Predictive Factors for 24-h Survival After Perioperative Cardiopulmonary Resuscitation: Single-Center Retrospective Cohort Study
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Setting
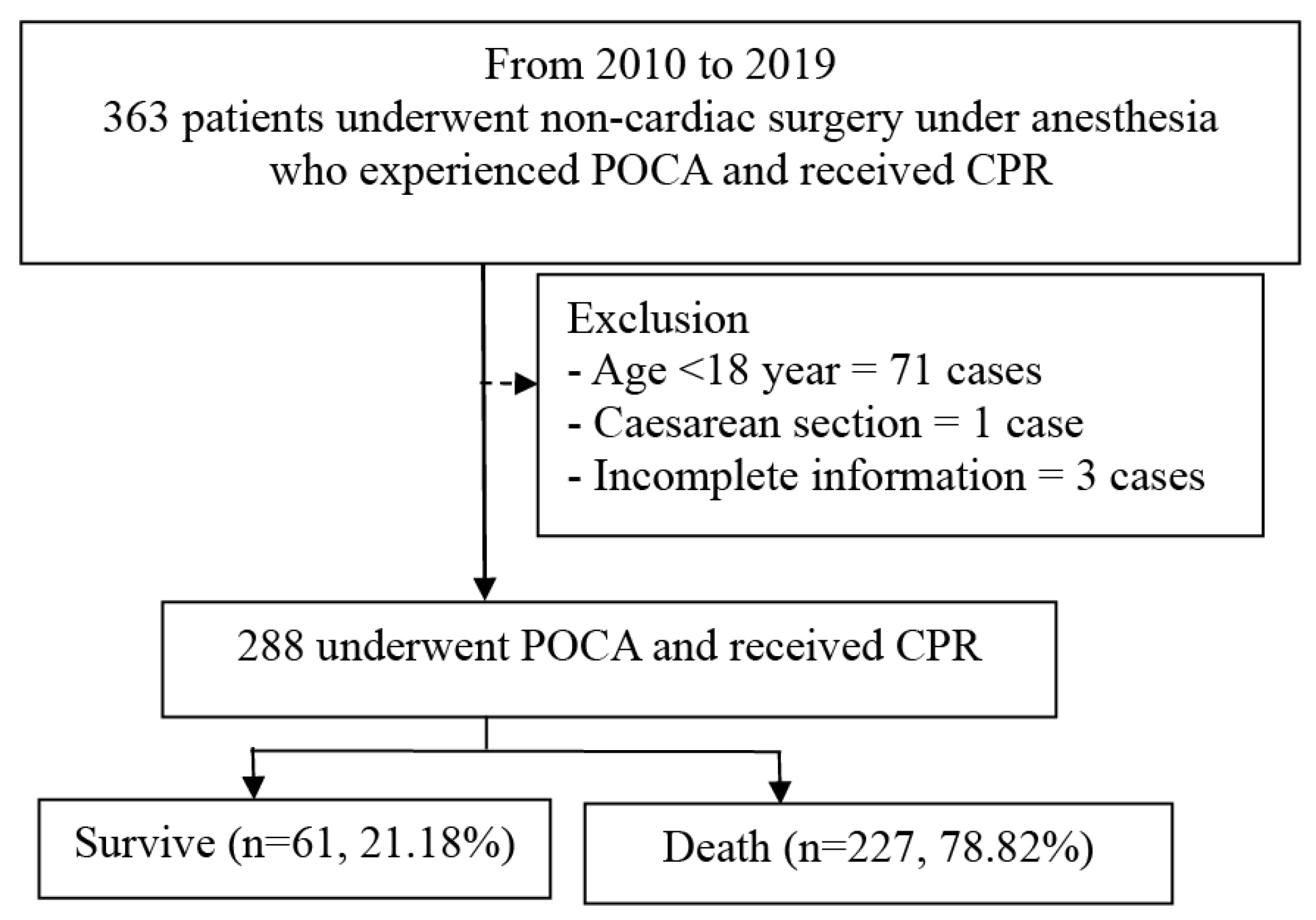
2.3. Participants
2.4. Variables
2.5. Data Sources/Measurement
2.6. Bias
2.7. Study Size
2.8. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| STROBE | Strengthening the Reporting of Observational studies in Epidemiology |
References
- Allen, M.B.; Orkaby, A.R.; Justice, S.; Hall, D.E.; Hu, F.Y.; Cooper, Z.; Bernacki, R.E.; Bader, A.M. Frailty and Outcomes Following Cardiopulmonary Resuscitation for Perioperative Cardiac Arrest. JAMA Netw. Open 2023, 6, e2321465. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Chu, Q.; Ji, M.; Guo, J.; Ye, H.; Zheng, S.; Yang, J. A retrospective study of mortality for perioperative cardiac arrests toward a personalized treatment. Sci. Rep. 2022, 12, 13709. [Google Scholar] [CrossRef] [PubMed]
- Charuluxananan, S.; Sriraj, W.; Punjasawadwong, Y.; Pitimana-aree, S.; Lekprasert, V.; Werawatganon, T.; Wasinwong, W.; Ratanachai, P.; Sriramatr, D.; Atichat, S.; et al. Perioperative and Anesthetic Adverse events in Thailand (PAAd Thai) incident reporting study: Anesthetic profiles and outcomes. Asian Biomed. 2017, 11, 21–32. [Google Scholar]
- Nair, A.; Naik, V.; Rayani, B. Letters to the Editor. Indian J. Crit. Care Med. 2017, 21, 713. [Google Scholar] [CrossRef] [PubMed]
- Sprung, J.; Flick, R.; Gleich, S.; Weingarten, T. Perioperative Cardiac Arrests. Signa Vitae 2008, 3, 8–12. [Google Scholar]
- Constant, A.L.; Montlahuc, C.; Grimaldi, D.; Pichon, N.; Mongardon, N.; Bordenave, L.; Soummer, A.; Sauneuf, B.; Ricome, S.; Misset, B.; et al. Predictors of functional outcome after intraoperative cardiac arrest. Anesthesiology 2014, 121, 482–491. [Google Scholar] [CrossRef]
- Vane, M.F.; Carmona, M.J.C.; Pereira, S.M.; Kern, K.B.; Timerman, S.; Perez, G.; Vane, L.A.; Otsuki, D.A.; Auler, J.O.C., Jr. Predictors and their prognostic value for no ROSC and mortality after a non-cardiac surgery intraoperative cardiac arrest: A retrospective cohort study. Sci. Rep. 2019, 9, 14975. [Google Scholar] [CrossRef]
- Goswami, S.; Brady, J.E.; Jordan, D.A.; Li, G. Intraoperative Cardiac Arrests in Adults Undergoing Noncardiac Surgery: Incidence, Risk Factors, and Survival Outcome. Anesthesiology 2012, 117, 1018–1026. [Google Scholar] [CrossRef]
- Gong, C.L.; Hu, J.P.; Qiu, Z.L.; Zhu, Q.Q.; Hei, Z.Q.; Zhou, S.L.; Li, X. A study of anaesthesia-related cardiac arrest from a Chinese tertiary hospital. BMC Anesthesiol. 2018, 18, 127. [Google Scholar] [CrossRef]
- Nunes, J.C.; Braz, J.R.C.; Oliveira, T.S.; de Carvalho, L.R.; Castiglia, Y.M.M.; Braz, L.G. Intraoperative and Anesthesia-Related Cardiac Arrest and Its Mortality in Older Patients: A 15-Year Survey in a Tertiary Teaching Hospital. PLoS ONE 2014, 9, e104041. [Google Scholar] [CrossRef][Green Version]
- Carlucci, M.T.O.; Braz, J.R.C.; do Nascimento, P., Jr.; de Carvalho, L.R.; Castiglia, Y.M.M.; Braz, L.G. Intraoperative Cardiac Arrest and Mortality in Trauma Patients. A 14-Yr Survey from a Brazilian Tertiary Teaching Hospital. PLoS ONE 2014, 9, e90125. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hinkelbein, J.; Andres, J.; Böttiger, B.W.; Brazzi, L.; De Robertis, E.; Einav, S.; Gwinnutt, C.; Kuvaki, B.; Krawczyk, P.; McEvoy, M.D.; et al. Cardiac arrest in the perioperative period: A consensus guideline for identification, treatment, and prevention from the European Society of Anaesthesiology and Intensive Care and the European Society for Trauma and Emergency Surgery. Eur. J. Trauma Emerg. Surg. Off. Publ. Eur. Trauma Soc. 2023, 49, 2031–2046. [Google Scholar] [CrossRef] [PubMed]
