Abstract
Objectives: Chronic kidney disease-associated pruritus (CKD-aP) is underdiagnosed and not fully understood by healthcare professionals, which leads to poor patient management and impacts patients’ quality of life (QoL). The aim of this study was to analyse unmet needs in CKD-aP management and explore the attributes/characteristics that the ideal CKD-aP treatment should have from the perspective of a group of nephrologists, hospital pharmacists, nurses, patient representatives, and regional health authorities in Spain. Methods: A descriptive, cross-sectional study was conducted using an e-survey including ad hoc questions (6-point Likert scale) related to unmet needs in CKD-aP and best–worst scaling (BWS) to prioritise the attributes/characteristics of the ideal CKD-aP treatment. The survey was developed from a literature review, a patient focus group, and a multidisciplinary expert committee. Results: A total of 21 people participated, and it was considered, among other aspects, that CKD-aP had a significant impact on patient QoL (4.29/5), but the diagnosis rate and knowledge level of agents involved, as well as current treatment efficacy and safety, were low (1.71/5, 2.19/5, 1.91/5, and 2.67/5, respectively). The attributes “improves overall QoL (physical and mental)”, “reduces itch with statistical significance”, and “treatment is supported by clinical development/high evidence and has AEMPS (Spanish Agency for Medicines and Medical Devices)-approved indication for pruritus” were selected as the most valued attributes. There was a positive balance between best–worst scores (86-5, 71-2, and 78-13 points, respectively). Conclusions: The results show the need to undertake actions to drive relevant changes in current clinical practice to improve CKD-aP diagnosis and management.
1. Introduction
Chronic kidney disease (CKD) is a complex condition that affects approximately 850 million people worldwide, over 85 million in Europe, and more than 6 million in Spain [1]. As CKD progresses, complications such as mineral bone disorders, anaemia, hypertension, and hypokalaemia may develop, leading to advanced stages of CKD, and patients may require renal replacement therapy and haemodialysis [2]. Patients undergoing haemodialysis report a high symptom burden, including fatigue, pain, mood disturbance, sleep disturbances, and pruritus [3]. Chronic kidney disease-associated pruritus (CKD-aP) is one of the most common and disabling comorbidities in patients with advanced CKD [4], affecting up to 80% of patients on haemodialysis (HD) [5]. Pruritus is defined as the sensation of itching in one area or all over the body that causes the need to scratch [6]. It is an extremely distressing and debilitating symptom comparable to chronic pain [7]. The severity varies from somewhat uncomfortable to extremely disturbing and inducing restlessness [8]. CKD-aP in these patients has a significant impact on their quality of life (QoL), leading to sleep disruption and mental disorders [9]. Pruritus is associated with an increased risk of dementia, cardiovascular disease, and mortality [10,11,12,13,14,15,16]. In addition, there is a statistically significant association between increased itchiness severity and worsening QoL [17,18].
It is an old and well-known health problem, the prevalence of which has decreased with the improvement of dialytic techniques, but it still persists [6]. In Spain, according to the National Registry of Renal Patients, the prevalence of patients with HD was 26,683 in 2021 [19]. A survey (n = 1605) conducted by the Spanish Society of Nephrology (SEN) in 2021, mostly on HD (92%), reported that 50.5% of patients suffered from pruritus and that 27% of the total suffered from moderate to severe pruritus [20]. An international cohort study of adult dialysis patients (Dialysis Outcomes and Practice Patterns Study (DOPPS)) reported similar figures: 59% of Spanish patients suffered from pruritus and 36% had moderate to severe pruritus [21]. However, despite having CKD-aP, 35% of HD patients stated that they never reported their pruritus symptoms to a healthcare professional [22], and only 16% of nephrologists in Spain indicated that the prevalence of pruritus in their centres was greater than 20%, which shows a clear inconsistency between prevalence and diagnosis [6]. Moreover, the use of scales to measure it and its codification in medical records is not well established. The diagnosis of CKD-aP is mainly based on direct communication of the patient’s symptoms to healthcare staff [6].
Recently, in Spain, a consensus document with recommendations for the diagnosis and management of CKD-aP in HD patients was published [4]. This document, which was subject to public consultation by all members of the Spanish Society of Nephrology (SEN) prior to publication, addresses some of the identified barriers to adequately managing this condition. These included which scales are more appropriate for CKD-aP diagnosis and recommendations for the codification of the disease in patient medical records with the tools available today.
The multifactorial pathogenesis of CKD-aP is still not fully understood, and different explanations have been considered possible [23,24]. Various pruritogens, receptors, neurons, and neurotransmitters have been identified as playing a role in the pathophysiology of itching [25]. It is likely that different itch syndromes involve distinct combinations of cells and molecules responsible for transmitting itch sensations [25]. While few studies have focused on the specific pathophysiological mechanisms of itch in CKD-aP, general theories have emerged from the existing literature [25]. CKD-aP has been associated with dermatological factors (xerosis or skin barrier dysfunction), systemic factors such as immune system dysfunction and the proinflammatory state inherent to CKD, neurological factors, and factors due to the accumulation of toxins and other metabolic substances, including parathyroid hormone, calcium, phosphorus, and aluminium [25]. The fact that healthcare professionals do not fully understand CKD-aP, together with the fact that it is underreported by patients, leads to underdiagnosis, and it usually goes unnoticed by most nephrologists [6,9].
Besides the challenges around diagnosis, there are also difficulties around the management of this disease, and there is not a commonly accepted clinical practice guideline. As there is currently no approved treatment for CKD-aP available in Spain [26], off-label pharmacological treatment approaches are used to tackle CKD-aP, such as antihistamines, pregabalin, and antidepressants. However, the evidence on the efficacy of these therapies in CKD-aP is limited [6,9]. In the limited available clinical evidence, some of these therapeutic approaches have shown good results in reducing itchiness, but they have many serious side effects, which make them inappropriate for use in most HD patients, as they are frail patients [6,9,27,28,29,30,31,32,33,34,35,36]. Furthermore, these are polymedicated patients, so it is a priority to treat pruritus with minimal pharmacological burden and safe drugs while avoiding drug–drug interactions as much as possible [9]. Therefore, patients experiencing CKD-aP face clear unmet medical needs, with studies reporting high numbers of untreated patients or, when under treatment, high patient dissatisfaction [37].
