Effect of Dental Throat Pack Used Under General Anesthesia in Children with Special Healthcare Needs on Postoperative Nausea, Vomiting, and Sore Throat: A Randomized Clinical Trial
Abstract
1. Introduction
2. Methods
2.1. Ethical Approval
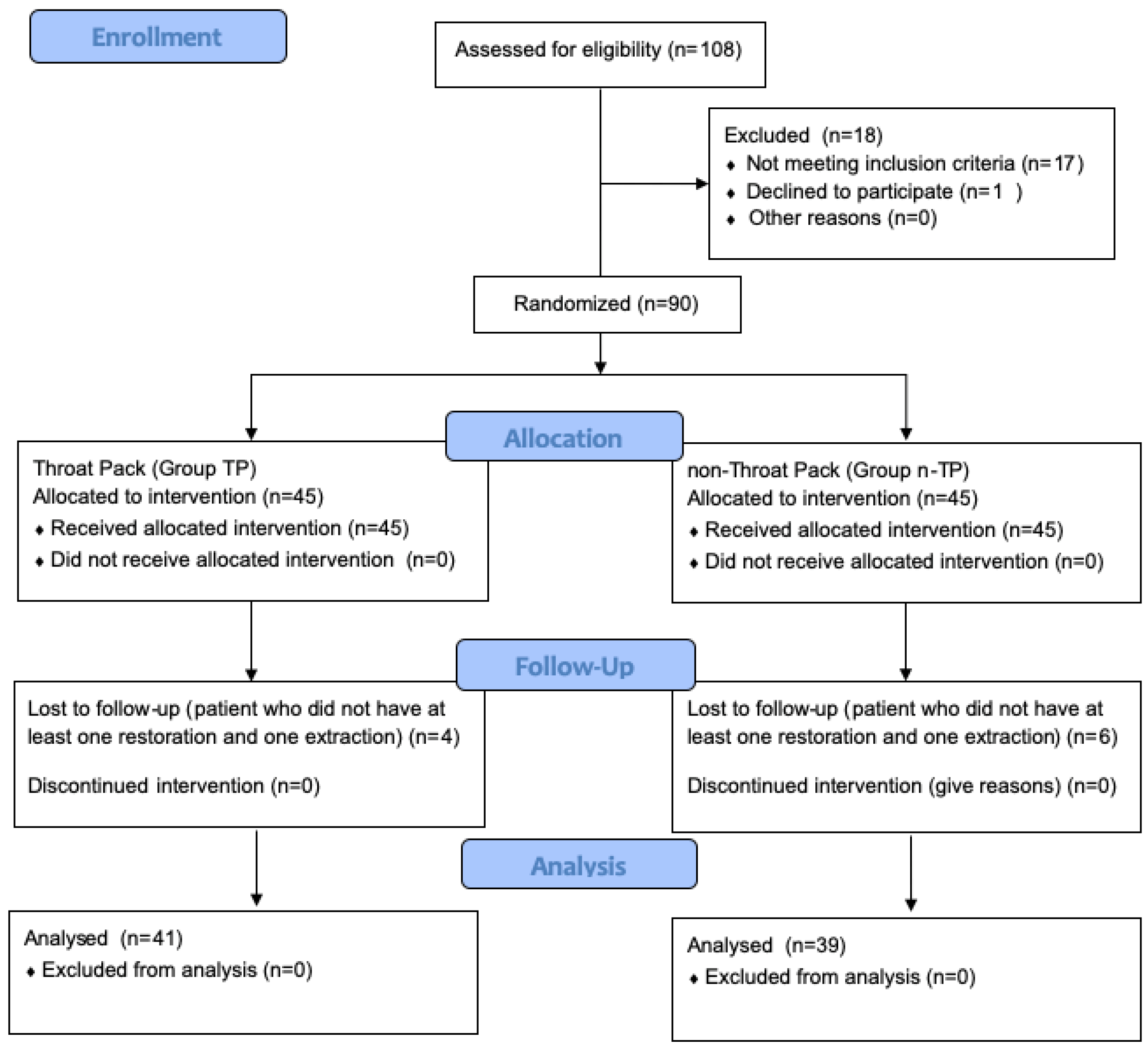
2.2. Sample Selection and Randomization
2.3. Procedures
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASA | American Society of Anesthesiologists |
| BARF | Baxter Retching Faces |
| ECG | Electrocardiography |
| Group n-TP | No throat packing |
| Group TP | Throat packing |
| NIBP | Noninvasive blood pressure |
| PONV | Postoperative nausea and vomiting |
| SHCN | Special healthcare needs |
| SpO2 | Oxygen saturation |
| SPSS | Statistical Package for the Social Sciences |
| VAS | Visual Analog Scale |
| VCV | Volume-controlled ventilation |
References
- Sexton, J.; Dohlman, L. Benefits of the pharyngeal pack. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 1989, 47, 891. [Google Scholar] [CrossRef]
- Apfel, C.C.; Heidrich, F.M.; Jukar-Rao, S.; Jalota, L.; Hornuss, C.; Whelan, R.P.; Zhang, K.; Cakmakkaya, O.S. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br. J. Anaesth. 2012, 109, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Turgut, H.C.; Alkan, M.; Kíp, G.; Ataç, M.S.; Altundağ, S.K.; Bozkaya, S.; Işik, B.; Arslan, M. Is age a determinant for nausea and vomiting in disabled patients after dental treatment under sedation? Niger. J. Clin. Pract. 2017, 20, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Athanassoglou, V.; Patel, A.; McGuire, B.; Higgs, A.; Dover, M.S.; Brennan, P.A.; Banerjee, A.; Bingham, B.; Pandit, J.J. Systematic review of benefits or harms of routine anaesthetist-inserted throat packs in adults: Practice recommendations for inserting and counting throat packs: An evidence-based consensus statement by the Difficult Airway Society (DAS), the British Association of Oral and Maxillofacial Surgery (BAOMS) and the British Association of Otorhinolaryngology, Head and Neck Surgery (ENT-UK). Anaesthesia 2018, 73, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Basha, S.I.; McCoy, E.; Ullah, R.; Kinsella, J.B. The efficacy of pharyngeal packing during routine nasal surgery–a prospective randomised controlled study. Anaesthesia 2006, 61, 1161–1165. [Google Scholar] [CrossRef] [PubMed]
- Council on Clinical Affairs. Guideline on management of dental patients with special health care needs. Pediatr. Dent. 2012, 34, 160–165. [Google Scholar]
- Phillips, C.; Brookes, C.D.; Rich, J.; Arbon, J.; Turvey, T.A. Postoperative nausea and vomiting following orthognathic surgery. Int. J. Oral Maxillofac. Surg. 2015, 44, 745–751. [Google Scholar] [CrossRef]
- Smarius, B.J.A.; Guillaume, C.H.A.L.; Jonker, G.; Mink van der Molen, A.B.; Breugem, C.C. The use of throat packs in pediatric cleft lip/palate surgery: A retrospective study. Clin. Oral Investig. 2018, 22, 3053–3059. [Google Scholar] [CrossRef]