- Alao, D.O.; Mohammed, N.A.; Hukan, Y.O.; Al Neyadi, M.; Jummani, Z.; Dababneh, E.H.; Cevik, A.A. The epidemiology and outcomes of adult in-hospital cardiac arrest in a high-income developing country. Resusc. Plus 2022, 10, 100220. [Google Scholar] [CrossRef] [PubMed]
- Hohn, A.; Machatschek, J.N.; Franklin, J.; Padosch, S.A. Incidence and risk factors of anaesthesia-related perioperative cardiac arrest: A 6-year observational study from a tertiary care university hospital. Eur. J. Anaesthesiol. 2018, 35, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, H.A.; Saied, N.N.; Kokoefer, A.S.; Saffour, L.; Zoller, J.K.; Helwani, M.A. Incidence and prediction of intraoperative and postoperative cardiac arrest requiring cardiopulmonary resuscitation and 30-day mortality in non-cardiac surgical patients. PLoS ONE 2020, 15, e0225939. [Google Scholar] [CrossRef]
- Kan, H.; Ding, Y.; Wu, S.; Zhang, Z. Retrospective study of perioperative cardiac arrest from a Chinese tertiary hospital. Medicine 2021, 100, e26890. [Google Scholar] [CrossRef]
- Morimatsu, H. Incidence of accidental events during anesthesia from 2012 to 2016: Survey on anesthesia-related events by the Japanese Society of Anesthesiologists. J. Anesth. 2021, 35, 206–212. [Google Scholar] [CrossRef]
- Braz, L.G.; Braz, J.R.C.; Modolo, M.P.; Corrente, J.E.; Sanchez, R.; Pacchioni, M.; Cury, J.B.; Soares, I.B.; Braz, M.G. Perioperative and anesthesia-related cardiac arrest and mortality rates in Brazil: A systematic review and proportion meta-analysis. PLoS ONE 2020, 15, e0241751. [Google Scholar] [CrossRef]
- Siriphuwanun, V.; Punjasawadwong, Y.; Lapisatepun, W.; Charuluxananan, S.; Uerpairojkit, K.; Patumanond, J. The initial success rate of cardiopulmonary resuscitation and its associated factors in patients with cardiac arrest within 24 hours after anesthesia for an emergency surgery. Risk Manag. Healthc. Policy 2014, 7, 65–76. [Google Scholar]
- Khan, N.U.; Razzak, J.A.; Ahmed, H.; Furqan, M.; Saleem, A.F.; Alam, H.; ul Huda, A.; Khan, U.R.; Rehmani, R. Cardiopulmonary resuscitation: Outcome and its predictors among hospitalized adult patients in Pakistan. Int. J. Emerg. Med. 2008, 1, 27–34. [Google Scholar] [CrossRef]
- Berry, W.R. Cardiac resuscitation in the operating room: Reflections on how we can do better. Can. J. Anesth. 2012, 59, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Aloweidi, A.; Alghanem, S.; Bsisu, I.; Ababneh, O.; Alrabayah, M.; Al-Zaben, K.; Qudaisat, I. Perioperative Cardiac Arrest: A 3-Year Prospective Study from a Tertiary Care University Hospital. Drug Healthc. Patient Saf. 2022, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Braz, L.; Morais, A.; Sanchez, R.; Porto, D.; Pacchioni, M.; Serafim, W.; Módolo, N.; Nascimento, P.D., Jr.; Braz, M.; Braz, J. Epidemiology of perioperative cardiac arrest and mortality in Brazil: Systematic review. Braz. J. Anesthesiol. (Engl. Ed.) 2020, 70, 82–89. [Google Scholar] [CrossRef]
- Murray, C.; Sasaki, S.S.; Berg, D. Local Anesthesia and Malignant Hyperthermia: Review of the Literature and Recommendations for the Dermatologic Surgeon. Dermatol. Surg. 1999, 25, 626–630. [Google Scholar] [CrossRef]