Understanding unmet needs, the impact on QoL, and patients’ and healthcare professionals’ perspectives about CKD-aP may help in clinical, licensing, reimbursement, and policy decisions. Therefore, the objective of this study was to analyse current unmet needs in CKD-aP management and preferences from a multi-stakeholder perspective regarding the attributes that the ideal CKD-aP treatment should have.
2. Materials and Methods
This is a descriptive, cross-sectional study based on an electronic questionnaire, including ad hoc questions related to unmet needs in CKD-aP, and a best–worst scaling (BWS) experiment.
2.1. Participants
The study sample included health professionals (nephrologists, hospital pharmacists, and nurses) who are experts in CKD management but have different levels of expertise in the disease, as well as representatives of patient advocacy groups and regional health authorities with decision-making responsibilities. The participants were identified and invited by the ALCER (Spanish Association for the Fight against Kidney Diseases). Fifty-three people received the invitation to participate in the project with the questionnaire link, username, and password (exclusive for each participant) via e-mail (between May 2023 and September 2023).
A multidisciplinary committee of experts (n = 4) consisting of one nephrologist expert in CKD-aP, one hospital pharmacist, one patient representative from ALCER, and one regional health authority participated in the design of the questionnaire and the interpretation of results.
2.2. Ethical Considerations
All procedures followed were in line with the ethical standards of the Declaration of Helsinki. Due to the nature of the study, which did not collect clinical data from participants, including data on drugs or interventions, and the fact that the questions were related to participants’ perceptions, the approval of an ethics committee was not required, as defined in the Royal Decree 957/2020 of 3 November [38] and the Memorandum of Cooperation between Ethics Committees [39]. Prior to beginning the questionnaire, all participants were informed of the study structure, scientific committee, and the confidentiality and data protection measures in place. Written informed consent was obtained by all participants by checking a box indicating that they read and approved the survey and agreed to participate in the study with all the conditions described.
2.3. Survey
The survey’s content was developed based on a literature review dealing with studies on the management of CKD-aP (Supplementary Methods and Table S1), information from a focus group with patients, and the opinion of the scientific committee. There was no developed survey to assess unmet needs in CKD-aP. Therefore, it was considered appropriate to conduct a literature review to identify those relevant aspects of the diagnosis and management of the disease where gaps exist in order to develop questions to explore the perspectives of different stakeholders.
The online patient focus group included five people who suffered or had suffered with CKD-aP—two with mild–moderate pruritus and three with moderate–severe pruritus—while on renal replacement therapy, and was conducted to identify their needs and demands about disease management. Patients were presented with the information identified in the literature. Different questions were asked for the discussion: the symptoms of the pathology, diagnosis, the impact of the disease and its treatment on their daily life, and their perception of its management during medical visits. Patients were contacted and invited to participate via the ALCER.
Finally, an online meeting was held with the multidisciplinary committee to review the information gathered from the literature and the focus group and to design the questionnaire (for the full questionnaire, see Table S2).
The first part of the questionnaire consisted of participant baseline characteristics (age, sex, autonomous region, profile). In the second part, unmet needs about CKD-aP pathology management were identified using a 6-point Likert scale for each item (Table 1). Depending on the question included in the topic, the unmet need is greater when the score is closer to 5 or 0.

Table 1.
Items assessing CKD-aP pathology management.
In the third part, we conducted a BWS exercise to elicit participants’ preferences about the most and least preferred attributes that an ideal treatment for CKD-aP should have. Our objective in the BWS analysis was to establish a ranking from the response counts, not to make inferences and comparisons from the sample of participants; so, considerations that might imply a more accurate sample size estimate were not required [40]. Once the attributes that would form part of the BWS were defined by the scientific committee (in this case, characteristics that would make an ideal treatment for CKD-aP), they were combined to form the choice scenarios using a balanced incomplete block design [41]. This design ensures that each attribute appears the same number of times within the design and that all pairs of attributes are combined together the same number of times [40,41]. The attributes were identified during the literature review, and the patient focus group validated the relevance of potential attributes from the Spanish patients´ perspectives. The attributes were also verified by the experts committee for inclusion in the BWS (Table 2).

Table 2.
Attributes included in the BWS.
Each scenario comprised four attributes, each attribute appeared in seven scenarios, and each pair of attributes appeared together three times.
2.4. Statistical Analysis
Stata version 14 was used for the statistical analysis. The participants’ characteristics were summarised through descriptive statistics (continuous variables were presented as means and standard deviations (SDs) and categorical variables as relative and absolute frequencies). The results from the Likert scale were presented using minimum and maximum values for continuous variables in addition to the mentioned statistics. For the BWS, a best-minus-worst score was calculated by subtracting the number of times an attribute was chosen as worst from the number of times it was chosen as best. Subgroup descriptive analyses were performed for each group of participants.
3. Results
3.1. Participants
The questionnaire was ultimately completed by 21 participants (a 40% response rate) from 11 regions of Spain: five nephrologists, five hospital pharmacists, three nurses, four patients (one patient advocacy group representative and three expert renal patients), and four health authorities. The mean age of all participants was 53 years old, and 57% were men (for all participants’ sociodemographic data, see Table S3).
