The Role of Impella in Cardiogenic Shock Complicated by an Acute Myocardial Infarction: A Meta-Analysis
Abstract
1. Introduction
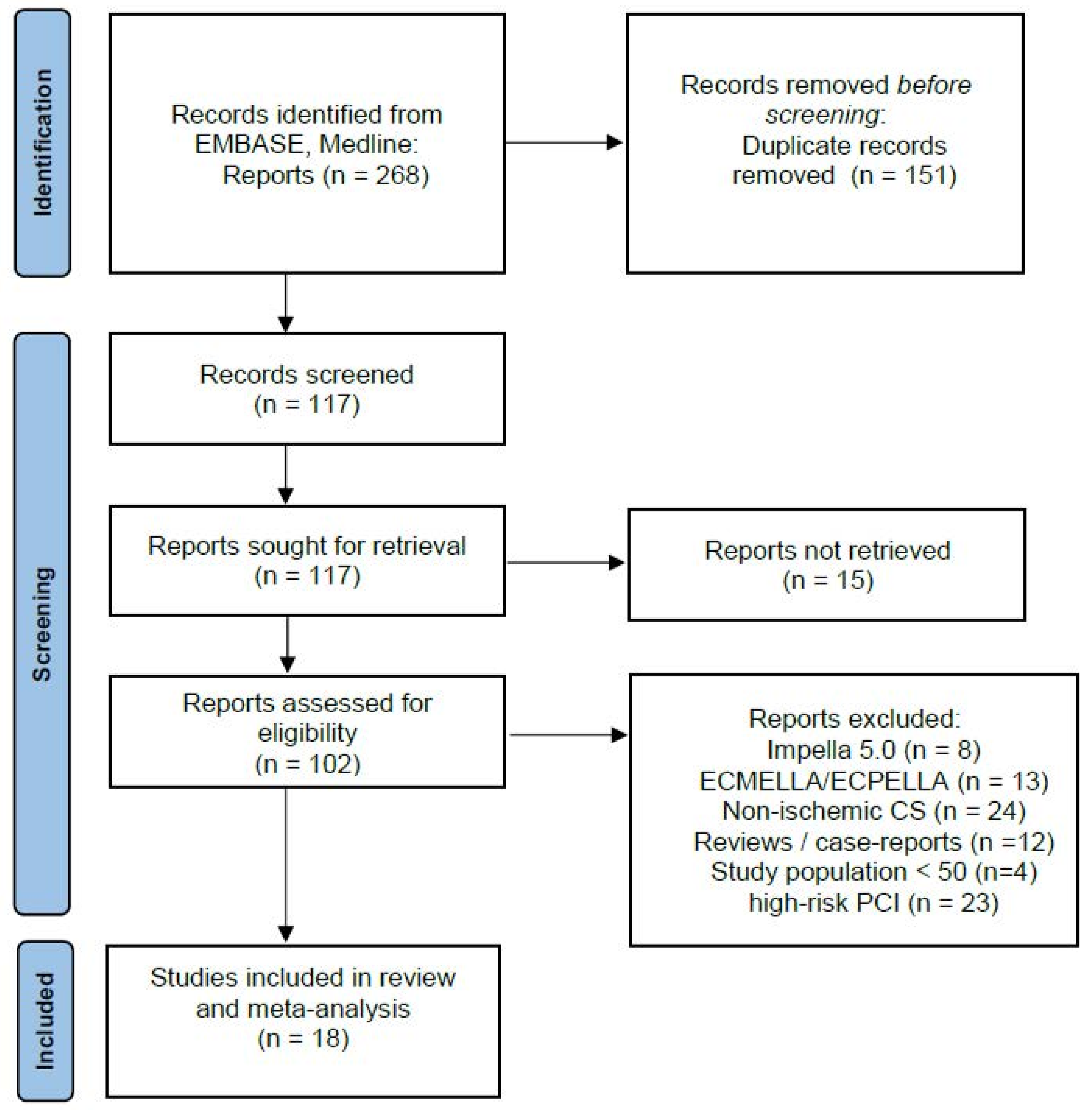