- Siriphuwanun, V.; Punjasawadwong, Y.; Lapisatepun, W.; Charuluxananan, S.; Uerpairojkit, K. Prognostic factors for death and survival with or without complications in cardiac arrest patients receiving CPR within 24 hours of anesthesia for emergency surgery. Risk Manag. Healthc. Policy 2014, 7, 199–210. [Google Scholar]
- Braz, L.G.; Módolo, N.S.P.; do Nascimento, P., Jr.; Bruschi, B.A.M.; Castiglia, Y.M.M.; Ganem, E.M.; de Carvalho, L.R.; Braz, J.R.C. Perioperative cardiac arrest: A study of 53 718 anaesthetics over 9 yr from a Brazilian teaching hospital. BJA Br. J. Anaesth. 2006, 96, 569–575. [Google Scholar] [CrossRef]
- Choi, Y.J.; Han, S.U.; Woo, S.; Ro, Y.J.; Yang, H.S. Perioperative cardiac arrest in 457,529 anesthetized patients at a single teaching hospital in Korea: A retrospective study. Anesth. Pain Med. 2014, 9, 144–151. [Google Scholar]
- Sobreira-Fernandes, D.; Teixeira, L.; Lemos, T.S.; Costa, L.; Pereira, M.; Costa, A.C.; Couto, P.S. Perioperative cardiac arrests—A subanalysis of the anesthesia-related cardiac arrests and associated mortality. J. Clin. Anesth. 2018, 50, 78–90. [Google Scholar] [CrossRef]
- Fernando, S.M.; Tran, A.; Cheng, W.; Rochwerg, B.; Taljaard, M.; Vaillancourt, C.; Rowan, K.M.; Harrison, D.A.; Nolan, J.P.; Kyeremanteng, K.; et al. Pre-arrest and intra-arrest prognostic factors associated with survival after in-hospital cardiac arrest: Systematic review and meta-analysis. BMJ 2019, 367, l6373. [Google Scholar] [CrossRef]
- Pignaton, W.; Braz, J.R.C.; Kusano, P.S.; Módolo, M.P.; de Carvalho, L.R.; Braz, M.G.; Braz, L.G. Perioperative and Anesthesia-Related Mortality: An 8-Year Observational Survey From a Tertiary Teaching Hospital. Medicine 2016, 95, e2208. [Google Scholar] [CrossRef]
- Kuan, K.K.; Rahalkar, K. Beyond 5Hs and 5Ts: A rare cause of cardiac arrest. Singap. Med. J. 2023, 64, 146–148. [Google Scholar] [CrossRef]
- Kaur, H.; Katyal, N.; Yelam, A.; Kumar, K.; Srivastava, H.; Govindarajan, R. Malignant Hyperthermia. Mo. Med. 2019, 116, 154–159. [Google Scholar] [PubMed]
- Gupta, P.K.; Bilmen, J.G.; Hopkins, P.M. Anaesthetic management of a known or suspected malignant hyperthermia susceptible patient. BJA Educ. 2021, 21, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Newland, M.C.; Ellis, S.J.; Lydiatt, C.A.; Peters, K.R.; Tinker, J.H.; Romberger, D.J.; Ullrich, F.A.; Anderson, J.R. Anesthetic-related cardiac arrest and its mortality: A report covering 72,959 anesthetics over 10 years from a US teaching hospital. Anesthesiology 2002, 97, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-H.; Huang, C.-H.; Chang, W.-T.; Tsai, M.-S.; Yu, P.-H.; Wang, A.-Y.; Chen, N.-C.; Chen, W.-J. Association between hemoglobin levels and clinical outcomes in adult patients after in-hospital cardiac arrest: A retrospective cohort study. Intern. Emerg. Med. 2016, 11, 727–736. [Google Scholar] [CrossRef]
- Brown, L.M.; Call, M.S.; Margaret Knudson, M.; Cohen, M.J.; Holcomb, J.B.; Wade, C.E.; Brasel, K.J.; Vercruysse, G.; MacLeod, J.; Dutton, R.P.; et al. A normal platelet count may not be enough: The impact of admission platelet count on mortality and transfusion in severely injured trauma patients. J. Trauma 2011, 71 (Suppl. S3), S337–S342. [Google Scholar] [CrossRef]
- Chae, Y.J.; Lee, J.; Park, J.H.; Han, D.G.; Ha, E.; Yi, I.K. Late Mortality Prediction of Neutrophil-to-Lymphocyte and Platelet Ratio in Patients with Trauma Who Underwent Emergency Surgery: A Retrospective Study. J. Surg. Res. 2021, 267, 755–761. [Google Scholar] [CrossRef]
