Abstract
Background: Preterm birth, even for moderate or late preterm infants (MLPIs), is associated with longer-term developmental challenges. Family Integrated Care (FICare) models of care, like Alberta FICare, aim to improve outcomes by integrating parents into neonatal care during hospitalization. This follow-up study examined the association between models of care (Alberta FICare versus standard care) and risk of child developmental delay at 18 months corrected age (CA) and explored the influences of maternal psychosocial distress. Methods: We assessed 257 mothers and 298 infants from a cluster randomized controlled trial (ID: NCT0279799) conducted in ten Level II NICUs in Alberta, Canada. Risk of delay was assessed using developmental screening tests. Maternal psychosocial distress was assessed using self-reported measures of depressive symptoms, anxiety, parenting stress, and self-efficacy. Results: There was no association between model of care and risk of developmental delay. Higher maternal parenting stress was associated with increased risk of developmental delay. Conclusions: Alberta FICare was not associated with decreased risk of developmental delay at 18 months CA. Maternal parenting stress may play an important role in the development of MLPIs and should be addressed post-discharge.
1. Introduction
Globally, preterm birth affects approximately 10% of live births [1]. Approximately 15% of preterm infants are born very preterm, at <32 weeks gestational age (GA); the remaining 85% are moderate or late preterm infants (MLPIs), born between 32 weeks and zero days and 36 weeks and 6 days GA [1,2]. Due to advances in neonatal intensive care, preterm infants are more likely to survive; however, many have morbidities [3]. As gestational age (GA) decreases, the risk of morbidity increases [4,5]. Compared to their full-term counterparts, MLPIs are at higher risk for (1) poor health (e.g., respiratory, growth, and feeding problems) [6,7,8], (2) poor developmental outcomes (e.g., neurodevelopmental disabilities, cognitive and language delays, and school-related problems) [6,9,10], and (3) emotional and behavioral problems [9,11,12,13]. At school entry, 8.3% of MLPIs have developmental delays, including fine motor, communication, and personal-social skills [14]. These deficits likely stem from early disruptions in neural development and postnatal environmental influences, which creates greater risk for developmental delay in MLPIs.
At birth, MLPIs often require care in a neonatal intensive care unit (NICU). NICUs are critical care environments where healthcare providers often unintentionally marginalize parents in the pursuit of lifesaving care of the infant [15,16]. The unexpected birth of a preterm infant often leaves parents in shock, feeling anxious, depressed, isolated, and unprepared to interact with, and care for, their newborn [15,17]. Preterm birth and experiences in the NICU also disrupt human milk feeding [18] and early parent–infant relationships [19,20], which are necessary conditions for optimal child development [20,21,22]. While maternal mental health does not affect their involvement in the care of very preterm infants while in hospital [23], the negative effects of the postnatal family environment on preterm child development after discharge is clear. For example, in a Danish population cohort population cohort (N = 19,017), postpartum depression, as measured by the Edinburgh Postnatal Depression Scale, and preterm birth (30 to 36 weeks’ gestation) predicted infant social withdrawal up to an infant age of 12 months [24]. Social withdrawal is considered prodromal to socio-emotional problems in childhood and mental illnesses in adulthood. In a narrative review, low maternal education and social disadvantage also negatively influenced cognitive, motor, and behavior development in children born at <28 weeks’ gestation [25]. Thus, preterm birth and family environment are associated with a complex variety of negative outcomes for the infant.
Family-integrated models of care aim to educate and support parents to become members of the NICU team [26]. Healthcare provider and parent roles shift dynamically as parents become more knowledgeable and confident in caring for their infant [27]. However, interventions to improve developmental outcomes in preterm infants delivered in the NICU and/or post discharge have demonstrated mixed results [28,29]. Several studies have demonstrated benefits to child development of integrating families into their preterm (<33 weeks’ gestation) infant’s care team during their Level III NICU stay [30,31,32]. In a systematic review and meta-analysis, the authors found that preterm (<37 weeks) infants who received a variety of interventions (e.g., environmental stress controls, individualized developmental care, and maternal education to understand infant behavioral cues) had significantly better cognitive and motor outcomes at 12 months and better psychomotor development at age 24 months [33]. This meta-analysis was limited by the heterogeneity of interventions and few randomized controlled trials. In another systematic review and meta-analysis of RCTs of family-centered care interventions in the NICU, the authors found no effect on infant neurobehavioral development, although this review included only three RCTs that assessed neurobehavioral development [34]. However, the included studies dated back to 2006 and pre-dated two cluster RCTs with positive results of developmental follow-up [35,36]. In a follow-up study of a Canadian subsample (N = 126) from a 25-site cluster randomized controlled trial (FICare n = 895; standard care n = 891) with preterm infants <33 weeks’ gestation [30], the authors found group differences in child internalizing and externalizing behavior problems favoring FICare, which were mediated by maternal hair cortisol levels but not subjective measures of stress [37]. Taken together, these results suggest that interventions to support preterm infant development that are delivered in the NICU may be effective for early preterm infants, but positive effects may erode over time. Also, most previous studies were conducted in Level III NICUs; none were focused exclusively on MLPIs, who typically receive care in a Level II NICU. Thus, follow-up studies on the association of models of neonatal intensive care and risk of developmental delays in MLPIs are needed to optimize models of care of these infants and their families in the NICU.