2. Methods
2.1. Studies Selection
2.2. Endpoints and Definitions
2.3. Statistical Analysis
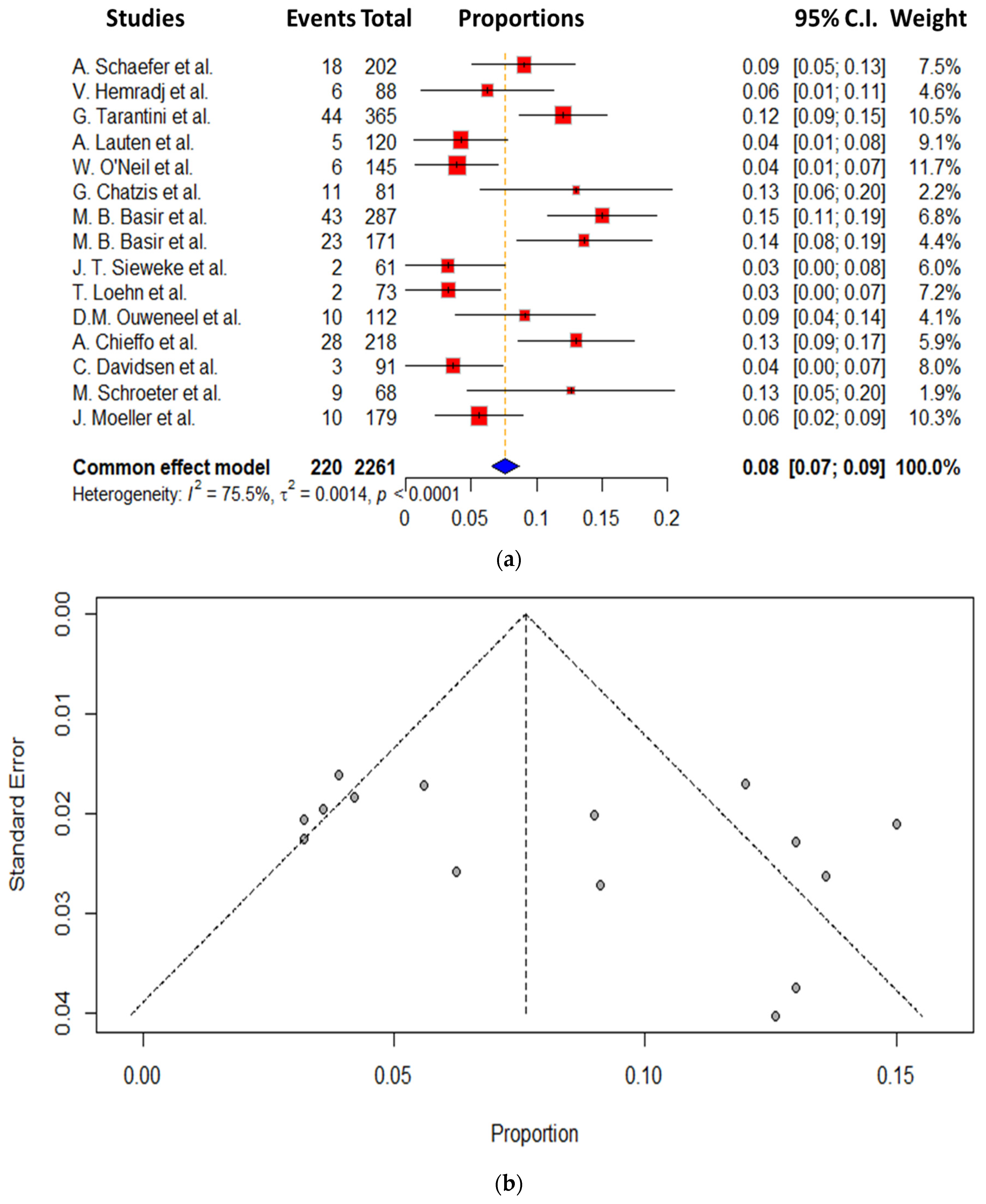