3.2. Unmet Needs in CKD-aP Management
Participants considered CKD-aP as a moderate to severe pathology (3.71/5) and that it had a very severe impact on patients’ QoL (4.29/5). In addition, they reported that the size of the population affected by pruritus is moderately high (3.24/5). However, the diagnosis rate was low (1.71/5). Otherwise, they identified that it was necessary to improve levels of knowledge (2.19/5) and the guidelines and consensus documents for CKD-aP management (3.0/5). In addition, the low–moderate score given for the efficacy and safety of current treatments (1.91 and 2.67, respectively) showed unmet needs in these areas. Furthermore, participants considered that the economic burden (3.38/5) of CKD-aP management was moderately high (Figure 1).
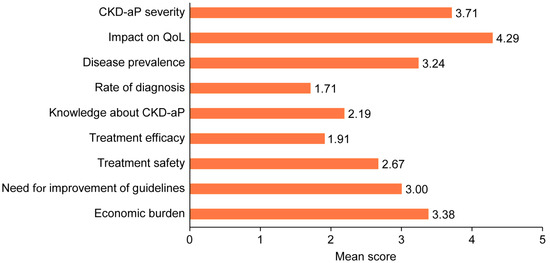
Figure 1.
Unmet needs identified by participants. CKD-aP: chronic kidney disease-associated pruritus; QoL: quality of life.
Nurses and nephrologists were the highest scoring profiles, and the regional health authorities’ profile was the lowest (Figure 2). The item “CKD-aP severity” obtained similar scores across all profiles but not the rest of the items (for the full unmet needs identified by profile, see Table S4). Regarding the “impact on patient QoL”, nurses and nephrologists stood out, although all scored close to 4. Concerning “disease prevalence”, nurses’ opinions were the most prominent (4.3/5) and regional health authorities were the least (2.2/5). Regarding “diagnosis rate”, all profiles provided low scores (maximum 2.2). The only item in which patients scored lower than the rest of the profiles was “knowledge about CKD-aP”. In this case, the health authorities’ group score was the highest. Concerning the efficacy of current treatments, low–medium scores were obtained, from 1.3 for nurses and 1.5 for patients to 2.4 for nephrologists. Regarding the safety of current treatments, its scores were better than for efficacy, but nurses and patients still scored lower than the others. The greatest variability in responses was in the item for the “need to improve guidelines and consensus documents” (details about variability are included in Table S5). In this case, hospital pharmacists indicated the highest score (3.8/5), followed by regional health authorities (3.2/5) and patients (3.0/5). Regarding the cost of disease management, all profiles, except for regional health authorities (2.5/5), obtained similar scores (between 3.2 and 4.0).
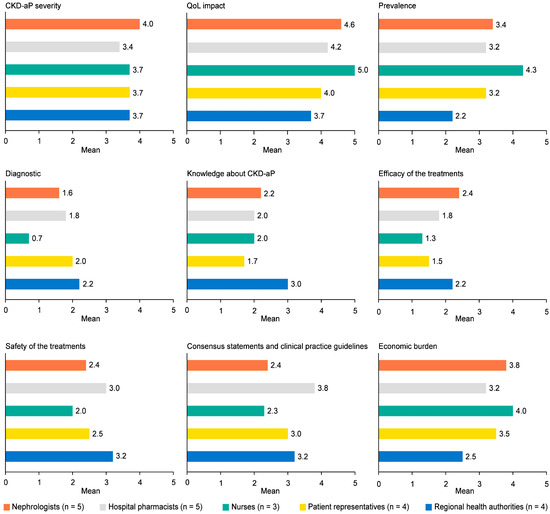
Figure 2.
Unmet needs identified by subgroups. CKD-aP: chronic kidney disease-associated pruritus; QoL: quality of life.
3.3. Attributes of Ideal CKD-aP Treatment by BWS
The preferences of respondents for each attribute were indicated by the distance between the least and most preferred levels (Table 3). According to the best-minus-worst score, the highest valued attribute level was “treatment improves the overall QoL (physical and mental)” and the lowest was “the treatment is administered in the dialysis circuit after each dialysis session”.

Table 3.
Participants’ preferences about which attributes the ideal CKD-aP treatment should have.
The three best attributes occupied the first three positions in all groups (Figure 3). However, hospital pharmacists prioritised “treatment is supported by clinical development/high evidence and has Spanish Agency of Medicines and Medical Devices-approved indication for pruritus”.
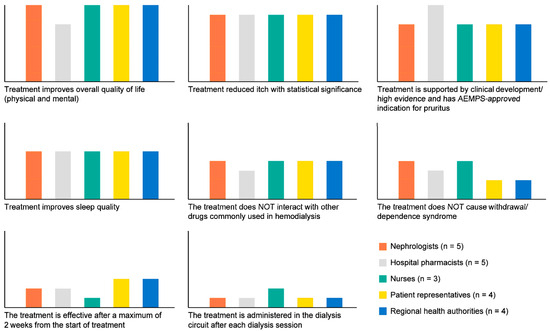
Figure 3.
Preferences about which attributes the ideal CKD-aP treatment should have by subgroups. AEMPS: Spanish Agency of Medicines and Medical Devices.
All groups ranked “treatment improves sleep quality” in the fourth position. Hospital pharmacists prioritised that the treatment did not cause withdrawal/dependence syndrome, and the remaining groups did not. Similarly, the nurse profile gave more importance to administering the drug than the other groups. Patient representatives prioritised “the treatment is effective after a maximum of 2 weeks from the start of treatment” over “the treatment does not cause withdrawal/dependence syndrome” and “the treatment is administered in the dialysis circuit after each dialysis session” (detailed results are included in Table S6 in Supplementary Materials).