1.1. Objectives
The objective of this study was to examine the association of models of neonatal intensive care (Alberta FICare versus standard care) and the risk of child development in MLPIs at age 18 months corrected age (CA). The primary research question was as follows: Compared to standard care in a Level II neonatal intensive care unit (NICU) and controlling for covariates, does Alberta FICare influence child global development of MLPIs at 18 months as measured by the Ages and Stages Questionnaires, 3rd Edition (ASQ-3) [38]. The secondary research questions were as follows: Compared to standard care in a Level II NICU and controlling for covariates, does Alberta FICare influence child social emotional and behavioral development at 18 months CA as measured by the Ages and Stages Questionnaires: Social-Emotional, 2nd Edition (ASQ:SE-2) [39], and the Brief Infant Toddler Social Emotional Assessment (BITSEA) [40]. As covariates, we used child and maternal characteristics that have been identified as contributing to child development. Maternal characteristics included depressive symptoms, anxiety, parenting stress, and self-efficacy, as measured by the Center for Epidemiologic Studies Depression Scale—Revised (CESD-R), [41] the State Trait Anxiety Inventory (STAI) [42] State Anxiety subscale, the Parenting Stress Index, 4th Edition Short Form (PSI-4-SF) [43], and the General Self-Efficacy (GSE) [44] scale. We hypothesized that, after controlling for child and maternal characteristics, Alberta FICare would decrease the risk of developmental delay at age 18 months CA.
1.2. Theoretical Framework
We applied Bronfenbrenner’s [45] theory of bio ecological development to this study. Bronfenbrenner asserts that multi-factorial interactions between individuals and micro-, meso-, exo-, and macro-systems influence children’s development. Specifically, at the micro-system level, interactions occur between individuals internal or external to the family. At the meso-system level, interactions occur between two or more micro-systems, such as family and health care settings. At the exo-system level, interactions between events and processes, and family and healthcare settings are included. Finally, at the macro-system level, culture is embedded across each of the previous systems and influences child development. Thus, child development is complex and influenced by multiple factors at multiple levels in their environment.
2. Materials and Methods
2.1. Study Design and Setting
This study was the 18-month-old follow-up to our cluster randomized controlled trial (cRCT) evaluating the effects of Alberta FICare in ten Level II NICUs in Alberta, Canada [27]. See clinicaltrials.gov ID: NCT0279799. Entire NICUs were randomized to five Alberta FICare sites and five standard care sites. At the time of this study, Alberta had a single integrated healthcare system serving a population of 4.4 million people, with approximately 50,000 births per year [46]. Alberta Health Services espoused the philosophy of family centered care [47]: none of the sites had previously implemented a family integrated model of care.
2.2. Sample
For the cRCT, we recruited mothers and their singleton or twin preterm infants born between 32 weeks and 0 days and 34 weeks and 6 days GA between December 2016 and July 2018 [27]. GA was based on the 12-week ultrasound. The upper limit of GA was selected to ensure that infants received at least one week exposure to Alberta FICare, because otherwise healthy MLPIs who are gaining weight are often discharged from Canadian NICUs at 36 weeks and zero days. Discharge criteria may vary by country. We excluded mothers with language or social issues as well as infants who required palliative care or had severe congenital or chromosomal anomalies. Follow-up data were collected between October 2017 and March 2020. Of the 614 mothers of 718 infants included in the cRCT outcome analyses, there were 257 mothers of 298 children included in the 18-month follow-up study. See Figure 1, CONSORT flow diagram. The final sample consisted of 111 mothers of 135 children in the Alberta FICare group and 146 mothers of 163 children in the standard care group. Compared to mothers who did not participate in the 18-month follow-up study, mothers who participated were more likely to be partnered (96.9% versus 91.7%, p = 0.01), Caucasian (76.0% versus 61.9%, p < 0.001), have post-secondary education (83.1% versus 74.8%, p = 0.02), and report an annual family income over $80,000 CAD (66.9% versus 47.1%, p < 0.001).
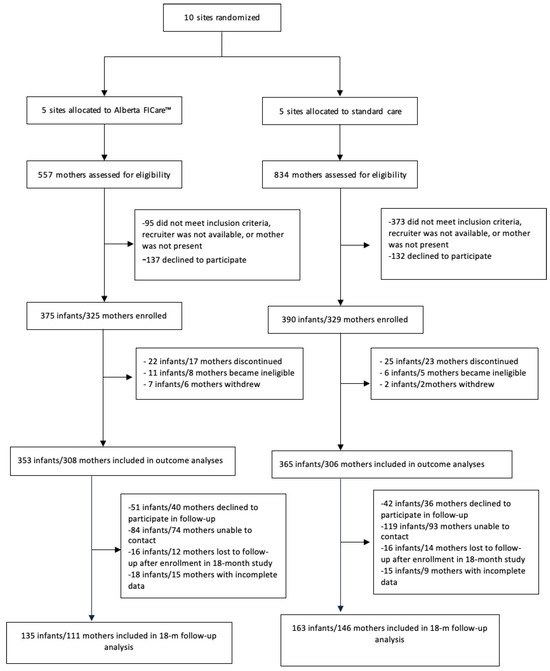
Figure 1.