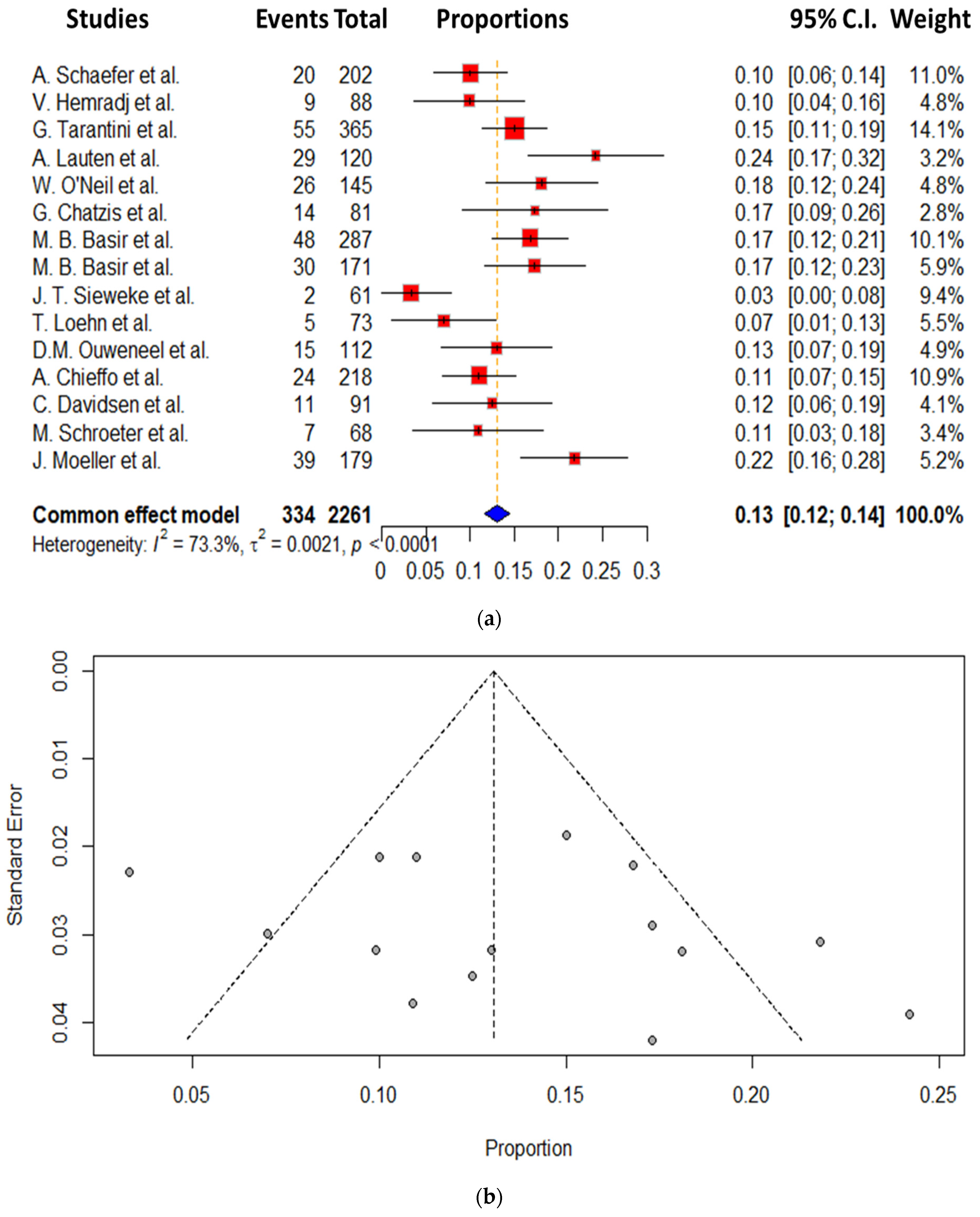
3. Results
Secondary End Points
4. Discussion
- The 30-day mortality rate for CS patients is still high even with the use of Impella.