4. Discussion
Pruritus is a common and burdensome issue reported by the general population, but its prevalence and severity are even higher in patients with CKD [42]. Furthermore, these individuals have an increased risk of developing skin ulcers that are difficult to treat (particularly in those with diabetes or arteriosclerosis). Thus, it would be interesting to include patients with pruritus in preventive treatment protocols for skin lesions [43,44,45]. In recent years, there has been growing national and international interest in gaining a better understanding of CKD-aP prevalence, symptoms, and treatment, especially its impact on patients’ health-related QoL [6,7,9,46,47]. This study reported the unmet needs for CKD-aP management and the preferences for the characteristics that a hypothetical, ideal CKD-aP treatment should have from the perspective of a group of nephrologists, hospital pharmacists, nurses, patient representatives, and regional health authorities. To our knowledge, this is the first such study to survey all stakeholders involved in disease management nationwide in Spain.
Our study finds that CKD-aP is considered a serious pathology that greatly impacts QoL in dialysis patients. This is consistent with numerous national and international studies on this topic [7,14,18,20,21,22,46,48,49,50,51,52]. In addition, the size of the population affected by pruritus is quite high; however, the low diagnosis rate suggests there may be a larger affected population than currently identified. This underdiagnosis could be due to the identified need to improve knowledge about CKD-aP and better information in guidelines and consensus documents. Other recent studies carried out in Spain [6,47] and other European countries [7] have shown results that are in line with those highlighted in our study. Accordingly, in a survey conducted by the European Kidney Patients Federation (EKPF), 75% of nephrologists interviewed agreed that CKD-aP is underdiagnosed in HD patients and emphasised the lack of guidelines and standardised scales to consistently diagnose and classify CKD-aP severity. In this context, the recently published consensus document [4] in Spain provides national recommendations for the diagnostic and therapeutic management of CKD-aP, aiming to address the current lack of specific guidelines for its diagnosis and management.
Besides the challenges around diagnosis, participants also identified difficulties around the medical needs of this disease. Currently, in Spain, there is no approved, available therapeutic option to tackle CKD-aP [26]. Furthermore, CKD-aP codification in medical records is not usual, as there are no specific codes for the disease in the ICD-9 or ICD-10 (Statistical Classification of Diseases and Related Health Problems in Spain). Previous work [53] has confirmed that the lack of specific codes reduces diagnosis by experts, and the results of this study would be in line with these findings. In addition, they pointed out that the current economic burden of CKD-aP management in dialysis patients is high.
Regarding the subgroup analysis and based on the opinion of the expert committee of this study, it was identified that nurses had the highest scores, likely because of their tremendous involvement in the daily care of dialysis patients. On the contrary, regional health authorities reported the lowest unmet needs, which aligns with their expected roles and responsibilities. In particular, regional health authorities highlighted items such as the size of the affected population, the level of knowledge that the agents involved in the pathology have, and the costs associated with managing patients with CKD-aP. On the other hand, it was expected that patient representatives would have higher scores than nurses; nevertheless, patients showed their objectivity as experts when assessing CKD-aP as one symptom among the diverse array of end-stage CKD symptoms in dialysis treatment as a whole. Hospital pharmacists showed a mid-level score profile and highlighted the need to improve guidelines and consensus documents, as they play an important part in their responsibilities.
CKD-aP severity and its impact on QoL are the items with the lowest variability in response across profiles (minimum: 3, maximum: 5.0, and SD: 0.64 and 0.56, respectively). The need to improve guidelines and consensus documents is the item with the greatest variability (minimum: 1.0, maximum: 5.0, and SD: 1.18). In this case, hospital pharmacists indicated the highest score, even higher than nephrologists or nurses, which may be because the latter two are regularly updated with scientific information and believe that they have adequate information for the proper management of these patients.
According to the best-minus-worst score, three of the four most valued attributes by the total sample were related to patient-reported outcomes (PROs): “treatment improves the overall QoL (physical and mental)”, “treatment reduces itch with statistical significance”, and “treatment improves sleep quality”. All groups also considered “treatment is supported by clinical development/high evidence and has Spanish Agency of Medicines and Medical Devices-approved indication for pruritus” as one of the most relevant attributes, being prioritised by hospital pharmacists. The remaining attributes varied in order, depending on the interests and responsibilities of each group. Therefore, pharmacists’ prioritisation could be due to their responsibilities to ensure effective, safe, and efficient treatment, while patients prioritised attributes with the aim of alleviating symptoms as soon as possible. Finally, it is noteworthy that patient representatives and regional health authorities had similar attribute prioritisation, reflecting the results of the efforts made in recent years to promote patient-centred care, now considered an essential goal of high-quality healthcare systems.
5. Limitations
This study had some limitations. First, the sample size was too small to be regarded as representative of the country. However, a balanced sample was included in each group of participants, ensuring that key multi-stakeholder perspectives were considered in analysing the different criteria for disease management. The sample was distributed among eleven different regions within the territory, which ensured broad representation despite the sample size. Notably, given the lack of formal guidance on the minimal sample size required for best–worst scaling [40,54] and considering that making inferences about the population represented via the sample or comparing scores between groups was not the objective of this study, we aimed to reach as many participants as possible. However, the response rate ultimately determined the sample proportion. The number of participants and analyses is in line with other studies featuring similar methodologies in the recent literature [55,56,57]. Secondly, participants’ preferences and choices are constrained within the attributes presented in BWS scenarios; thus, societal preferences for the ideal treatment of CKD-aP may include attributes not explored in this study. Nonetheless, the attributes tested herein were selected according to the literature and the opinions of a multidisciplinary committee of experts. The BWS method is becoming increasingly popular in eliciting preferences in healthcare [58] because it helps collect more data compared to discrete choice experiments and has several advantages over these [59]. On the one hand, respondents are provided with scenarios one by one rather than two (or more) at a time; thus, BWS is considered less cognitively demanding for participants. On the other hand, respondents make choices within scenarios rather than between scenarios.