CONSORT flow diagram. Note: FICare = Family Integrated Care.
2.3. Intervention
Alberta FICare is a psycho-educational intervention with three components, which empower parents to sequentially build their knowledge, skill, and confidence so they are well prepared to care for their preterm infant before discharge. See Figure 2. Parents are educated and coached to provide routine, non-medical care; healthcare providers continue to provide medical and technical care, such as intravenous medications and procedures, legal documentation, and professional support for families [48]. In the cRCT, compared to infants in the standard care group, infants in the Alberta FICare group were discharged 2.55 days sooner without concomitant increases in emergency department visits and readmissions [27]. When Alberta FICare was scaled to all 14 NICUs in the province, there were lower-than-expected lengths of stay, emergency department visits, and readmissions [49].
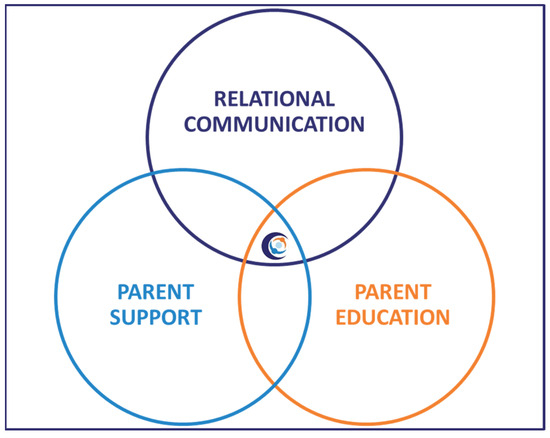
Figure 2.
Components of Alberta Family Integrated Care.
2.4. Measures
See Table 1 for characteristics of measures used in the follow-up study. Maternal psychological distress was measured at 18 months using the Center for Epidemiological Studies Depression scale—Revised (CESD-R) [41], State Trait Anxiety Scale (STAI) [42], and Parenting Stress Index (PSI) [43]. Child age was adjusted for prematurity for all measures of child development.

Table 1.
Characteristics of measures.
2.5. Procedures
To retain participants in the study, we maintained contact through quarterly newsletters to participants and a birthday card sent to the individual child. Approximately one month prior to the child turning 18 months CA, research assistants contacted eligible mothers to inquire about their interest in the follow-up study. For interested mothers, we sent a link to an electronic informed consent form. After mothers completed the consent form, we sent a link to the surveys, which were completed at admission and discharge and at 18 months CA. For mothers who did not complete the survey within two weeks, we sent two reminder emails. At 18 months, eleven mothers completed the online survey outside the time frame for the ASQ-3. In those cases, we excluded the ASQ-3 scores from analysis; however, we retained scores on the other measures, as they were valid. Six children were already past 18 months CA when recruitment for this follow-up study started. Therefore, four mothers completed the 20-month version, and two mothers completed the 22-month version of the ASQ-3
2.6. Data Analysis
We examined data for missing values and patterns of missing values. Where possible, we replaced missing values according to scale developers’ recommendations. In the absence of recommendations, we replaced up to 20% of the missing values with the group mean on that item. We analyzed data using Chi-square tests for categorical variables and t-tests for continuous variables. We estimated Pearson’s bivariate correlations between child development risk on three assessments (ASQ-3, ASQ:SE-2, and BITSEA) and potential covariates (infant and maternal characteristics). Independent variables with correlations of p < 0.01 were included in the final logistic regression models. The p-value was set to 0.05. IBM SPSS Version 28 was used for statistical analyses.
3. Results
3.1. Child and Maternal Characteristics by Group
In the follow-up sample, there were significant differences between groups in the proportion of singletons and maternal ethnicity. For the follow-up sample, mothers in the Alberta FICare group reported significantly more singleton births and Caucasian ethnicity than mothers in the standard care group. There were no other group differences in child or maternal socio-demographic characteristics. See Table 2.

Table 2.
Comparison of child and maternal characteristics by Alberta FICare versus standard care group.