- Our findings do, however, indicate that vascular complications remain high, being, however, quite heterogeneous between the studies.
4.1. Study Limitations
4.2. Future Perspective
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bellumkonda, L.; Gul, B.; Masri, S.C. Evolving Concepts in Diagnosis and Management of Cardiogenic Shock. Am. J. Cardiol. 2018, 122, 1104–1110. [Google Scholar] [CrossRef]
- Squara, P.; Hollenberg, S.; Payen, D. Reconsidering Vasopressors for Cardiogenic Shock: Everything Should Be Made as Simple as Possible, but Not Simpler. Chest 2019, 156, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Dünser, M.W.; Takala, J.; Brunauer, A.; Bakker, J. Re-thinking resuscitation: Leaving blood pressure cosmetics behind and moving forward to permissive hypotension and a tissue perfusion-based approach. Crit. Care 2013, 17, 326. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, F.H.; Dupont, M.; Steels, P.; Grieten, L.; Malbsrain, M.; Tang, W.H.; Mullens, W. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J. Am. Coll. Cardiol. 2013, 62, 485–495. [Google Scholar] [CrossRef]
- Burzotta, F.; Trani, C.; Doshi, S.N.; Townend, J.; van Geuns, R.J.; Hunziker, P.; Schieffer, B.; Karatolios, K.; Møller, J.E.; Ribichini, F.L.; et al. Impella ventricular support in clinical practice: Collaborative viewpoint from a European expert user group. Int. J. Cardiol. 2015, 201, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Markus, B.; Patsalis, N.; Chatzis, G.; Luesebrink, U.; Ahrens, H.; Schieffer, B.; Karatolios, K. Impact of microaxillar mechanical left ventricular support on renal resistive index in patients with cardiogenic shock after myocardial infarction: A pilot trial to predict renal organ dysfunction in cardiogenic shock. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 158–163. [Google Scholar] [CrossRef]
- Schiller, P.; Vikholm, P.; Hellgren, L. The Impella® Recover mechanical assist device in acute cardiogenic shock: A single-centre experience of 66 patients. Interact. Cardiovasc. Thorac. Surg. 2016, 22, 452–458. [Google Scholar] [CrossRef]
- Casassus, F.; Corre, J.; Leroux, L.; Chevalereau, P.; Fresselinat, A.; Seguy, B.; Calderon, J.; Coste, P.; Ouattara, A.; Roques, X.; et al. The use of Impella 2.5 in severe refractory cardiogenic shock complicating an acute myocardial infarction. J. Interv. Cardiol. 2015, 28, 41–50. [Google Scholar] [CrossRef]
- Karatolios, K.; Chatzis, G.; Markus, B.; Luesebrink, U.; Ahrens, H.; Dersch, W.; Betz, S.; Ploeger, B.; Boesl, E.; O’Neill, W.; et al. Impella support compared to medical treatment for post-cardiac arrest shock after out of hospital cardiac arrest. Resuscitation 2018, 126, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Chatzis, G.; Markus, B.; Luesebrink, U.; Ahrens, H.; Divchev, D.; Syntila, S.; Scheele, N.; Al Eryani, H.; Tousoulis, D.; Schieffer, B.; et al. Early Impella Support in Postcardiac Arrest Cardiogenic Shock Complicating Acute Myocardial Infarction Improves Short- and Long-Term Survival. Crit. Care Med. 2021, 49, 943–955. [Google Scholar] [CrossRef]
