Cavanagh, E.M.V.; Inserra, F.; Ferder, L. Renin-angiotensin system inhibitors positively impact on multiple aging regulatory pathways: Could they be used to protect against human aging? Physiol. Rep. 2024, 12, e16094. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wang, J.; Zhang, R.; Zhang, Y.; Geng, J.; Cao, L.; Zhao, X.; Geng, J.; Du, X.; Hu, Y.; et al. Angiotensin II mediates cardiomyocyte hypertrophy in atrial cardiomyopathy via epigenetic transcriptional regulation. Comput. Math. Methods Med. 2022, 2022, 6312100. [Google Scholar] [CrossRef]
- Bryniarski, P.; Nazimek, K.; Marcinkiewicz, J. Immunomodulatory activity of the most commonly used antihypertensive drugs-angiotensin converting enzyme inhibitors and angiotensin II receptor blockers. Int. J. Mol. Sci. 2022, 23, 1772. [Google Scholar] [CrossRef]
- Fatima, N.; Patel, S.N.; Hussain, T. Angiotensin II Type 2 Receptor: A target for protection against hypertension, metabolic dysfunction, and organ remodeling. Hypertension 2021, 77, 1845–1856. [Google Scholar] [CrossRef] [PubMed]
- Gwathmey, T.M.; Shaltout, H.A.; Pendergrass, K.D.; Pirro, N.T.; Figueroa, J.P.; Rose, J.C.; Diz, D.I.; Chappell, M.C. Nuclear angiotensin II type 2 (AT2) receptors are functionally linked to nitric oxide production. Am. J. Physiol. Ren. Physiol. 2009, 296, F1484–F1493. [Google Scholar] [CrossRef] [PubMed]
- Savoia, C.; Arrabito, E.; Parente, R.; Nicoletti, C.; Madaro, L.; Battistoni, A.; Filippini, A.; Steckelings, U.M.; Touyz, R.M.; Volpe, M. Mas receptor activation contributes to the improvement of nitric oxide bioavailability and vascular remodeling during chronic AT1R (Angiotensin Type-1 Receptor) blockade in experimental hypertension. Hypertension 2020, 76, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Welsh, P.; Campbell, R.T.; Mooney, L.; Kimenai, D.M.; Hayward, C.; Campbell, A.; Porteous, D.; Mills, N.L.; Lang, N.N.; Petrie, M.C.; et al. Reference ranges for NT-proBNP (N-Terminal Pro-B-Type Natriuretic Peptide) and risk factors for higher NT-proBNP concentrations in a large general population cohort. Circ. Heart Fail. 2022, 15, e009427. [Google Scholar] [CrossRef]
- Shetty, N.S.; Patel, N.; Gaonkar, M.; Li, P.; Arora, G.; Arora, P. Natriuretic peptide normative levels and deficiency: The National Health and Nutrition Examination Survey. JACC Heart Fail. 2024, 12, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.E.; Januzzi, J.L., Jr. NT-proBNP concentrations in the community: Elevation, deficiency, and everything in between. JACC Heart Fail. 2024, 12, 64–66. [Google Scholar] [CrossRef] [PubMed]
- ESC Cardiovasc Risk Collaboration. SCORE2 working group and ESC Cardiovascular risk collaboration. SCORE2 risk prediction algorithms: New models to estimate 10-year risk of cardiovascular disease in Europe. Eur. Heart J. 2021, 42, 2439–2454. [Google Scholar] [CrossRef] [PubMed]
| Measure of cumulative exposure to relevant stressors across life |
| Biomarker able to capture early end-organ damage in apparently asymptomatic individuals |
| Aging biomarker, including the prediction of cognitive decline and dementia |
| Inhibition of: ACE AT1R RENIN ALDOSTERONE NEP |
| Activation of: AT2R ACE2 Ang (1-7)/Mas receptor Ang (1-9) Alamandine ANP, BNP, CNP, NPRA |
| Peptides analogs: MANP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubattu, S.; Gallo, G.; Volpe, M. The Balance Between the Natriuretic Peptides and the Renin-Angiotensin-Aldosterone System in the Preservation of Ideal Cardiovascular Health. J. Clin. Med. 2025, 14, 626. https://doi.org/10.3390/jcm14020626
Rubattu S, Gallo G, Volpe M. The Balance Between the Natriuretic Peptides and the Renin-Angiotensin-Aldosterone System in the Preservation of Ideal Cardiovascular Health. Journal of Clinical Medicine. 2025; 14(2):626. https://doi.org/10.3390/jcm14020626
Chicago/Turabian StyleRubattu, Speranza, Giovanna Gallo, and Massimo Volpe. 2025. "The Balance Between the Natriuretic Peptides and the Renin-Angiotensin-Aldosterone System in the Preservation of Ideal Cardiovascular Health" Journal of Clinical Medicine 14, no. 2: 626. https://doi.org/10.3390/jcm14020626
APA StyleRubattu, S., Gallo, G., & Volpe, M. (2025). The Balance Between the Natriuretic Peptides and the Renin-Angiotensin-Aldosterone System in the Preservation of Ideal Cardiovascular Health. Journal of Clinical Medicine, 14(2), 626. https://doi.org/10.3390/jcm14020626