3.2. Risk of Child Developmental Delay and Maternal Scale Scores
See Table 3 for frequencies and percentages of risk for developmental delay on the ASQ-3, ASQ:SE-2, and BITSEA domains by group (Alberta FICare versus standard care). See Table 4 for scores on maternal depressive symptoms, anxiety, parenting stress, and self-efficacy scales. In three logistic regression models, we examined the associations between maternal socio-demographic characteristics, maternal psychosocial distress, maternal self-efficacy, and group with child global development (ASQ-3) and social and emotional difficulties (ASQ:SE-2 and BITSEA). We found that mothers who were not partnered reported higher risk of global delay (ASQ-3) and social-emotional problems (ASQ: SE-2) for their child. Mothers who were born in Canada reported a lower risk of social-emotional difficulties (ASQ:SE-2 and BITSEA) and behavioral problems (BITSEA). Mothers who reported higher parenting stress reported a higher risk of global delay (ASQ-3) and of social emotional difficulties (ASQ:SE-2 and BITSEA) and behavioral problems (BITSEA). Maternal age, education, depressive symptoms, anxiety, and self-efficacy did not contribute to the risk of child developmental delay at 18 months CA. Contrary to our hypothesis, group did not contribute significantly to risk of child developmental delay. See Table 5.

Table 3.
Frequencies and percentages of risk for child developmental delay by group.

Table 4.
Means and standard deviations on maternal scales by group.

Table 5.
Logistic regression models for associations between maternal socio-demographic characteristics, maternal psychosocial distress, maternal self-efficacy, and group and child global development (ASQ-3) and social emotional and behavioral difficulties (ASQ:SE-2 and BITSEA).
See Supplementary Table S1 for Pearson’s correlations to identify covariates that were included in the models. Except for group, which we hypothesized would make a difference in risk of child developmental delay at age 18 months, we included covariates in the models that were significant at p < 0.01. No other variables that were collected at admission, discharge, or 18 months were associated with risk of child developmental delay.
4. Discussion
In this follow-up study of mothers and their MLPIs from a cRCT (N = 654), Alberta FICare in Level II NICUs was not associated with decreased risk of child global development delay or social-emotional and behavioral difficulties at age 18 months CA. However, increased maternal parenting stress was associated with increased risk of developmental delay on each measure. The results of our follow-up study of MLPIs in Level II NICUs contrast with results from two follow-up studies of early preterm infants (<33 weeks’ gestation) from a cRCT (N = 1786) of FICare in Level III NICUs [37,50]. In a subsample (N = 123) between 18 and 21 months, FICare was associated with lower child dysregulation, suggesting that FICare had a sustained positive effect on infant behavior [50]. In a similar subsample (N = 126) at 18 months, FICare was associated with decreased child internalizing and externalizing problems, and this association was mediated by lower maternal stress hormones [37]. One explanation for the lack of significant differences in the risk of developmental delay between MLPIs in Level II NICUs and early preterm in Level III NICUs may be related to the relatively shorter length of stay of MLPIs. A shorter length of stay limits exposure to FICare practices and may reduce the potential for a longer-term influence of the model of care. Also, MLPIs generally have fewer health complications than early preterm infants, reducing the opportunity for interventions like FICare to influence their neurodevelopment [6].
4.1. Maternal Psychosocial Distress and Child Development
In contrast to the results of a systematic review and meta-analysis of psycho-educational interventions for parents of preterm infants that identified positive effects on maternal depression and stress [51], in our study, Alberta FICare was unrelated to maternal psychosocial distress. Shorter length of NICU stay, and the limited exposure of mothers of MLPIs to the model of care may explain why there was no association between Alberta FICare and maternal psychosocial distress.
In our follow-up study, maternal parenting stress was associated with increased risk of child developmental delay for both the Alberta FICare and standard care groups. These results suggest that maternal psychosocial distress after discharge may have a stronger influence on the developmental outcomes of MLPI infants than exposure to FICare. These results are consistent with other studies that suggest post-discharge environments and maternal factors play a more significant role in a child’s development than interventions in the NICU [52,53].
4.2. Maternal Self-Efficacy and Child Development
In our study, Alberta FICare was not related to maternal general self-efficacy. In contrast, a systematic review reported that psychosocial interventions in the NICU and community increased self-efficacy for mothers of preterm infants, but only up to 6 months [28]. It is possible that any positive influence of Alberta FICare on maternal self-efficacy eroded by 18 months. Alternatively, in our study we used a measure of general self-efficacy. The use of a measure specific to parenting self-efficacy for a preterm infant may have yielded different results.
4.3. Maternal Demographic Characteristics and Child Development
In our follow-up study, maternal marital status and whether the mother was born in Canada were associated with the risk of child developmental delay. Partnered mothers reported fewer developmental concerns for their child compared to mothers who were parenting alone. Our results are consistent with previous research with mothers of infants (N = 2023) with a birth weight of 1250 g [54]. Compared to mothers in two-parent families, mothers who parented alone were younger and had less education and income [54]. At age 3 years CA, infants living in lone-parent families had greater diagnosed mild/moderate impairments [54]. In a systematic review, the quality of family (defined as > two individuals) interactions was more important for cognitive and behavioral development in preterm than term children [55]. This review did not include studies of families with lone-parent mothers, who face greater emotional and financial stress. Together, these results reinforce the need for supportive interventions for interactions in lone-parent families to improve developmental outcomes, even for children who are born moderate and late preterm. In our study, mothers who were not born in Canada reported higher levels of child social-emotional and behavioral difficulties. This may be influenced by differences in values, beliefs, and parenting practices in different cultures [56]. Divergence in developmental expectations that parents have for their children in their current context and country of origin may conflict. These differences may explain why mothers who were not born in Canada reported higher levels of social-emotional and behavioral difficulties for their child.