- Syntila, S.; Chatzis, G.; Markus, B.; Ahrens, H.; Waechter, C.; Luesebrink, U.; Divchev, D.; Schuett, H.; Tsalouchidou, P.E.; Jerrentrup, A.; et al. Comparison of Mechanical Support with Impella or Extracorporeal Life Support in Post-Cardiac Arrest Cardiogenic Shock: A Propensity Scoring Matching Analysis. J. Clin. Med. 2021, 10, 3583. [Google Scholar] [CrossRef] [PubMed]
- Thiele, H.; Moller, J.E.; Henriques, J.P.S.; Bogerd, M.; Seyfarth, M.; Burkhoff, D.; Ostadal, P.; Rokyta, R.; Belohlavek, J.; Massberg, S.; et al. Temporary mechanical circulatory support in infarct-related cardiogenic shock: An individual patient data meta-analysis of randomised trials with 6-month follow-up. Lancet 2024, 404, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Moller, J.E.; Engstrom, T.; Jensen, L.O.; Eiskjaer, H.; Mangner, N.; Polzin, A.; Schulze, P.C.; Skurk, C.; Nordbeck, P.; Clemmensen, P.; et al. Microaxial Flow Pump or Standard Care in Infarct-Related Cardiogenic Shock. N. Engl. J. Med. 2024, 390, 1382–1393. [Google Scholar] [CrossRef]
- Basir, M.B.; Schreiber, T.L.; Grines, C.L.; Dixon, S.R.; Moses, J.W.; Maini, B.S.; Khandelwal, A.K.; Ohman, E.M.; O’Neill, W.W. Effect of Early Initiation of Mechanical Circulatory Support on Survival in Cardiogenic Shock. Am. J. Cardiol. 2017, 119, 845–851. [Google Scholar] [CrossRef]
- Basir, M.B.; Kapur, N.K.; Patel, K.; Salam, M.A.; Schreiber, T.; Kaki, A.; Hanson, I.; Almany, S.; Timmis, S.; Dixon, S.; et al. Improved Outcomes Associated with the use of Shock Protocols: Updates from the National Cardiogenic Shock Initiative. Catheter. Cardiovasc. Interv. 2019, 93, 1173–1183. [Google Scholar] [CrossRef]
- Chieffo, A.; Ancona, M.B.; Burzotta, F.; Pazzanese, V.; Briguori, C.; Trani, C.; Piva, T.; De Marco, F.; Di Biasi, M.; Pagnotta, P.; et al. Observational multicentre registry of patients treated with IMPella mechanical circulatory support device in ITaly: The IMP-IT registry. EuroIntervention 2020, 15, e1343–e1350. [Google Scholar] [CrossRef]
- Davidsen, C.; Packer, E.J.S.; Løland, K.H.; Rotevatn, S.; Nygreen, E.L.; Eriksen, E.; Øksnes, A.; Herstad, J.; Haaverstad, R.; Bleie, Ø.; et al. Impella use in acute myocardial infarction complicated by cardiogenic shock and cardiac arrest: Analysis of 10 years registry data. Resuscitation 2019, 140, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Loehn, T.; O’Neill, W.W.; Lange, B.; Pfluecke, C.; Schweigler, T.; Mierke, J.; Waessnig, N.; Mahlmann, A.; Youssef, A.; Speiser, U.; et al. Long term survival after early unloading with Impella CP(®) in acute myocardial infarction complicated by cardiogenic shock. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Lauten, A.; Engström, A.E.; Jung, C.; Empen, K.; Erne, P.; Cook, S.; Windecker, S.; Bergmann, M.W.; Klingenberg, R.; Lüscher, T.F.; et al. Percutaneous left-ventricular support with the Impella-2.5-assist device in acute cardiogenic shock: Results of the Impella-EUROSHOCK-registry. Circ. Heart Fail. 2013, 6, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Hanson, I.D.; Tagami, T.; Mando, R.; Kara Balla, A.; Dixon, S.R.; Timmis, S.; Almany, S.; Naidu, S.S.; Baran, D.; Lemor, A.; et al. SCAI shock classification in acute myocardial infarction: Insights from the National Cardiogenic Shock Initiative. Catheter. Cardiovasc. Interv. 