Despite these limitations, this study provides valuable insights into current CKD-aP management, an underdiagnosed comorbidity that significantly impacts patients’ quality of life and presents unmet needs recognised by different stakeholders involved in its management. Beyond raising awareness of the relevance of CKD-aP, this study serves as a starting point for establishing measures from both clinical and health policymaker perspectives to address the identified needs related to diagnosis, management, and treatment availability for this population. Future efforts should focus on identifying and diagnosing this pathology, measuring its severity and impact on patients’ lives, and optimising treatment, favouring the use of safe and effective treatments for these patients. Pruritus should always be considered a key component in any preventive treatment protocols for skin lesions.
6. Conclusions
Based on the perspectives of a multidisciplinary panel of experts, this study has established the unmet needs and preferred attributes of an ideal treatment for CKD-aP management in Spain. The results suggest that CKD-aP is a severe pathology with a high impact on QoL in dialysis patients. Moreover, it is underdiagnosed or not fully understood by healthcare professionals. Thus, strengthening our understanding and knowledge of CKD-aP should be a priority.
This study concludes that the ideal CKD-aP treatment should improve overall QoL (physical and mental) and have an approved indication for CKD-aP supported by clinical development/high-quality evidence, according to participants’ perspectives.
This study serves as a starting point to raise awareness about CKD-aP—an enduring health problem that still persists in our society—and to create an action framework with all stakeholders involved in CKD-aP management to provide viable solutions for patients with this disease.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm14020624/s1, Table S1: List of terms in the literature search strategy; Table S2: Online questionnaire; Table S3: Sociodemographic characteristics; Table S4: Unmet needs identified by profile; Table S5: Unmet needs. Variability in responses; Table S6: Preferences about which attributes the ideal CKD-aP treatment should have, detailed per group.
Author Contributions
Conceptualisation, P.D.S., J.M.M.-S., I.R. and J.C.J.-M.; methodology, A.C. and S.A.; validation, P.D.S., J.M.M.-S., I.R. and J.C.J.-M.; formal analysis, A.C. and S.A.; visualisation, O.R.-A.; writing—original draft preparation, P.D.S., J.M.M.-S., I.R., J.C.J.-M., A.C. and S.A.; writing—review and editing, P.D.S., J.M.M.-S., I.R., J.C.J.-M., A.C. and S.A.; funding acquisition, O.R.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Vifor Fresenius Medical Care Renal Pharma España S.L.
Institutional Review Board Statement
All procedures followed were in line with the ethical standards of the Declaration of Helsinki. Due to the nature of the study, ethics committee approval was not required, while written informed consent was obtained by all participants. In addition, some of them gave their consent to appear in the acknowledgements.
Informed Consent Statement
Written informed consent was obtained by all participants by checking a box indicating that they read and approved the survey and agreed to participate in the study with all the conditions described.
Data Availability Statement
All data presented in this study are available on the supplementary.
Acknowledgments
The authors acknowledge José Luis Trillo, Alfonso Alonso, Javier Deira, Pablo Bouza, Jose Ignacio Minguela, Itziar Castaño, Pere Ventayol, Ana Regueira, Carlos Martínez, Juan Francisco Pulido, Patricia Arribas, Marta Moreno, Gregorio Vega, and Alberto Garrido for answering the questionnaire as participants, as well as Carla Garí and Asís Ariznavarreta from Outcomes’10 (a ProductLife Group Company) for their support during project development.
Conflicts of Interest
P.D.S., J.M.M.-S., I.R., and J.C.J.-M. declare that they (or their institutions) have received fees from Vifor Fresenius Medical Care Renal Pharma España S.L. for their advisory roles as members of the Scientific Committee of the project. O.R.-A. is a full-time Vifor Fresenius Medical Care Renal Pharma España S.L. employee. A.C. and S.A. work for an independent research entity that received funding from Vifor Fresenius Medical Care Renal Pharma España S.L. for contributing to the study design; the collection, analyses, or interpretation of the data; project coordination; and the drafting of this manuscript.
Correction Statement
This article has been republished with a minor correction to the Informed Consent Statement. This change does not affect the scientific content of the article.
References
- Memoria de Actividades ALCER. 2023. Available online: https://alcer.org/wp-content/uploads/2024/06/memoria-2023-.pdf (accessed on 19 December 2024).
- Evans, M.; Lewis, R.D.; Morgan, A.R.; Whyte, M.B.; Hanif, W.; Bain, S.C.; Davies, S.; Dashora, U.; Yousef, Z.; Patel, D.C.; et al. A Narrative Review of Chronic Kidney Disease in Clinical Practice: Current Challenges and Future Perspectives. Adv. Ther. 2022, 39, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, R.; Davison, S.N.; Farrington, K.; Flythe, J.E.; Foo, M.; Madero, M.; Morton, R.L.; Tsukamoto, Y.; Unruh, M.L.; Cheung, M.; et al. Managing the symptom burden associated with maintenance dialysis: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2023, 104, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Buades, J.M.; Figueras-Nart, I.; Goicoechea, M.; Villanueva, R.J.S.; Serra-Baldrich, E. Information and consensus document for the diagnostic and therapeutic management of pruritus associated with chronic kidney disease in patients on haemodialysis in Spain. Nefrología 2024, 44, 465–474. [Google Scholar] [CrossRef]
- Ramírez-Guerrero, G.; Ronco, C.; Reis, T. Chronic Kidney Disease-Associated Pruritus: Nomenclature and Treatment—We Need to Take Two Steps Forward. Blood Purif. 2023, 52, 821–823. [Google Scholar] [CrossRef]
- Goicoechea, M.; Arenas-Jimenez, M.D.; Areste, N.; Perez-Morales, R.E.; Esteve, V.; Sanchez-Alvarez, E.; Bezhold, G.A.; Blanco, A.; Sanchez-Villanueva, R.; Molina, P.; et al. Perception of Spanish nephrologists on an old unsolved problem: Pruritus associated with chronic kidney disease (CKD-aP). Nefrología 2023, 43, 102–110. [Google Scholar] [CrossRef] [PubMed]
- EKPF White Paper—Improving Chronic Kidney Disease-Associated Pruritus (CKD-aP) Patients’ Quality of Life. Available online: https://ekpf.eu/wp-content/uploads/2023/10/EKPF-draft-report-final_WEB.pdf (accessed on 8 November 2023).