- Hong, S.I.; Kim, Y.J.; Cho, Y.J.; Huh, J.W.; Hong, S.B.; Kim, W.Y. Predictive value of pre-arrest albumin level with GO-FAR score in patients with in-hospital cardiac arrest. Sci. Rep. 2021, 11, 10631. [Google Scholar] [CrossRef]
- Barnett, S.; Moonesinghe, S.R. Clinical risk scores to guide perioperative management. Postgrad. Med. J. 2011, 87, 535. [Google Scholar] [CrossRef]
- Han, F.; Wang, Y.; Wang, Y.; Dong, J.; Nie, C.; Chen, M.; Hou, L. Intraoperative cardiac arrest: A 10-year study of patients undergoing tumorous surgery in a tertiary referral cancer center in China. Medicine 2017, 96, e6794. [Google Scholar] [CrossRef]
- Moitra, V.; Einav, S.; Thies, K.-C.; Nunnally, M.; Gabrielli, A.; Maccioli, G.; Weinberg, G.; Banerjee, A.; Ruetzler, K.; Dobson, G.; et al. Cardiac Arrest in the Operating Room: Resuscitation and Management for the Anesthesiologist Part 1. Anesth. Analg. 2018, 127, e49–e50. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.K.; Mhyre, J.; Kheterpal, S.; Christensen, R.E.; Tallman, K.; Morris, M.; Chan, P.S. Predictors of survival from perioperative cardiopulmonary arrests: A retrospective analysis of 2,524 events from the Get With The Guidelines-Resuscitation registry. Anesthesiology 2013, 119, 1322–1339. [Google Scholar] [CrossRef] [PubMed]
- Sprung, J.; Warner, M.E.; Contreras, M.G.; Schroeder, D.R.; Beighley, C.M.; Wilson, G.A.; Warner, D.O. Predictors of survival following cardiac arrest in patients undergoing noncardiac surgery: A study of 518,294 patients at a tertiary referral center. Anesthesiology 2003, 99, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Kalkman, S.; Hooft, L.; Meijerman, J.M.; Knape, J.T.A.; van Delden, J.J.M. Survival after Perioperative Cardiopulmonary Resuscitation: Providing an Evidence Base for Ethical Management of Do-not-resuscitate Orders. Anesthesiology 2016, 124, 723–729. [Google Scholar] [CrossRef]
- Rukewe, A.; Fatiregun, A.; Osunlaja, T.O. Cardiac arrest during anesthesia at a university Hospital in Nigeria. Niger. J. Clin. Pract. 2014, 17, 28–31. [Google Scholar] [CrossRef]
- Krittayaphong, R.; Saengsung, P.; Chawaruechai, T.; Yindeengam, A.; Udompunturak, S. Factors predicting outcome of cardiopulmonary resuscitation in a developing country: The Siriraj cardiopulmonary resuscitation registry. J. Med. Assoc. Thail. = Chotmaihet Thangphaet 2009, 92, 618–623. [Google Scholar]
- Markovic, D.; Jevtovic-Stoimenov, T.; Stojanovic, M.; Vukovic, A.; Dinic, V.; Markovic-Zivkovic, B.; Jankovic, R.J. Addition of clinical risk scores improves prediction performance of American Society of Anesthesiologists (ASA) physical status classification for postoperative mortality in older patients: A pilot study. Eur. Geriatr. Med. 2018, 9, 51–59. [Google Scholar] [CrossRef]
- Salottolo, K.M.; Mains, C.W.; Offner, P.J.; Bourg, P.W.; Bar-Or, D. A retrospective analysis of geriatric trauma patients: Venous lactate is a better predictor of mortality than traditional vital signs. Scand. J. Trauma Resusc. Emerg. Med. 2013, 21, 7. [Google Scholar] [CrossRef]
- An, J.-X.; Zhang, L.-M.; Sullivan, E.A.; Guo, Q.-L.; Williams, J.P. Intraoperative cardiac arrest during anesthesia: A retrospective study of 218 274 anesthetics undergoing non-cardiac surgery in a US teaching hospital. Chin. Med. J. 2011, 124, 227–232. [Google Scholar]