2020, 96, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Hemradj, V.V.; Karami, M.; Sjauw, K.D.; Engström, A.E.; Ouweneel, D.M.; de Brabander, J.; Vis, M.M.; Wykrzykowska, J.J.; Beijk, M.A.; Koch, K.T.; et al. Pre-PCI versus immediate post-PCI Impella initiation in acute myocardial infarction complicated by cardiogenic shock. PLoS ONE 2020, 15, e0235762. [Google Scholar] [CrossRef]
- Meraj, P.M.; Doshi, R.; Schreiber, T.; Maini, B.; O’Neill, W.W. Impella 2.5 initiated prior to unprotected left main PCI in acute myocardial infarction complicated by cardiogenic shock improves early survival. J. Interv. Cardiol. 2017, 30, 256–263. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, W.W.; Schreiber, T.; Wohns, D.H.; Rihal, C.; Naidu, S.S.; Civitello, A.B.; Dixon, S.R.; Massaro, J.M.; Maini, B.; Ohman, E.M. The current use of Impella 2.5 in acute myocardial infarction complicated by cardiogenic shock: Results from the USpella Registry. J. Interv. Cardiol. 2014, 27, 223. [Google Scholar] [CrossRef] [PubMed]
- Ouweneel, D.M.; de Brabander, J.; Karami, M.; Sjauw, K.D.; Engstrom, A.E.; Vis, M.M.; Wykrzykowska, J.J.; Beijk, M.A.; Koch, K.T.; Baan, J.; et al. Real-life use of left ventricular circulatory support with Impella in cardiogenic shock after acute myocardial infarction: 12 years AMC experience. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.; Westenfeld, R.; Sieweke, J.T.; Zietzer, A.; Wiora, J.; Masiero, G.; Sanchez Martinez, C.; Tarantini, G.; Werner, N. Complete Revascularisation in Impella-Supported Infarct-Related Cardiogenic Shock Patients Is Associated With Improved Mortality. Front. Cardiovasc. Med. 2021, 8, 678748. [Google Scholar] [CrossRef]
- Scherer, C.; Lüsebrink, E.; Kupka, D.; Stocker, T.J.; Stark, K.; Stremmel, C.; Orban, M.; Petzold, T.; Germayer, A.; Mauthe, K.; et al. Long-Term Clinical Outcome of Cardiogenic Shock Patients Undergoing Impella CP Treatment vs. Standard of Care. J. Clin. Med. 2020, 9, 3803. [Google Scholar] [CrossRef]
- Schroeter, M.R.; Köhler, H.; Wachter, A.; Bleckmann, A.; Hasenfuß, G.; Schillinger, W. Use of the Impella Device for Acute Coronary Syndrome Complicated by Cardiogenic Shock—Experience From a Single Heart Center With Analysis of Long-term Mortality. J. Invasive Cardiol. 2016, 28, 467–472. [Google Scholar]
- Sieweke, J.T.; Berliner, D.; Tongers, J.; Napp, L.C.; Flierl, U.; Zauner, F.; Bauersachs, J.; Schäfer, A. Mortality in patients with cardiogenic shock treated with the Impella CP microaxial pump for isolated left ventricular failure. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Tarantini, G.; Masiero, G.; Burzotta, F.; Pazzanese, V.; Briguori, C.; Trani, C.; Piva, T.; De Marco, F.; Di Biasi, M.; Pagnotta, P.; et al. Timing of Impella implantation and outcomes in cardiogenic shock or high-risk percutaneous coronary revascularization. Catheter. Cardiovasc. Interv. 2021, 98, E222–E234. [Google Scholar] [CrossRef] [PubMed]