- Lamperti, M.; Romero, C.S.; Guarracino, F.; Cammarota, G.; Vetrugno, L.; Tufegdzic, B.; Lozsan, F.; Frias, J.J.M.; Duma, A.; Bock, M.; et al. Preoperative assessment of adults undergoing elective noncardiac surgery Update guidelines from the European Society of Anaesthesiology and Intensive Care. Eur. J. Anaesthesiol. 2025, 42, 1–35. [Google Scholar] [CrossRef] [PubMed]
- White, P.F.; Eng, M.R. Ambulatory (Outpatinet) Anesthesia. In Miller’s Anesthesia, 7th ed.; Miller, R.D., Ericson, L.I., Fleisher, L.A., Wiener-Kronish, J.P., Young, W.L., Eds.; Churchill Livingstone, Elsevier: New York, NY, USA, 2010; pp. 2419–2460. [Google Scholar]
- Aldrete, J.A. The post-anesthesia recovery score revisited. J. Clin. Anesth. 1995, 7, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Baxter, A.L.; Watcha, M.F.; Baxter, W.V.; Leong, T.; Wyatt, M.M. Development and validation of a pictorial nausea rating scale for children. Pediatrics 2011, 127, e1542–e1549. [Google Scholar] [CrossRef]
- Anderson, C.R.; Premakumar, Y.; Navaratnam, A.V.; Rouhani, M.; Singh, A. The use of throat packs in ear, nose and throat, oral and dental surgery: A systematic review. Rhinology 2020, 58, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Al-Lami, A.; Amonoo-Kuofi, K.; Kulloo, P. A study evaluating the effects of throat packs during nasal surgery: A randomised controlled trial. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 3109–3114. [Google Scholar] [CrossRef] [PubMed]
- Green, R.; Konuthula, N.; Sobrero, M.; Saini, A.; Parasher, A.; Pool, C.; Levine, A.I.; DeMaria, S.; Tufts, R.; Govindaraj, S.; et al. Use of pharyngeal packs in functional endoscopic sinus surgery: A randomized controlled trial. Laryngoscope 2017, 127, 2460–2465. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, M.M.; Singh, R.B.; Rasheed, M.A.; Sarkar, A. Effects of different types of pharyngeal packing in patients undergoing nasal surgery: A comparative study. Anesth. Essays Res. 2015, 9, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Pabst, A.; Müller, D.; Thiem, D.G.; Scherhag, A.; Krüger, M.; Heimes, D.; Kämmerer, P.W. Effects of throat packs in upper airway surgery under intubation anesthesia: A randomized controlled trial. Clin. Oral Investig. 2022, 26, 6795–6804. [Google Scholar] [CrossRef]
- Jin, H.J.; Kim, S.; Hwang, S.H. Can pharyngeal packing prevent postoperative nausea and vomiting in nasal surgery? Laryngoscope 2019, 129, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Borna, R.; McCleary, S.; Wang, L.; Lee, A.; Saadat, S.; Grogan, T.; Partownavid, P.; Roostaeian, J. Effect of throat pack placement on the incidence of sore throat and postoperative nausea and vomiting in septorhinoplasty patients: A randomized controlled trial. Aesthetic Surg. J. 2022, 42, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Borna, R.; McCleary, S.; Wang, L.; Lee, A.; Saadat, S.; Grogan, T.; Partownavid, P.; Roostaeian, J. A randomized, double-blind study of the ultrasound assessment of the effect of pharyngeal packing on perioperative gastric volume in nasal surgery. BMC Anesthesiol. 2019, 19, 2–8. [Google Scholar] [CrossRef]