- Hanif, A.A.; Rachman, I.A.; Yuwono, H.S. Factors Influencing the Success Rate of Cardiopulmonary Resuscitation. Althea Med. J. 2015, 2, 615–619. [Google Scholar] [CrossRef][Green Version]
- Ahmed, A.; Ali, M.; Khan, F.A.; Khan, M.U. An Audit of Perioperative Cardiac Arrests in a Southeast Asian University Teaching Hospital Over 15 Years. Anaesth. Intensive Care 2008, 36, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Leidel, B.A.; Kanz, K.G.; Kirchhoff, C.; Bürklein, D.; Wismüller, A.; Mutschler, W. Cardiac arrest following blunt chest injury. Emergency thoracotomy without ifs or buts? Der Unfallchirurg 2007, 110, 884–890. [Google Scholar] [CrossRef]
- Fitzgerald, M.; Spencer, J.; Johnson, F.; Marasco, S.; Atkin, C.; Kossmann, T. Definitive management of acute cardiac tamponade secondary to blunt trauma. Emerg. Med. Australas. EMA 2005, 17, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Yongyukantorn, K.; Oofuvong, M. Risk factors of intraoperative and 24-hour postoperative cardiac arrest in geriatric patients in non-cardiac surgery. J. Gerontol. Geriatr. 2020, 68, 159–167. [Google Scholar] [CrossRef]
- Kim, I.J.; Yang, P.S.; Kim, T.H.; Uhm, J.S.; Pak, H.N.; Lee, M.H.; Sung, J.H.; Joung, B. Relationship Between Anemia and the Risk of Sudden Cardiac Arrest—A Nationwide Cohort Study in South Korea. Circ. J. Off. J. Jpn. Circ. Soc. 2018, 82, 2962–2969. [Google Scholar] [CrossRef]
- Shor, L.; Helviz, Y.; Einav, S. Anemia before in-hospital cardiac arrest and survival from cardio-pulmonary resuscitation—A retrospective cohort study. J. Anesth. Analg. Crit. Care 2022, 2, 51. [Google Scholar] [CrossRef]
- Eagle, K.A.; Berger, P.B.; Calkins, H.; Chaitman, B.R.; Ewy, G.A.; Fleischmann, K.E.; Fleisher, L.A.; Froehlich, J.B.; Gusberg, R.J.; Leppo, J.A.; et al. ACC/AHA Guideline Update for Perioperative Cardiovascular Evaluation for Noncardiac Surgery—Executive Summary A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). J. Am. Coll. Cardiol. 2002, 105, 1257–1267. [Google Scholar]
- Weil, I.A.; Kumar, P.; Seicean, S.; Neuhauser, D.; Seicean, A. Platelet count abnormalities and peri-operative outcomes in adults undergoing elective, non-cardiac surgery. PLoS ONE 2019, 14, e0212191. [Google Scholar] [CrossRef]
- Wheeler, I.; Price, C.; Sitch, A.; Banda, P.; Kellett, J.; Nyirenda, M.; Rylance, J. Early warning scores generated in developed healthcare settings are not sufficient at predicting early mortality in Blantyre, Malawi: A prospective cohort study. PLoS ONE 2013, 8, e59830. [Google Scholar] [CrossRef]
- Blankush, J.M.; Freeman, R.; McIlvaine, J.; Tran, T.; Nassani, S.; Leitman, I.M. Implementation of a novel postoperative monitoring system using automated Modified Early Warning Scores (MEWS) incorporating end-tidal capnography. J. Clin. Monit. Comput. 2017, 31, 1081–1092. [Google Scholar] [CrossRef]
- Paiva, E.F.; Paxton, J.H.; O’Neil, B.J. The use of end-tidal carbon dioxide (ETCO(2)) measurement to guide management of cardiac arrest: A systematic review. Resuscitation 2018, 123, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chomchoey, C.; Thawitsri, T. The incidence of postoperative cardiac arrest and pre-resuscitation factors associated with post- cardiopulmonary resuscitation mortality: A single-center study in Thailand. Clin. Crit. Care 2021, 29, e0007. [Google Scholar] [CrossRef]