- Cheng, A.Y.; Wong, L.S. Uremic Pruritus: From Diagnosis to Treatment. Diagnostics 2022, 12, 1108. [Google Scholar] [CrossRef] [PubMed]
- Santos-Alonso, C.; Martín, M.M.; Villanueva, R.S.; García, L.Á.; Gallardo, M.A.V.; Rubio, M.A.B.; del Peso Gilsanz, G.; González, M.O.; Gutiérrez, R.S. Pruritus in dialysis patients. Review and new perspectives. Nefrología 2022, 42, 15–21. [Google Scholar] [CrossRef]
- Baglioni, C.; Battagliese, G.; Feige, B.; Spiegelhalder, K.; Nissen, C.; Voderholzer, U.; Lombardo, C.; Riemann, D. Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. J. Affect. Disord. 2011, 135, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Bertisch, S.M.; Pollock, B.D.; Mittleman, M.A.; Buysse, D.J.; Bazzano, L.A.; Gottlieb, D.J.; Redline, S. Insomnia with objective short sleep duration and risk of incident cardiovascular disease and all-cause mortality: Sleep Heart Health Study. Sleep 2018, 41, zsy047. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.L.; Anderson, M.L.; Dublin, S.; Hanlon, J.T.; Hubbard, R.; Walker, R.; Yu, O.; Crane, P.K.; Larson, E.B. Cumulative use of strong anticholinergics and incident dementia: A prospective cohort study. JAMA Intern. Med. 2015, 175, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Hoevenaar-Blom, M.P.; Spijkerman, A.M.; Kromhout, D.; Van Den Berg, J.F.; Verschuren, W.M. Sleep duration and sleep quality in relation to 12-year cardiovascular disease incidence: The MORGEN study. Sleep 2011, 34, 1487–1492. [Google Scholar] [CrossRef]
- Pisoni, R.L.; Wikström, B.; Elder, S.J.; Akizawa, T.; Asano, Y.; Keen, M.L.; Saran, R.; Mendelssohn, D.C.; Young, E.W.; Port, F.K. Pruritus in haemodialysis patients: International results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol. Dial. Transplant. 2006, 21, 3495–3505. [Google Scholar] [CrossRef] [PubMed]
- Sabia, S.; Fayosse, A.; Dumurgier, J.; van Hees, V.T.; Paquet, C.; Sommerlad, A.; Kivimäki, M.; Dugravot, A.; Singh-Manoux, A. Association of sleep duration in middle and old age with incidence of dementia. Nat. Commun. 2021, 12, 2289. [Google Scholar] [CrossRef]
- Fang, H.; Tu, S.; Sheng, J.; Shao, A. Depression in sleep disturbance: A review on a bidirectional relationship, mechanisms and treatment. J. Cell. Mol. Med. 2019, 23, 2324–2332. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, K.; Bond, T.C.; Claxton, A.; Sood, V.C.; Kootsikas, M.; Agnese, W.; Sibbel, S. Clinical characteristics and outcomes of end-stage renal disease patients with self-reported pruritus symptoms. Int. J. Nephrol. Renovasc. Dis. 2013, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sukul, N.; Karaboyas, A.; Csomor, P.A.; Schaufler, T.; Wen, W.; Menzaghi, F.; Rayner, H.C.; Hasegawa, T.; Al Salmi, I.; Al-Ghamdi, S.M.; et al. Self-reported Pruritus and Clinical, Dialysis-Related, and Patient-Reported Outcomes in Hemodialysis Patients. Kidney Med. 2021, 3, 42–53.e41. [Google Scholar] [CrossRef] [PubMed]
- Registro Español de Enfermos Renales (REER): Informe 2021-Datos Preliminares. 52 Congreso SEN. 12–14 November 2022. Available online: https://www.setrasplante.org/posts/post/registro-espanol-de-enfermos-renales-reer-informe-2021-datos-preliminares (accessed on 15 October 2023).
- Areste-Fosalba, N.; Emilio, S.A.J.; Henriquez, F.; Jesus Lloret, M.; Ulloa, C.; Buades, F.J.M.; Mascarós, V.; Isabel Martínez Puerto, A.; Dolores Arenas, M.; Yetman, D.; et al. MO131: Prevalence of Pruritus in Spanish Patients with Chronic Kidney Disease and Affectation of Quality of Life. Nephrol. Dial. Transplant. 2022, 37, gfac066.033. [Google Scholar] [CrossRef]
- Rayner, H.C.; Larkina, M.; Wang, M.; Graham-Brown, M.; van der Veer, S.N.; Ecder, T.; Hasegawa, T.; Kleophas, W.; Bieber, B.A.; Tentori, F.; et al. International Comparisons of Prevalence, Awareness, and Treatment of Pruritus in People on Hemodialysis. Clin. J. Am. Soc. Nephrol. 2017, 12 (Suppl. S3), 2000–2007. [Google Scholar] [CrossRef] [PubMed]
- Reyes, V.; Caro, J.; Pais, B.; Buades, F.J.M.; De Sequera Ortiz, P.; Espín, J.; Tombás, A.; Moreno, M.; Julián Mauro, J.C. A Survey on the Impact of Pruritus Associated with Chronic Kidney Disease (CKD-AP) in the Quality of Life of Patients Undergoing Haemodialysis in Spain. Nephrol. Dial. Transplant. 2022, 37, gfac083.022. [Google Scholar] [CrossRef]
- Esteve-Simó, V.; Perez-Morales, R.; Buades-Fuster, J.M.; Arenas Jimenez, M.D.; Areste-Fosalba, N.; Alcalde Bezhold, G.; Blanco Santos, A.; Sanchez Álvarez, E.; Sanchez Villanueva, R.; Molina, P.; et al. Chronic Kidney Disease-Associated Pruritus and Quality of Life: Learning from Our Patients. J. Clin. Med. 2023, 12, 4505. [Google Scholar] [CrossRef]
- Hercz, D.; Jiang, S.H.; Webster, A.C. Interventions for itch in people with advanced chronic kidney disease. Cochrane Database Syst. Rev. 2020, 12, CD011393. [Google Scholar] [CrossRef]
- Verduzco, H.A.; Shirazian, S. CKD-Associated Pruritus: New Insights into Diagnosis, Pathogenesis, and Management. Kidney Int. Rep. 2020, 5, 1387–1402. [Google Scholar] [CrossRef]
- Bifimed. Buscador de la Información Sobre la Situación de Financiación de los Medicamentos. Available online: https://www.sanidad.gob.es/eu/profesionales/medicamentos.do (accessed on 15 January 2024).