4.4. Limitations and Future Research
Strengths of this follow-up study include a larger sample than many previous studies, which is likely to decrease selection bias. However, loss to follow-up resulted in low completion rates and significant group differences in singleton versus twin births and ethnicity at 18 months. Loss to follow-up also resulted in a smaller-than-expected sample size. With small group differences (15.1% versus 12.5%) on the primary outcome (proportion at risk for delay on the ASQ-3), this study was underpowered to find group differences. In addition, this study included rigorous measurement of maternal characteristics and child development using reliable and valid scales. However, this study has several limitations. While FICare encourages parental involvement during the NICU stay, it may not adequately address the post-discharge needs of families. Future research should explore extending parental support after discharge, especially in addressing maternal psychosocial distress and parenting self-efficacy. While we measured whether mothers were partnered, we did not measure the quality of support from the partner. Future studies should assess the quality of social support from the mother’s partner. Additionally, this study relied on self-reported data for maternal psychosocial distress, which could introduce bias. Objective measures, such as physiological indicators of stress (e.g., cortisol levels), may provide more accurate assessments in future studies [37]. Moreover, this study focused exclusively on MLPIs, who generally have better developmental outcomes compared to early preterm infants [14]. Future studies should explore whether different subgroups of preterm infants benefit more from FICare interventions, particularly those born at earlier gestational ages, who may experience more severe developmental challenges. Assessment at 6, 12, and 18 months CA would provide information about erosion of the influence of Alberta FICare on child and maternal outcomes. A longer follow-up period, beyond 18 months, may also provide insights into the enduring effects of FICare on developmental outcomes at school age and beyond [14].
5. Conclusions
In conclusion, while the Alberta FICare model provides valuable support to mothers of MLPIs during the NICU stay, post-discharge maternal psychosocial distress and family environments played a more important role in determining risk of global and social-emotional developmental delays. Interventions targeting maternal psychosocial distress and parenting self-efficacy, particularly after discharge, may be key to reducing the risk of child developmental delays. Future research should aim to further understand the complex interplay between NICU interventions and post-discharge family environments, with a focus on developing longer-term support systems for mothers of MLPI infants with psychosocial distress.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm14020586/s1, Table S1: Pearson’s correlations for modeled variables.
Author Contributions
Conceptualization, K.M.B.; methodology, K.M.B.; validation, D.A.M.; formal analysis, K.M.B. and F.C.B.; resources, K.M.B.; data curation, K.M.B. and F.C.B.; writing—original draft, K.M.B.; writing—review and editing, F.C.B. and D.A.M.; Funding acquisition, K.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Alberta Innovates-Health Solutions, Partnership for Innovation in Health Services Research (Grant No. 201400399), Alberta Children’s Hospital Research Institute, and Canadian Institutes for Health Research (Grant No. 371297), with in-kind support from Alberta Health Services, Covenant Health, and the Faculty of Nursing, University of Calgary.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the University of Calgary, Conjoint Health Research Ethics Board (CHREB ID 17-0079), on 17 August 2017; the University of Alberta, Health Research Ethics Board (Pro00075902) on 18 October 2017; and the Covenant Health Research Ethics Board (NCT02879799) on 18 October 2017.
Informed Consent Statement
Informed consent was obtained from all participants involved in the study.
Data Availability Statement
Data are available to qualified researchers upon reasonable request to the corresponding author.
Conflicts of Interest
Fiona C. Bartram and Deborah A. McNeil declare no competing interests. Karen M. Benzies is the founder and CEO of Liminality Innovations Inc., a social enterprise that makes Alberta FICare™ accessible in jurisdictions outside Alberta.