- Wayangankar, S.A.; Bangalore, S.; McCoy, L.A.; Jneid, H.; Latif, F.; Karrowni, W.; Charitakis, K.; Feldman, D.N.; Dakik, H.A.; Mauri, L.; et al. Temporal Trends and Outcomes of Patients Undergoing Percutaneous Coronary Interventions for Cardiogenic Shock in the Setting of Acute Myocardial Infarction: A Report From the CathPCI Registry. JACC Cardiovasc. Interv. 2016, 9, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Thiele, H.; Zeymer, U.; Neumann, F.J.; Ferenc, M.; Olbrich, H.G.; Hausleiter, J.; de Waha, A.; Richardt, G.; Hennersdorf, M.; Empen, K.; et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): Final 12 month results of a randomised, open-label trial. Lancet 2013, 382, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Aissaoui, N.; Puymirat, E.; Tabone, X.; Charbonnier, B.; Schiele, F.; Lefevre, T.; Durand, E.; Blanchard, D.; Simon, T.; Cambou, J.P.; et al. Improved outcome of cardiogenic shock at the acute stage of myocardial infarction: A report from the USIK 1995, USIC 2000, and FAST-MI French nationwide registries. Eur. Heart J. 2012, 33, 2535–2543. [Google Scholar] [CrossRef] [PubMed]
- De Ferrari, T.; Pistelli, L.; Franzino, M.; Molinero, A.E.; De Santis, G.A.; Di Carlo, A.; Vetta, G.; Parlavecchio, A.; Fimiani, L.; Picci, A.; et al. MI2AMI-CS: A meta-analysis comparing Impella and IABP outcomes in Acute Myocardial Infarction-related Cardiogenic Shock. Int. J. Cardiol. 2024, 414, 132411. [Google Scholar] [CrossRef] [PubMed]
- Iannaccone, M.; Albani, S.; Giannini, F.; Colangelo, S.; Boccuzzi, G.G.; Garbo, R.; Brilakis, E.S.; D’Ascenzo, F.; de Ferrari, G.M.; Colombo, A. Short term outcomes of Impella in cardiogenic shock: A review and meta-analysis of observational studies. Int. J. Cardiol. 2021, 324, 44–51. [Google Scholar] [CrossRef] [PubMed]

| Author | Publication Date | Journal | Country | Study Design | Clinical Endpoint |
|---|---|---|---|---|---|
| B. Basir [15] | 2017 | AJC | USA | Retrospective | 30 days mortality and overall survival to discharge |
| B. Basir [16] | 2019 | CCI | USA | Prospective Multicenter | In hospital survival and 30 days mortality |
| Chatzis [10] | 2021 | CCM | USA | Retrospective | Overall survival to discharge and at 6 months |
| Chieffo [17] | 2020 | EI | Italy | Retrospective Multicenter | All-cause mortality at hospital and at one year |
| Davidsen [18] | 2019 | ERC | Norway | Retrospective | 30 days outcome |
| Loehn [19] | 2018 | ACC-EHJ | Germany | Retrospective | Survival at discharge, 30 and 90 days |
| Lauten [20] | 2012 | CHF | Europe | Retrospective Multicenter | 30 days mortality |
| Hanson [21] | 2020 | CCI | USA | Prospective blinded | Survival to discharge and 30 days mortality |
| Hemradj [22] | 2020 | PLO | Netherlands | Retrospective | 30 days mortality |
| M. Meraj [23] | 2017 | JIC | USA | Retrospective | 30 days mortality |
| O`Neil [24] | 2014 | JIC | USA | Retrospective | Survival to discharge and 30 days mortality |
| Ouweneel [25] | 2018 | EHJ | Netherlands | Retrospective | 30 days mortality and 6 months mortality |
| Schäfer [26] | 2021 | FCM | Germany | Retrospective | 30 days mortality |
| Scherer [27] | 2020 | JCM | Germany | Retrospective | 1 year mortality |
| Schroeter [28] | 2016 | JIC | Germany | Retrospective | 1 year mortality |