- Ficha Técnica Mirtazapina. CIMA-AEMPS. Available online: https://cima.aemps.es/cima/pdfs/es/ft/67068/FT_67068.pdf (accessed on 15 January 2024).
- Gabapentinoides: Nuevas Evidencias Para Reconsiderar su Uso. SESCAM. 2019. Vol.XX, Nº2. Available online: https://sanidad.castillalamancha.es/sites/sescam.castillalamancha.es/files/documentos/farmacia/hem_2_2019_gabapentinoides_nuevas_evidencias_para_reconsiderar_su_uso_0.pdf (accessed on 15 November 2023).
- Gabapentina y Pregabalina: Entre el Uso y el Abuso. Osakidetza. 2014. Infac. Volumen 22, Nº4. Available online: https://www.euskadi.eus/contenidos/informacion/cevime_infac_2014a/es_def/adjuntos/INFAC_Vol_22_4_Gabapentina_Pregabalina_es.pdf (accessed on 15 November 2023).
- Avaliación Farmacoteraéutica de Novos Medicamentos. SERGAS. 2005. Nº8. Available online: https://www.sergas.es/Asistencia-sanitaria/Documents/399/Pregabalina.pdf (accessed on 15 November 2023).
- Ficha Técnica Pregabalina. CIMA-AEMPS. Available online: https://cima.aemps.es/cima/dochtml/ft/04279018/FT_04279018.html (accessed on 15 January 2024).
- Gabapentin FDA Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020235s064_020882s047_021129s046lbl.pdf (accessed on 15 November 2023).
- Boletín Mensual de Seguridad de la AEMPS Sobre Medicamentos de uso Humano del Mes de Octubre de 2022|AEMPS. Available online: https://www.aemps.gob.es/informa/boletin-mensual-de-seguridad-de-la-aemps-sobre-medicamentos-de-uso-humano-del-mes-de-octubre-de-2022/ (accessed on 15 November 2023).
- Pregabalin (Lyrica), Gabapentin (Neurontin) and Risk of Abuse and Dependence: New Scheduling Requirements from 1 April—Gov.UK. Available online: https://www.gov.uk/drug-safety-update/pregabalin-lyrica-gabapentin-neurontin-and-risk-of-abuse-and-dependence-new-scheduling-requirements-from-1-april#risk-of-abuse-and-dependence (accessed on 15 November 2023).
- Tambon, M.; Ponté, C.; Jouanjus, E.; Fouilhé, N.; Micallef, J.; Lapeyre-Mestre, M.; French Addictovigilance Network (FAN). Gabapentinoid Abuse in France: Evidence on Health Consequences and New Points of Vigilance. Front. Psychiatry 2021, 12, 639780. [Google Scholar] [CrossRef] [PubMed]
- Ishida, J.H.; McCulloch, C.E.; Steinman, M.A.; Grimes, B.A.; Johansen, K.L. Gabapentin and Pregabalin Use and Association with Adverse Outcomes among Hemodialysis Patients. J. Am. Soc. Nephrol. 2018, 29, 1970–1978. [Google Scholar] [CrossRef] [PubMed]
- de Sequera, P.; Buades, J.M.; Reyes-Alcázar, V.; Pais, B.; Espín, J.; Tombás, A.; Moreno, M.; Julián, J.C. Impact of pruriture associated with chronic renal disease (PaCKD) on the quality of life of patients in hemodialysis in Spain. Nefrología 2023, 43, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de la Presidencia Rclcymd. Real Decreto 957/2020, de 3 de Noviembre, por el que se Regulan los Estudios Observacionales con Medicamentos de uso Humano. 2020. Available online: https://www.boe.es/buscar/doc.php?id=BOE-A-2020-14960 (accessed on 15 January 2024).
- Grupo de Trabajo de los CEIMs con la AEMPS. Memorando de Colaboración entre los Comités de Ética de la Investigación con Medicamentos para la Evaluación y Gestión de los Estudios Observacionales con Medicamentos. 2021. Available online: https://www.aemps.gob.es/investigacionClinica/medicamentos/docs/estudios-PA/Memorando_CEIMS.pdf (accessed on 15 January 2024).