- Ames, W.A.; Machovec, K. An update on the management of PONV in a pediatric patient. Best Pract. Res. Clin. Anaesthesiol. 2020, 34, 749–758. [Google Scholar] [CrossRef]
- Schaefer, M.S.; Kranke, P.; Weibel, S.; Kreysing, R.; Ochel, J.; Kienbaum, P. Total intravenous anesthesia vs single pharmacological prophylaxis to prevent postoperative vomiting in children: A systematic review and meta-analysis. Pediatr. Anesth. 2017, 27, 1202–1209. [Google Scholar] [CrossRef]
- Goebel, U.; Schumann, S.; Wirth, S. Peak airway pressure is lower during pressure-controlled than during manual facemask ventilation for induction of anesthesia in pediatric patients—A randomized, clinical crossover trial. J. Anesth. 2019, 33, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, X.; Gong, W.; Li, S.; Wang, F.; Fu, S.; Zhang, M.; Hang, Y. Correlations between controlled endotracheal tube cuff pressure and postprocedural complications: A multicenter study. Anesth. Analg. 2010, 111, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Qi, Y.; Wu, L.; Jiang, G. Comparative efficacy of 6 topical pharmacological agents for preventive interventions of postoperative sore throat after tracheal intubation: A systematic review and network meta-analysis. Anesth. Analg. 2021, 133, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Powell, K.; Amin, D.; Sesanto, R.; Bryant, A.; Kukreja, P.; Waite, P. Do oropharyngeal throat packs prevent fluid ingestion during orthognathic surgery? Int. J. Oral Maxillofac. Surg. 2022, 51, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Ansari, L.; Bohluli, B.; Mhaseni, H.; Valaei, N.; Sadr-Eshkevari, P.; Rashad, A. The effect of endotracheal tube cuff pressure control on postextubation throat pain in orthognathic surgeries: A randomized double-blind controlled clinical trial. Br. J. Oral Maxillofac. Surg. 2014, 52, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Faro, T.F.; de Oliveira ESilva, E.D.; Campos, G.J.; Duarte, N.M.; Caetano, A.M.M.; Lureano Filho, J.R. Effects of throat packs during orthognathic surgery: A double-blind randomized controlled clinical trial. Int. J. Oral Maxillofac. Surg. 2021, 50, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Bhakta, P.; Srinivasan, S.; Khan, R.M.; Kaul, N. Acute tongue enlargement secondary to pharyngeal packing after tracheal intubation. Middle East. J. Anesthesiol. 2012, 21, 761–764. [Google Scholar] [PubMed]
- Basha, M.S. Missing pharyngeal pack endoscopically retrieved: An avoidable complication. Ann. Maxillofac. Surg. 2018, 8, 342–343. [Google Scholar] [CrossRef] [PubMed]