Abbreviations
| ASQ-3 | Ages and Stages Questionnaire version |
| ASQ:SE-2 | Ages and Stages Questionnaire version |
| BITSEA | Brief Infant and Toddler Social Emotional Assessment |
| CA | corrected age |
| CESD-R | Center for Epidemiological Studies Depression scale, Revised |
| FICare | Family Integrated Care |
| GA | gestational age |
| GSE | General Self-Efficacy Scale |
| MLPI | moderate and late preterm infant |
| NICU | neonatal intensive care unit |
| PSI-4-SF | Parenting Stress Index version—short form |
| STAI | State Trait Anxiety Scale |
References
- Ward, V.C.; Lee, A.C.; Hawken, S.; Otieno, N.A.; Mujuru, H.A.; Chimhini, G.; Darmstadt, G.L. Overview of the global and US burden of preterm birth. Clin. Perinatol. 2024, 51, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Chawanpaiboon, S.; Vogel, P.; Moller, A.B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Health 2019, 7, e37–e46. [Google Scholar] [CrossRef] [PubMed]
- Crump, C. An overview of adult health outcomes after preterm birth. Early Hum. Dev. 2020, 150, 105187. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.L.; Wiingreen, R.; Jensen, A.; Rackauskaite, G.; Laursen, B.; Hansen, B.M.; Hoei-Hansen, C.E.; Greisen, G. The effect of gestational age on major neurodevelopmental disorders in preterm infants. Pediatr. Res. 2022, 91, 1906–1912. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.H.; Chou, J.; Brown, K.A. Neurodevelopmental outcomes of preterm infants: A recent literature review. Transl. Pediatr. 2020, 9, S3–S8. [Google Scholar] [CrossRef]
- Natarajan, G.; Shankaran, S. Short-and long-term outcomes of moderate and late preterm infants. Am. J. Perinatol. 2016, 33, 305–317. [Google Scholar]
- Huff, K.; Rose, R.S.; Engle, W.A. Late preterm infants: Morbidities, mortality, and management recommendations. Pediatr. Clin. 2019, 66, 387–402. [Google Scholar]
- Karnati, S.; Kollikonda, S.; Abu-Shaweesh, J. Late preterm infants-changing trends and continuing challenges. Int. J. Pediatr. Adolesc. Med. 2020, 7, 38–46. [Google Scholar] [CrossRef]
- Jin, J.H.; Yoon, S.W.; Song, J.; Kim, S.W.; Chung, H.J. Long-term cognitive, executive, and behavioral outcomes of moderate and late preterm at school age. Clin. Exp. Pediatr. 2020, 63, 219–225. [Google Scholar] [CrossRef]
- de Gamarra-Oca, L.F.; Ojeda, N.; Gómez-Gastiasoro, A.; Peña, J.; Ibarretxe-Bilbao, N.; García-Guerrero, M.A.; Loureiro, B. Long-term neurodevelopmental outcomes after moderate and late preterm birth: A systematic review. J. Pediatr. 2021, 237, 168–176. [Google Scholar] [CrossRef]
- Dotinga, B.M.; Winter, A.F.D.; Bocca-Tjeertes, I.F.; Kerstjens, J.M.; Reijneveld, S.A.; Bos, A.F. Longitudinal growth and emotional and behavioral problems at age 7 in moderate and late preterms. PLoS ONE 2019, 14, e0211427. [Google Scholar] [CrossRef] [PubMed]
- Faleschini, S.; Matte-Gagné, C.; Côté, S.; Tremblay, R.E.; Boivin, M. Trajectories of behavioral problems among moderate-late preterm children from 4 to 10 years: A prospective population-based study. Early Hum. Dev. 2020, 143, 104964. [Google Scholar] [CrossRef]
- Cheong, J.; Doyle, L.; Burnett, A.; Lee, K.; Walsh, J.; Potter, C.; Treyvaud, K.; Thompson, D.; Olsen, J.; Anderson, P. Association between moderate and late preterm birth and neurodevelopment and social-emotional development at age 2 years. JAMA Pediatr. 2017, 171, e16485. [Google Scholar] [CrossRef] [PubMed]
- Synnes, A.; Hicks, M. Neurodevelopmental outcomes of preterm children at school age and beyond. Clin. Perinatol. 2018, 45, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Haward, M.F.; Lantos, J.; Janvier, A. Helping parents cope in the NICU. Pediatrics 2020, 145, e20193567. [Google Scholar] [CrossRef] [PubMed]
- Labrie, N.H.; Veenendaal, N.R.V.; Ludolph, R.A.; Ket, J.C.; Schoor, S.R.V.D.; Kempen, A.A.V. Effects of parent-provider communication during infant hospitalization in the NICU on parents: A systematic review with meta-synthesis and narrative synthesis. Patient Educ. Couns. 2021, 104, 1526–1552. [Google Scholar] [CrossRef]
- Roque, A.T.F.; Lasiuk, G.C.; Radünz, V.; Hegadoren, K. Scoping review of the mental health of parents of infants in the NICU. J. Obstet. Gynecol. Neonatal Nurs. 2017, 46, 576–587. [Google Scholar] [CrossRef]
- Quitadamo, P.A.; Zambianco, F.; Palumbo, G.; Wagner, X.; Gentile, M.A. Monitoring the use of human milk, the ideal food for very low-birth-weight infants—A narrative review. Foods 2024, 13, 649. [Google Scholar] [CrossRef]