- Okubo, M.; Komukai, S.; Andersen, L.W.; Berg, R.A.; Kurz, M.C.; Morrison, L.J.; Callaway, C.W. Duration of cardiopulmonary resuscitation and outcomes for adults with in-hospital cardiac arrest: Retrospective cohort study. BMJ 2024, 384, e076019. [Google Scholar] [CrossRef] [PubMed]
- Maeda, M.; Hirata, N.; Chaki, T.; Yamakage, M. Risk factors of cardiac arrest and failure to achieve return of spontaneous circulation during anesthesia: A 20-year retrospective observational study from a tertiary care university hospital. J. Anesth. 2022, 36, 221–229. [Google Scholar] [CrossRef]
| Variables. | Survival (n = 61) | Death (n = 227) | RR | 95%CI | p-Value |
|---|---|---|---|---|---|
| Age (years) | |||||
| ≥65, n (%) | 28 (45.90) | 69 (30.40) | 1.67 | 1.07–2.59 | 0.024 * |
| <65, n (%) | 33 (54.10) | 158 (69.60) | Ref. | ||
| Mean ± SD | 59.16 ± 19.77 | 53.09 ± 20.03 | |||
| Gender | |||||
| Female, n (%) | 27 (44.26) | 67 (29.52) | 1.63 | 1.05–2.55 | 0.028 * |
| Male, n (%) | 34 (55.74) | 160 (70.48) | Ref. | ||
| ASA physical status, n (%) | |||||
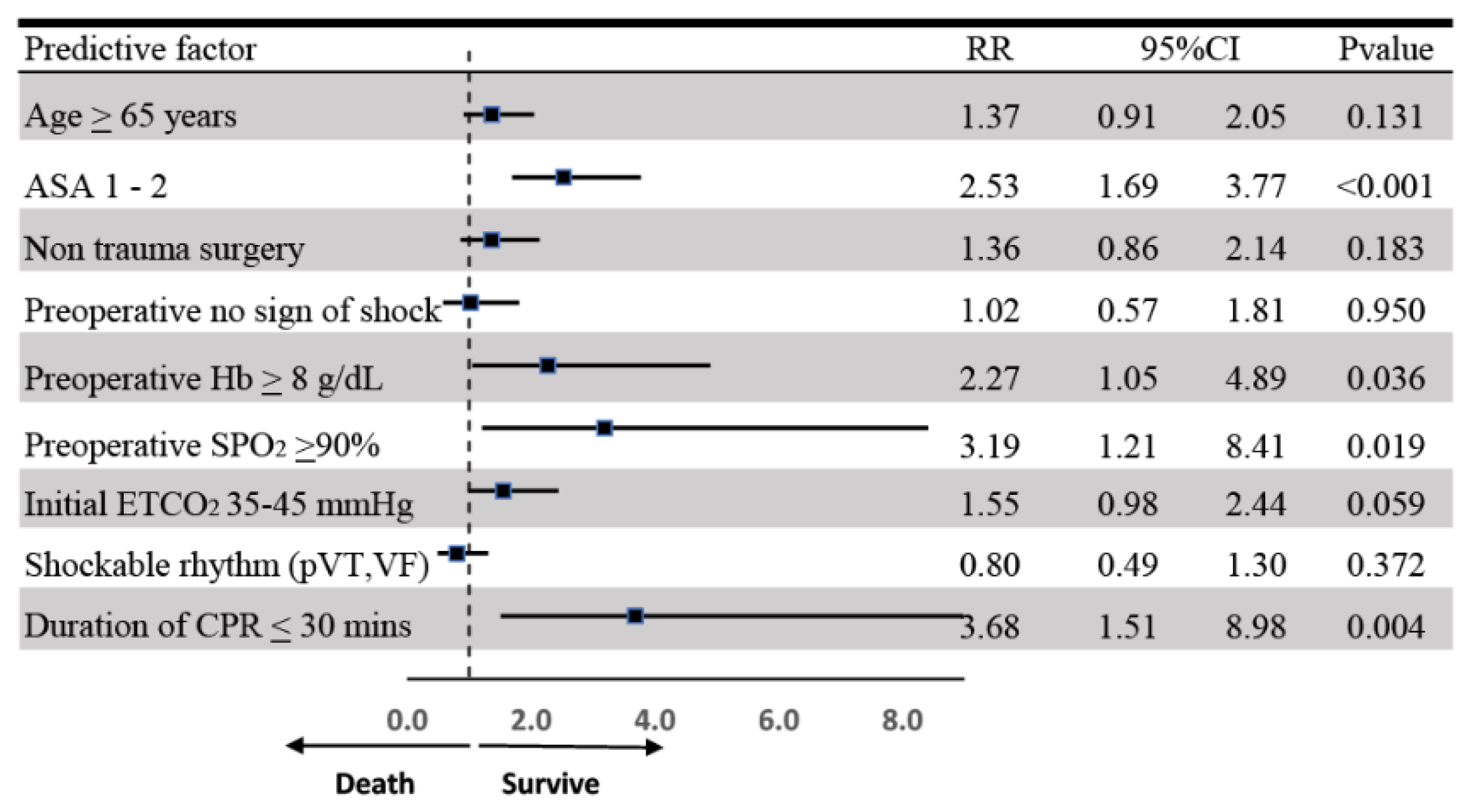
| 1–2 | 20 (32.79) | 6 (2.64) | 4.92 | 3.46–6.99 | <0.001 * |
| 3–5 | 41 (67.21) | 221 (97.36) | Ref. | ||
| Non-smoker, n (%) | |||||
| Yes | 52 (85.25) | 189 (83.26) | 1.13 | 0.59–2.13 | 0.713 |
| No | 9 (14.75) | 38 (16.74) | Ref. | ||
| Preoperative comorbidities, n (%) | |||||
| Diabetes mellitus | 9 (14.75) | 28 (12.33) | 1.17 | 0.63–2.18 | 0.611 |
| Hypertension | 29 (47.54) | 57 (25.11) | 2.13 | 1.38–3.29 | <0.001 * |
| Congestive heart failure | 3 (4.92) | 14 (6.17) | 0.82 | 0.28–2.36 | 0.720 |
| Ischemic heart disease | 6 (9.84) | 15 (6.61) | 1.39 | 0.67–2.84 | 0.371 |
| Respiratory disease | 9 (14.75) | 28 (12.33) | 1.17 | 0.63–2.18 | 0.611 |
| Renal disease | 15 (24.59) | 29 (12.78) | 1.81 | 1.11–2.94 | 0.017 * |
| TIA/stroke | 5 (8.20) | 13 (5.73) | 1.34 | 0.61–2.93 | 0.464 |
| Sepsis | 4 (6.56) | 13 (5.73) | 1.12 | 0.46–2.72 | 0.805 |
| Solid cancer | 13 (21.31) | 14 (6.17) | 2.62 | 1.64–4.18 | <0.001 * |
| Current medication, n (%) | |||||
| Anticoagulant | 15 (24.59) | 28 (12.33) | 1.86 | 1.14–3.01 | 0.012 * |
| Antihypertensive drug | 20 (32.79) | 55 (24.23) | 1.38 | 0.87–2.21 | 0.170 |
| Steroid | 7 (11.48) | 9 (3.96) | 2.20 | 1.20–4.04 | 0.011 * |
| Insulin | 2 (3.28) | 3 (1.32) | 1.92 | 0.64–5.76 | 0.245 |
| Preoperative sign of shock, n (%) | |||||
| No | 49 (80.33) | 132(58.15) | 2.41 | 1.34–4.33 | 0.003 * |
| Yes | 12 (19.67) | 95 (41.85) | Ref. | ||
| Preoperative mechanical ventilation, n (%) | |||||
| No | 22 (36.07) | 48 (21.15) | 1.76 | 1.12–2.75 | 0.014 * |
| Yes | 39 (63.93) | 179 (78.85) | Ref. | ||
| Variables | Survival (n = 61) | Death (n = 227) | RR | 95%CI | p-Value |
|---|---|---|---|---|---|
| Elective surgery, n (%) | |||||
| Yes | 16 (26.23) | 14 (6.17) | 3.06 | 1.99–4.69 | <0.001 * |
| No | 45 (73.77) | 213 (93.83) | Ref. | ||
| Site of operation, n (%) | |||||
| Upper intra-abdominal | 16 (26.23) | 83 (36.56) | 0.67 | 0.40–1.14 | 0.132 |
| Major vascular | 5 (8.20) | 42 (18.50) | 0.45 | 0.19–1.08 | 0.053 |
| Intracranial | 9 (14.75) | 37 (16.30) | 0.91 | 0.48–1.72 | 0.769 |
| Intrathoracic | 11 (18.03) | 38 (16.74) | 1.07 | 0.60–1.91 | 0.811 |
| Orthopedic | 7 (11.48) | 9 (3.96) | 2.20 | 1.20–4.03 | 0.023 * |
| Others | 13 (21.31) | 20 (8.81) | 2.09 | 1.27–3.43 | 0.006 * |
| Non-trauma surgery, n (%) | |||||
| Yes | 40 (63.57) | 99 (43.61) | 2.04 | 1.26–3.28 | 0.003 * |
| No | 21 (34.43) | 128 (56.39) | Ref. | ||
| Supine position, n (%) | |||||
| Yes | 49 (83.05) | 212 (93.39) | 2.13 | 1.24–3.67 | 0.006 * |