- Louviere, J.; Lings, I.; Islam, T.; Gudergan, S.; Flynn, T. An introduction to the application of (case 1) best–worst scaling in marketing research. Int. J. Res. Mark. 2013, 30, 292–303. [Google Scholar] [CrossRef]
- Wakeling, I.N.; Buck, D. Balanced incomplete block designs useful for consumer experimentation. Food Qual. Prefer. 2001, 12, 265–268. [Google Scholar] [CrossRef]
- Sommer, R.; Ständer, S.; Augustin, M. Skin Lesions, Skin Care, and Characteristics of Pruritus in Patients Undergoing Haemodialysis. Skin. Pharmacol. Physiol. 2022, 35, 87–93. [Google Scholar] [CrossRef]
- Dòria, M.; Betriu, À.; Belart, M.; Rosado, V.; Hernández, M.; Sarro, F.; Real, J.; Castelblanco, E.; Pacheco, L.R.; Fernández, E.; et al. High Incidence of Adverse Outcomes in Haemodialysis Patients with Diabetes with or without Diabetic Foot Syndrome: A 5-Year Observational Study in Lleida, Spain. J. Clin. Med. 2021, 10, 1368. [Google Scholar] [CrossRef] [PubMed]
- Goel, V.; Sil, A.; Das, A. Cutaneous Manifestations of Chronic Kidney Disease, Dialysis and Post-Renal Transplant: A Review. Indian J. Dermatol. 2021, 66, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Mancin, S.; Mazzoleni, B.; Reggiani, F.; Calatroni, M.; Alterchi, E.; Donizzetti, D.; Finazzi, S.; Soekeland, F.; Sguanci, M.; Badalamenti, S. Integrated protocol for the prevention and treatment of skin ulcers in patients with end-stage renal disease. MethodsX 2023, 11, 102482. [Google Scholar] [CrossRef] [PubMed]
- Aresté, N.; Sanchez-Alvarez, J.E.; Prieto-Velasco, M.; Molina, P.; Esteve-Simó, V.; Ojeda, R.; Buades, J.M.; Goicoechea, M.; Sanchez-Villanueva, R.; Bezhold, G.A.; et al. Prevalence and severity of pruritus in Spanish patients with chronic kidney disease and impact on quality of life: A cross-sectional study. Clin. Kidney J. 2023, 16, 1035–1037. [Google Scholar] [CrossRef] [PubMed]
- Burton, J.; Topf, J.; Fishbane, F.; Weiner, D. Unmet Medical Needs in Chronic-Kidney-Disease-Associated Pruritus. EMJ Nephrol. 2021, 9, 2–11. [Google Scholar]
- Grochulska, K.; Ofenloch, R.F.; Mettang, T.; Weisshaar, E. Mortality of Haemodialysis Patients with and Without Chronic Itch: A Follow-up Study of the German Epidemiological Hemodialysis Itch Study (GEHIS). Acta Derm. Venereol. 2019, 99, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Lanot, A.; Bataille, S.; Rostoker, G.; Bataille, P.; Chauveau, P.; Touzot, M.; Misery, L. Moderate-to-severe pruritus in untreated or non-responsive hemodialysis patients: Results of the French prospective multicenter observational study Pruripreva. Clin. Kidney J. 2023, 16, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.B.; Nogueira, F.C.P.; de Souza, M.R.; Penalva, M.A.; de Amorim, J.L.; Pisoni, R.L.; Robinson, B.M.; Lopes, A.A. Assessment of the psychological burden associated with pruritus in hemodialysis patients using the kidney disease quality of life short form. Qual. Life Res. 2012, 21, 603–612. [Google Scholar] [CrossRef]
- van der Willik, E.M.; Lengton, R.; Hemmelder, M.H.; Hoogeveen, E.K.; Bart, H.A.; van Ittersum, F.J.; Ten Dam, M.A.; Bos, W.J.W.; Dekker, F.W.; Meuleman, Y. Itching in dialysis patients: Impact on health-related quality of life and interactions with sleep problems and psychological symptoms-results from the RENINE/PROMs registry. Nephrol. Dial. Transplant. 2022, 37, 1731–1741. [Google Scholar] [CrossRef]
- Weiss, M.; Mettang, T.; Tschulena, U.; Passlick-Deetjen, J.; Weisshaar, E. Prevalence of chronic itch and associated factors in haemodialysis patients: A representative cross-sectional study. Acta Derm. Venereol. 2015, 95, 816–821. [Google Scholar] [CrossRef] [PubMed]
- eCIE10ES Diagnósticos. 2022. Available online: https://eciemaps.mscbs.gob.es/ecieMaps/browser/index_10_mc.html (accessed on 15 January 2024).
- Hollin, I.L.; Paskett, J.; Schuster, A.L.R.; Crossnohere, N.L.; Bridges, J.F.P. Best-Worst Scaling and the Prioritization of Objects in Health: A Systematic Review. Pharmacoeconomics 2022, 40, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Dams, F.; Gonzalez Rodriguez, J.L.; Cheung, K.L.; Wijnen, B.F.M.; Hiligsmann, M. Relevance of barriers and facilitators in the use of health technology assessment in Colombia. J. Med. Econ. 2018, 21, 510–517. [Google Scholar] [CrossRef] [PubMed]
- van der Putten, I.M.; Paulus, A.T.; Evers, S.M.; Hutubessy, R.C.; Hiligsmann, M. Identification and Prioritization of the Economic Impacts of Vaccines. Biomed. Res. Int. 2016, 2016, 6267343. [Google Scholar] [CrossRef]
- Wallar, L.E.; McEwen, S.A.; Sargeant, J.M.; Mercer, N.J.; Papadopoulos, A. Prioritizing professional competencies in environmental public health: A best–worst scaling experiment. Environ. Health Rev. 2018, 61, 50–63. [Google Scholar] [CrossRef]
- Cheung, K.L.; Wijnen, B.F.; Hollin, I.L.; Janssen, E.M.; Bridges, J.F.; Evers, S.M.; Hiligsmann, M. Using Best-Worst Scaling to Investigate Preferences in Health Care. Pharmacoeconomics 2016, 34, 1195–1209. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Chen, H.; Gao, Y.; Xiang, Y.; Wang, F.; Ni, Z.; Wang, X.; Huang, X. Best-Worst Scaling Survey of Inpatients’ Preferences in Medical Decision-Making Participation in China. Healthcare 2023, 11, 323. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).