- Øberg, G.K.; Sørvoll, M.; Labori, C.; Girolami, G.L.; Håkstad, R.B. A systematic synthesis of qualitative studies on parents’ experiences of participating in early intervention programs with their infant born preterm. Front. Psychol. 2023, 14, 1172578. [Google Scholar] [CrossRef]
- Givrad, S.; Hartzell, G.; Scala, M. Promoting infant mental health in the neonatal intensive care unit (NICU): A review of nurturing factors and interventions for NICU infant-parent relationships. Early Hum. Dev. 2021, 154, 105281. [Google Scholar] [CrossRef]
- Hartzell, G.; Shaw, R.J.; Givrad, S. Preterm infant mental health in the neonatal intensive care unit: A review of research on NICU parent-infant interactions and maternal sensitivity. Infant Ment. Health J. 2023, 44, 837–856. [Google Scholar] [CrossRef] [PubMed]
- Treyvaud, K.; Spittle, A.; Anderson, P.J.; O’Brien, K. A multilayered approach is needed in the NICU to support parents after the preterm birth of their infant. Early Hum. Dev. 2019, 139, 104838. [Google Scholar] [CrossRef] [PubMed]
- Dubner, S.E.; Morales, V.A.M.C.; Marchman, R.J.; Shaw, K.E.; Melissa, T.; Scala, M. Maternal mental health and engagement in developmental care activities with preterm infants in the NICU. J. Perinatol. 2023, 43, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Stuart, A.C.; Egmose, M.; Guedeney, I.; Væver, A.S.M. Associations between symptoms of maternal postpartum depression, gestational age and infant social withdrawal: A longitudinal study in a community cohort. Br. J. Dev. Psychol. 2022, 40, 371–383. [Google Scholar] [CrossRef]
- Spittle, A.; Orton, J.; Anderson, P.J.; Boyd, R.; Doyle, L.W. Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants. Cochrane Database Syst. Rev. 2015, 11, CD005495. [Google Scholar] [CrossRef]
- Franck, L.S.; Waddington, C.; O’Brien, K. Family integrated care for preterm infants. Crit. Care Nurs. Clin. N. Am. 2020, 32, 149–165. [Google Scholar] [CrossRef]
- Benzies, K.M.; Aziz, K.; Shah, V.; Faris, P.; Isaranuwatchai, W.; Scotland, J.; Larocque, J.; Mrklas, K.J.; Naugler, C.; Stelfox, H.T.; et al. Effectiveness of Alberta Family Integrated Care on infant length of stay in level II neonatal intensive care units: A cluster randomized controlled trial. BMC Pediatr. 2020, 20, 535. [Google Scholar] [CrossRef]
- Benzies, K.M.; Magill-Evans, J.E.; Hayden, K.A.; Ballantyne, M. Key components of early intervention programs for preterm infants and their parents: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2013, 13, S10. [Google Scholar] [CrossRef]
- Zhang, X.; Kurtz, M.; Lee, S.-Y.; Liu, H. Early intervention for preterm infants and their mothers: A systematic review. J. Perinat. Neonatal Nurs. 2021, 35, E69–E82. [Google Scholar] [CrossRef]
- O’Brien, K.; Robson, K.; Bracht, M.; Cruz, M.; Lui, K.; Alvaro, R.; Silva, O.D.; Monterrosa, L.; Narvey, M.; Ng, E. Effectiveness of Family Integrated Care in neonatal intensive care units on infant and parent outcomes: A multicentre, multinational, cluster- randomized controlled trial. Lancet Child Adolesc. Health 2018, 2, 245–254. [Google Scholar] [CrossRef]
- He, F.B.; Axelin, A.; Ahlqvist-Bjorkroth, S.; Raiskola, S.; Loyttyniemi, E. Effectiveness of the Close Collaboration with Parents intervention on parent-infant closeness in the NICU. Transl. Pediatr. 2020, 9, S3–S8. [Google Scholar] [CrossRef]
- Hei, M.; Gao, X.; Li, Y.; Gao, X.; Li, Z.; Xia, S.; Zhang, Q.; Han, S.; Gao, H.; Nong, S. Family integrated care for preterm infants in China: A cluster randomized controlled trial. J. Pediatr. 2021, 228, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, F.; Azari, N.; Ghiasvand, H.; Shahrokhi, A.; Rahmani, N. Do NICU developmental care improve cognitive and motoroutcomes for preterm infants? A systematic review and meta-analysis. BMC Pediatr. 2020, 20, 67. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhu, L.; Zhang, R.; Wang, L.; Wang, T.T.; Latour, J.M. Effects of family-centred care interventions on preterm infants and parents in neonatal intensive care units: A systematic review and meta-analysis of randomized controlled trials. Aust. Crit. Care 2019, 32, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Moe, A.M.; Kurilova, J.; Afzal, A.R.; Benzies, K.M. Effects of Alberta Family Integrated Care (FICare) on preterm infant development: Two studies at 2 months and between 6- and 24-months corrected age. J. Clin. Med. 2022, 11, 1684. [Google Scholar] [CrossRef]
- Synnes, A.R.; Petrie, J.; Grunau, R.E.; Church, P.; Kelly, E.; Moddemann, D.; Ye, X.; Lee, S.K.; O’Brien, K. Family integrated care: Very preterm neurodevelopmental outcomes at 18 months. Arch. Dis. Child. Fetal Neonatal Ed. 2022, 107, 76–81. [Google Scholar] [CrossRef]