- Cousin, G.C.S.; Markose, G. The incidental finding of a retained ‘throat pack’. Ann. R. Coll. Surg. Engl. 2020, 102, e125. [Google Scholar] [CrossRef] [PubMed]
- Curran, J.; Ward, M.; Knepil, G. Reducing the Risk of Retained Throat Packs after Surgery; National Patient Safety Agency: London, UK, 2009; pp. 1–12. [Google Scholar]
| Group TP (n = 41) n (%) | Group n-TP (n = 39) n (%) | p-Value a | |
|---|---|---|---|
| Sex | |||
| Male | 24 (58.5) | 25 (64.1) | 0.610 |
| Female | 17 (41.5) | 14 (35.9) | |
| mean ± SD | mean ± SD | p-Value b | |
| Age | 8.59 ± 3.2 | 8.28 ± 2.8 | 0.808 |
| Weight | 26.2 ± 10.9 | 26.1 ± 12.0 | 0.965 |
| Group TP (n = 41) Mean ± SD | Group n-TP (n = 39) Mean ± SD | p-Value b | |
|---|---|---|---|
| Operation time | 68.5 ± 26.4 | 69.6 ± 27.9 | 0.843 |
| Dental procedure | |||
| Restorative treatments | 7.40 ± 4.7 | 6.75 ± 2.5 | 0.984 |
| Extractions | 6.06 ± 3.8 | 5.17 ± 3.1 | 0.306 |
| Group TP (n = 41) n (%) | Group n-TP (n = 39) n (%) | p-Value a | |
|---|---|---|---|
| Bloody | - | 8 (20.5) | 0.002 * |
| Not bloody | 41 (100) | 31 (79.5) |
| Group TP (n = 41) Mean ± SD | Group n-TP (n = 39) Mean ± SD | p-Value b | |
|---|---|---|---|
| Sore throat, VAS at 1 h | 1.32 ± 1.7 | 0.21 ± 0.9 | <0.001 ** |
| Sore throat, VAS at 2 h | 0.61 ± 1.1 | 0.05 ± 0.3 | 0.002 * |
| Sore throat, VAS at 4 h | 0.17 ± 0.6 | 0.0 ± 0.0 | 0.087 |
| PONV at 1 h | 0.0 ± 0.0 | 1.23 ± 1.7 | <0.001 ** |
| PONV at 2 h | 0.0 ± 0.0 | 0.26 ± 0.7 | 0.019 * |
| PONV at 4 h | 0.0 ± 0.0 | 0.05 ± 0.3 | 0.305 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tetiker, S.; Akdogan, H.N.; Alpay, N.; Dogan, M.C. Effect of Dental Throat Pack Used Under General Anesthesia in Children with Special Healthcare Needs on Postoperative Nausea, Vomiting, and Sore Throat: A Randomized Clinical Trial. J. Clin. Med. 2025, 14, 567. https://doi.org/10.3390/jcm14020567
Tetiker S, Akdogan HN, Alpay N, Dogan MC. Effect of Dental Throat Pack Used Under General Anesthesia in Children with Special Healthcare Needs on Postoperative Nausea, Vomiting, and Sore Throat: A Randomized Clinical Trial. Journal of Clinical Medicine. 2025; 14(2):567. https://doi.org/10.3390/jcm14020567
Chicago/Turabian StyleTetiker, Sibel, Hacer Nida Akdogan, Nilgun Alpay, and Muharrem Cem Dogan. 2025. "Effect of Dental Throat Pack Used Under General Anesthesia in Children with Special Healthcare Needs on Postoperative Nausea, Vomiting, and Sore Throat: A Randomized Clinical Trial" Journal of Clinical Medicine 14, no. 2: 567. https://doi.org/10.3390/jcm14020567
APA StyleTetiker, S., Akdogan, H. N., Alpay, N., & Dogan, M. C. (2025). Effect of Dental Throat Pack Used Under General Anesthesia in Children with Special Healthcare Needs on Postoperative Nausea, Vomiting, and Sore Throat: A Randomized Clinical Trial. Journal of Clinical Medicine, 14(2), 567. https://doi.org/10.3390/jcm14020567