| Sieweke [29] | 2018 | EHJ | Germany | Prospective | 30 days mortality |
| Tarantini [30] | 2020 | CCI | Italy | Retrospective | 1 year mortality |
| Moeller [14] | 2024 | NEJM | Denmark Germany | Prospective Multicenter | Survival on 6 months |
| Author | No. of Patients | 30-Day Mortality (%) | Ischemic Events (%) | Lactate (mmol/L) | Age (Years) | Bleeding (%) | LVEF (%) | Prior CPR (%) | pH |
|---|---|---|---|---|---|---|---|---|---|
| B. Basir [15] | 287 | 56% | 5% | 5.8 | 66 | 17.0% | 22% | 52% | n.a. |
| B. Basir [16] | 171 | 28% | 13.6% | 5.4 | 63 | 17.3% | n.a. | 49% | n.a. |
| Chatzis [10] | 81 | 41% | 13% | 8.6 | 68 | 17% | 33% | n.a. | n.a. |
| Chieffo [17] | 218 | 41% | 13% | 6.1 | 64 | 11% | 35% | n.a. | 7.3 |
| Davidson [18] | 91 | 55% | 3.6% | n.a. | 61 | 12.5% | 26% | 74% | n.a. |
| Loehn [19] | 73 | 64% | 3.2% | 6.3 | 69 | 7% | 29% | 43% | 7.3 |
| Lauten [20] | 120 | 64% | 4.2% | 5.8 | 64 | 24% | 27% | 41% | n.a. |
| Hanson [21] | 269 | 29% | n.a. | n.a. | 64 | n.a. | n.a. | n.a. | n.a. |
| Hemradj [22] | 88 | 58% | 6.25% | 5.1 | 60 | 9.9% | n.a | 58% | 7.2 |
| M. Meraj [23] | 36 | 36% | 3% | 4.7 | 70 | 3% | 25% | 44% | n.a. |
| O`Neil [24] | 145 | 52% | 3.9% | 4.1 | 64 | 18% | 26% | 23% | n.a. |
| Ouweneel [25] | 112 | 56% | 9.1% | 6.2 | 60 | 13% | n.a. | 60% | 7.2 |
| Schäfer [26] | 202 | 47% | 9% | 5.2 | 66 | 9% | 26% | n.a. | n.a. |
| Scherer [27] | 54 | 54% | 5% | 3.4 | 67 | 23% | n.a. | 51% | n.a. |
| Schroeter [28] | 68 | 55% | 12.6% | n.a. | 63 | 10.9% | 27% | n.a. | n.a. |
| Sieweke [29] | 61 | 48% | 3.2% | n.a. | 62 | 3.3% | n.a. | n.a. | n.a. |
| Tarantini [30] | 365 | 36% | 12% | 4.7 | 66 | 15% | 25% | n.a. | 7.4 |
| Moeller [14] | 179 | 39% | 10% | 4.6 | 67 | 22% | 25% | 22% | n.a. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sassani, K.; Waechter, C.; Syntila, S.; Kreutz, J.; Markus, B.; Patsalis, N.; Di Vece, D.; Schieffer, B.; Templin, C.; Chatzis, G. The Role of Impella in Cardiogenic Shock Complicated by an Acute Myocardial Infarction: A Meta-Analysis. J. Clin. Med. 2025, 14, 611. https://doi.org/10.3390/jcm14020611
Sassani K, Waechter C, Syntila S, Kreutz J, Markus B, Patsalis N, Di Vece D, Schieffer B, Templin C, Chatzis G. The Role of Impella in Cardiogenic Shock Complicated by an Acute Myocardial Infarction: A Meta-Analysis. Journal of Clinical Medicine. 2025; 14(2):611. https://doi.org/10.3390/jcm14020611
Chicago/Turabian StyleSassani, Kiarash, Christian Waechter, Styliani Syntila, Julian Kreutz, Birgit Markus, Nikolaos Patsalis, Davide Di Vece, Bernhard Schieffer, Christian Templin, and Georgios Chatzis. 2025. "The Role of Impella in Cardiogenic Shock Complicated by an Acute Myocardial Infarction: A Meta-Analysis" Journal of Clinical Medicine 14, no. 2: 611. https://doi.org/10.3390/jcm14020611
APA StyleSassani, K., Waechter, C., Syntila, S., Kreutz, J., Markus, B., Patsalis, N., Di Vece, D., Schieffer, B., Templin, C., & Chatzis, G. (2025). The Role of Impella in Cardiogenic Shock Complicated by an Acute Myocardial Infarction: A Meta-Analysis. Journal of Clinical Medicine, 14(2), 611. https://doi.org/10.3390/jcm14020611