- Mclean, M.A.; Scoten, O.C.; Yu, W.; Xiang, Y.; Ye, J.; Petrie, P.T.; Church, A.S.; Soraisham, L.S.M.; Weinberg, J.; Synnes, A.R. Lower maternal chronic physiological stress and better child behavior at 18 months: Follow-up of a cluster randomized trial of Neonatal Intensive Care Unit Family Integrated Care. J. Pediatr. 2022, 243, 107–115. [Google Scholar] [CrossRef]
- Squires, J.; Bricker, D. Ages & Stages Questionnaires—Third Edition (ASQ-3): A Parent-Completed Child Monitoring System; Paul H Brookes Publishing: Baltimore, MD, USA, 2009. [Google Scholar]
- Squires, J.; Bricker, D.; Twombly, E. Ages & Stages Questionnaires®: Social-Emotional, Second Edition (ASQ:SE-2TM); Paul H Brookes Publishing: Baltimore, MD, USA, 2015. [Google Scholar]
- Briggs-Gowan, M.J.; Carter, A.S. Manual for the Brief Infant-Toddler Social and Emotional Assessment (BITSEA) Version 2; Psychological Corporation: San Antonio, TX, USA, 2006. [Google Scholar]
- Eaton, W.; Muntaner, C.; Smith, C.; Tien, A.; Ybarra, M. Center for Epidemiologic Studies Depression Scale: Review and revision (CESD and CESD-R). In The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. Volume 3: Instruments for Adults, 3rd ed.; Maruish, M., Ed.; Lawrence Erlbaum: Mahwah, NJ, USA, 2004; pp. 363–377. [Google Scholar]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.E. Test manual for the State-Trait Anxiety Inventory; Consulting Psychologists Press: Palo Alto, CA, USA, 1970. [Google Scholar]
- Abidin, R.R. Parenting Stress Index, Fourth Edition Short Form; Psychological Assessment Resource: Lutz, FL, USA, 2012. [Google Scholar]
- Schwarzer, R.; Jerusalem, M. Generalized Self-Efficacy scale. In Measures in Health Psychology: A User’s Portfolio. Causal and Control Beliefs; Weinman, J., Wright, S., Johnston, M., Eds.; NFER-NELSON: Windsor, UK, 1995; pp. 33–39. [Google Scholar]
- Bronfenbrenner, U. The bioecological theory of human development. In Making Human Beings Human: Biological Perspectives on Human Development; Bronfenbrenner, U., Ed.; Sage: Thousand Oaks, CA, USA, 2005; pp. 3–15. [Google Scholar]
- Alberta Health Services. About AHS. 2023. Available online: https://www.albertahealthservices.ca/about/about.aspx (accessed on 14 January 2025).
- Yui, V.; Gordon, D.; Woods, S.; Pougnet, J. The Patient First Strategy; Alberta Health Services: Edmonton, AB, Canada, 2015. [Google Scholar]
- Benzies, K.M.; Shah, V.; Aziz, K.; Isaranuwatchai, W.; Palacio-Derflingher, L.; Scotland, J.; Larocque, J.; Mrklas, K.; Suter, E. Family Integrated Care (FICare) in Level II Neonatal Intensive Care Units: A study protocol for a cluster randomized controlled trial. Trials 2017, 18, 467. [Google Scholar] [CrossRef]
- Wasylak, T.; Benzies, K.; Mcneil, D.; Zanoni, P.; Osiowy, K.; Mullie, T.; Chuck, A. Creating value through Learning Health Systems: The Alberta Strategic Clinical Network experience. Nurs. Adm. Q. 2023, 47, 20–30. [Google Scholar] [CrossRef]
- Church, P.T.; Grunau, R.E.; Mirea, L.; Petrie, J.; Soraisham, A.S.; Synnes, A. Family Integrated Care (FICare): Positive impact on behavioural outcomes at 18 months. Early Hum. Dev. 2020, 151, 105196. [Google Scholar] [CrossRef]
- Chan, S.H.; Shefaly, S. Effectiveness of psychosocial interventions on the psychological outcomes of parents with preterm infants: A systematic review and meta-analysis. J. Pediatr. Nurs. 2024, 74, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.S.; Connell, A.; Dishion, T.J.; Wilson, M.N.; Gardner, F. Improvements in maternal depression as a mediator of intervention effects on early childhood problem behavior. Dev. Psychopathol. 2009, 21, 417–439. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, X.; Wang, Q.; Liu, L. Family-centered care interventions for preterm infants in the NICU: A systematic review and meta-analysis of randomized controlled trials. J. Clin. Nurs. 2021, 30, 651–662. [Google Scholar]
- Lodha, A.; Lakhani, J.; Ediger, K.; Tang, S.; Lodha, A.; Gandhi, V.; Creighton, D. Do preterm infants with a birth weight ≤ 1250g born to single-parent families have poorer neurodevelopmental outcomes at age 3 than those born to two-parent families? J. Perinatol. 2018, 38, 900–907. [Google Scholar] [CrossRef]
- Larsson, J.; Nyborg, L.; Psouni, E. The role of family function and triadic interaction on preterm child development: A systematic review. Children 2022, 9, 1695. [Google Scholar] [CrossRef]
- Meléndez, L. Parental beliefs and practices around early self-regulation: The impact of culture and immigration. Infants Young Child. 2005, 18, 136–146. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).