| No | 10 (16.95) | 15 (6.61) | Ref. | ||
| Intraoperative blood loss, n (%) | |||||
| ≤3000 mL | 50 (81.97) | 166 (73.13) | 1.52 | 0.84–2.75 | 0.172 |
| >3000 mL | 11 (18.03) | 61 (26.87) | Ref. | ||
| Median [Q1, Q3] | 500 [200, 2000] | 1500 [200, 3900] | |||
| Variables | Survival (n = 61) | Death (n = 227) | RR | 95%CI | p-Value | Missing Data, n (%) |
|---|---|---|---|---|---|---|
| Blood glucose level mg/dL, n (%) | 35 (12.51) | |||||
| ≤70 | 1 (2.08) | 14 (6.90) | 1.97 | 0.19- 20.26 | 0.570 | |
| 71–239 | 45 (93.75) | 132 (65.02) | 7.50 | 1.88–29.97 | 0.004 * | |
| ≥240 | 2 (4.17) | 57 (28.08) | Ref. | |||
| Mean ± SD | 147.23 ± 66.59 | 192.41 ± 98.19 | ||||
| Hemoglobin ≥ 8 g/dL, n (%) | 3 (1.04) | |||||
| Yes | 53 (86.89) | 130 (58.04) | 3.69 | 1.83–7.47 | <0.001 * | |
| No | 8 (13.11) | 94 (41.96) | Ref. | |||
| Mean + SD | 10.59 ± 2.59 | 8.83 ± 3.58 | ||||
| Platelet count ≥ 100 × 103 cells/µL, n (%) | 3 (1.04) | |||||
| Yes | 54 (88.52) | 151 (67.41) | 3.01 | 1.43–6.34 | 0.004 * | |
| No | 7 (11.48) | 73 (32.59) | Ref. | |||
| Median [Q1, Q3] | 201 [153, 291] | 137 [86.5, 217] | ||||
| Neutrophil–to-lymphocyte ratio (NLR) ≤ 9, n (%) | 3 (1.04) | |||||
| Yes | 42 (70) | 166 (74.11) | 0.85 | 0.52–1.39 | 0.520 | |
| No | 18 (30) | 58 (25.89) | Ref. | |||
| Median [Q1, Q3] | 5.63 [2.62, 12.38] | 4.71 [2.37, 10.13] | ||||
| Serum albumin > 3.5 g/dL, n (%) | 22 (7.66) | |||||
| Yes | 21 (37.50) | 49 (23.33) | 1.68 | 1.05–2.68 | 0.030 * | |
| No | 35 (62.50) | 161 (76.67) | Ref. | |||
| Mean ± SD | 3.06 ± 0.92 | 2.46 ± 1.13 | ||||
| Serum creatinine < 2 mg/dL, n (%) | 2 (0.69) | |||||
| Yes | 47 (77.05) | 173 (76.89) | 1.00 | 0.52–1.98 | 0.979 | |
| No | 14 (22.95) | 52 (23.11) | Ref. | |||
| Median [Q1, Q3] | 1.2 [0.8, 1.9] | 1.3 [0.9, 1.9] | ||||
| Sodium (mmol/L), n (%) | 2 (0.69) | |||||
| <135 | 8 (13.11) | 26 (11.56) | 3.59 | 1.21–10.42 | 0.026 * | |
| 135–145 | 49 (80.33) | 142 (63.11) | 3.91 | 1.16–11.07 | 0.006 * | |
| >145 | 4 (6.56) | 57 (25.33) | Ref. | |||
| Mean ± SD | 139.11 ± 5.38 | 141.84 ± 7.50 | ||||
| Potassium (mmol/L), n (%) | 2 (0.69) | |||||
| ≤3 | 9 (14.75) | 41 (18.22) | 2.07 | 0.68–6.28 | 0.199 | |
| 3.1–4.9 | 48 (78.69) | 142 (63.11) | 2.91 | 1.10–7.66 | 0.031 * | |
| ≥5 | 4 (6.56) | 42 (18.67) | Ref. | |||
| Mean ± SD | 3.82 ± 0 74 | 4.09 ± 1.19 | ||||
| Variables | Survival (n = 61) | Death (n = 227) | RR | 95%CI | p-Value | Missing Data, n (%) |
|---|---|---|---|---|---|---|
| Heart rate (bpm), n (%) | 8 (2.78) | |||||
| >100 | 19 (31.15) | 115 (52.51) | 1.70 | 8.40–3.43 | <0.001 * | |
| 51–100 | 42 (68.85) | 92 (42.01) | 2.21 | 1.36–3.59 | 0.001 * | |
| ≤50 | 0 (0) | 12 (5.48) | Ref. | |||
| Mean ± SD | 96.62 ± 21.16 | 101.82 ± 28.58 | ||||
| Systolic blood pressure (mmHg) | 12 (4.16) | |||||
| >140 | 13 (21.31) | 35 (16.28) | 3.72 | 1.47–7.47 | 0.004 * | |
| 81–140 | 40 (65.57) | 90 (41.86) | 3.77 | 1.85–7.69 | <0.001 * | |
| ≤80 | 8 (13.11) | 90 (41.86) | Ref. | |||
| Mean ± SD | 114.98 ± 26.69 | 98.65 ± 38.17 | ||||
| Diastolic blood pressure (mmHg) | 12 (4.16) | |||||
| >80 | 8 (13.11) | 97 (45.12) | 3.94 | 1.74–8.93 | 0.001 * | |
| 51–80 | 41 (67.21) | 90 (411.86) | 4.11 | 2.01–8.30 | <0.001 * | |
| <50 | 12 (19.67) | 28 (13.02) | Ref. | |||
| Mean ± SD | 69.18 ± 16.74 | 57.81 ± 25.15 | ||||
| Mean arterial pressure (mmHg) | 12 (4.16) | |||||
| >95 | 14 (22.95) | 41 (19.07) | 2.88 | 1.33–6.24 | 0.007 * | |
| 65–95 | 38 (62.30) | 81 (37.67) | 3.62 | 1.84–7.13 | <0.001 * | |
| <65 | 9 (14.75) | 93 (43.26) | Ref. | |||
| Mean ± SD | 84.44 ± 19.09 | 71.14 ± 28.45 | ||||
| Preoperative oxygen saturation | 12 (4.16) | |||||
| ≥90% | 56 (91.80) | 137(63.72) | 4.82 | 1.99–11.61 | <0.001 * | |
| <90% | 5 (8.20) | 78 (36.28) | Ref. | |||
| Median [Q1, Q3] | 99 [96, 100] | 97 [86, 100] | ||||
| The initial end-tidal carbon-dioxide (mmHg) | 37 (12.89) | |||||
| 35–45 | 16 (29.09) | 18 (10.06) | 2.41 | 1.53–3.80 | <0.001 * | |
| <35 | 39 (70.91) | 161 (89.94) | Ref | |||
| Mean ± SD | 29.34 ± 7.97 | 23.91 ± 8.91 | ||||
| Variables | Survival (n = 61) | Death (n = 227) | RR | 95%CI | p-Value |
|---|---|---|---|---|---|
| Time of cardiac arrest | |||||
| Working hours | 33 (54.10) | 87 (38.33) | 1.65 | 1.05–2.58 | 0.028 * |
| Non-working hours | 28 (45.90) | 140 (61.67) | Ref. | ||
| Initial rhythm at time of CPR | |||||
| Shockable rhythm (pVT/VF) | 14 (22.95) | 30 (13.22) | 1.65 | 0.99–2.73 | 0.050 * |
| Non-shockable rhythm | |||||
| (PEA/asystole) | 47 (77.05) | 197 (86.78) | Ref. | ||
| Place where the cardiac arrest occurred | |||||
| In the operating room | 58 (95.08) | 194 (85.46) | 2.76 | 0.91–8.37 | 0.072 |
| ICU/PACU | 3 (4.92) | 33 (14.54) | Ref. | ||
| Duration of CPR | |||||
| ≤30 min | 57 (93.44) | 160 (70.48) | 4.66 | 1.75–12.42 | 0.002 * |
| >30 min | 4 (6.56) | 67 (29.52) | Ref. | ||
| Median [Q1, Q3] | 15 [5, 20] | 20 [10, 35] | |||
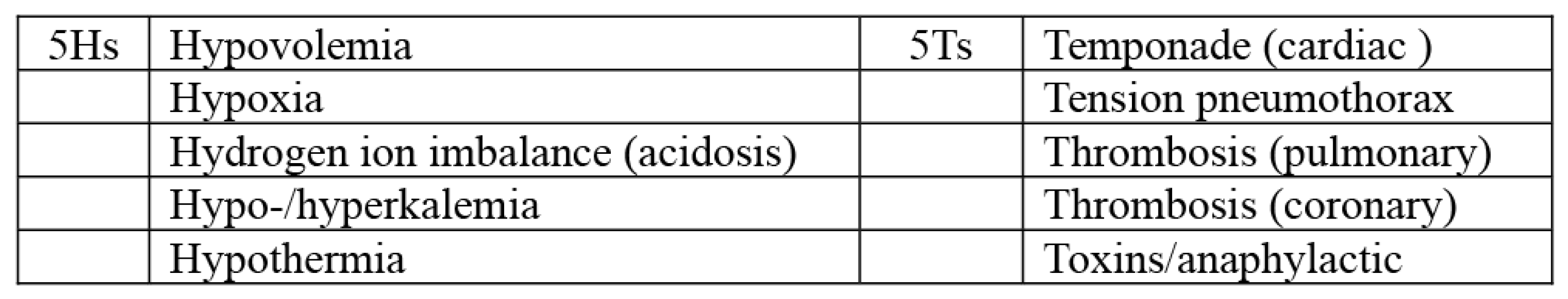
| Cause of POCA | Total (n = 288) | Survive (n = 61) | Death (n = 227) |
|---|---|---|---|
| Hypovolemia | 214 (74.30%) | 37 (60.66) | 177 (77.97) |
| Hypoxia | 28 (9.72%) | 9 (14.75) | 19 (8.37) |
| Hydrogen ions (acidosis) | 18 (6.25%) | 5 (8.20) | 13 (5.73) |
| Hypothermia | 3 (1.04%) | 0 (0) | 3 (1.32) |
| Cardiac tamponade | 1 (0.35%) | 1 (1.64) | 0 (0) |
| Tension pneumothorax | 4 (1.39%) | 1 (1.64) | 3 (1.32) |
| Pulmonary thrombosis | 1 (0.35%) | 0 (0) | 1 (0.44) |
| Coronary thrombosis | 16 (5.56%) | 5 (8.20) | 11 (4.85) |
| Toxins/anaphylactic | 3 (1.04%) | 3 (4.92) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chungsaengsatitayaporn, S.; Pipanmekaporn, T.; Khorana, J.; Leurcharusmee, P.; Boonsri, S.; Siriphuwanun, V. Predictive Factors for 24-h Survival After Perioperative Cardiopulmonary Resuscitation: Single-Center Retrospective Cohort Study. J. Clin. Med. 2025, 14, 599. https://doi.org/10.3390/jcm14020599
Chungsaengsatitayaporn S, Pipanmekaporn T, Khorana J, Leurcharusmee P, Boonsri S, Siriphuwanun V. Predictive Factors for 24-h Survival After Perioperative Cardiopulmonary Resuscitation: Single-Center Retrospective Cohort Study. Journal of Clinical Medicine. 2025; 14(2):599. https://doi.org/10.3390/jcm14020599
Chicago/Turabian StyleChungsaengsatitayaporn, Soontarin, Tanyong Pipanmekaporn, Jiraporn Khorana, Prangmalee Leurcharusmee, Settapong Boonsri, and Visith Siriphuwanun. 2025. "Predictive Factors for 24-h Survival After Perioperative Cardiopulmonary Resuscitation: Single-Center Retrospective Cohort Study" Journal of Clinical Medicine 14, no. 2: 599. https://doi.org/10.3390/jcm14020599
APA StyleChungsaengsatitayaporn, S., Pipanmekaporn, T., Khorana, J., Leurcharusmee, P., Boonsri, S., & Siriphuwanun, V. (2025). Predictive Factors for 24-h Survival After Perioperative Cardiopulmonary Resuscitation: Single-Center Retrospective Cohort Study. Journal of Clinical Medicine, 14(2), 599. https://doi.org/10.3390/jcm14020599






